Introduction
Primary gallbladder cancer (GBC), though generally
considered rare, is the most common malignancy of the biliary
tract, ranking fifth in all types of tumors (1). Risk factors for GBC are manifold and
include ethnic background, region, age and genetics (2). For the previous few years,
epidemiological studies have revealed that an increased frequency
of GBC occurs in northern India, Pakistan and Korea (3,4).
Therefore, increased attention should be given to GBC in these
regions.
Due to vague and nonspecific symptoms during the
early stages, the majority of GBC patients present with advanced
disease at the time of initial diagnosis (5). The overall mean survival time for
patients with GBC is just 6 months, with a 5-year survival rate of
5% (6). In addition, ~50% patients
succumb to disease within 1 year of diagnosis (6). To date, surgical resection has been the
mainstay of therapy for GBC (1).
Although reports have demonstrated an increase in survival time for
patients who undergo surgical resection, only 20~30% of patients
are considered as suitable candidates for surgery (1). However, the patients who underwent
radical resections do not demonstrated significant differences in
5-year survival rates (5,7). Chemotherapy and radiation, the main
adjunctive therapies for surgical resection, have demonstrated no
statistically significant benefit for the treatment of advanced GBC
(8).
Due to only a small number of studies investigating
pathogenesis (9), our knowledge of
GBC holds back progress in the treatment of this tumor. It is now
widely accepted that the majority of tumors are linked to chronic
inflammatory states, particularly certain epithelial tumors
(10). Inflammatory mediators,
including cytokines, chemokines, nitrogen species and free radicals
may act as contributing factors during the process of GBC (2). Chronic exposure to these mediators leads
to release of growth factors by tumor cells themselves, which
results in the development and progression of cancer (10). For example, IL-8 is able to provoke
tumor cell proliferation by activating downstream signals of
epidermal growth factor receptor, and regulate tumor metastasis
through the cyclin D1 signaling pathway (11,12).
Although direct association between chronic cholecystitis and GBC
has been observed (13), the
underlying molecular mechanism remains to be elucidated.
The present study reports a promoting role of
exogenous IL-1β on the proliferation and migration of GBC cell
lines GBC-SD and SGC996. Additional investigation identified that
promotion depends on Twist, suggesting Twist may have a critical
role in IL-1β associated gallbladder carcinogenesis. Taken
together, the results of the present study reveal that inflammatory
mediators have a specific link to GBC.
Materials and methods
Cell culture and treatment
The GBC-SD GBC cell line was obtained from the China
Center for Type Culture Collection (Wuhan University, Wuhan,
China). The SGC996 cell line was provided by Tongji University
School of Medicine (Shanghai, China). The HIBEpiC cell line was
purchased from Sciencell Research Laboratories (Carlsbad, CA, USA).
GBC-SD and SGC996 cells were maintained at 37°C in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; GE Healthcare Life Sciences, Logan, UT, USA), 1 mM
non-essential amino acid (Sigma-Aldrich; EMD Millipore, Billerica,
MA, USA) and 1% penicillin/streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) in a 5% CO2 incubator. HIBEpiC cells
were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.).
Stock cultures were maintained at 80% confluence and passaged in
0.25% trypsin (Gibco; Thermo Fisher Scientific, Inc.) and 1%
ethylenediaminetetraacetic acid in Ca2+- and
Mg2+-free phosphate-buffered saline (PBS). Experimental
cells were subcultured in 25 or 75 cm2 flasks overnight
at 37°C.
Lentiviral transfection
To produce cells deficient in Twist,
Lenti-Twist-small hairpin RNA (shRNA) (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) was applied. In brief, GBC-SD and SGCC996
cell lines were cultured in 12-well plates for 24 h at 37°C, then 5
µg/ml Lenti-Twist-shRNA or Lenti-control was added. Following 24 h
of transfection at 37°C, cells were cultured in complete media
overnight at 37°C. To screen out stable transfected cells lines,
cells were subcultured in 25 cm2 flasks with 5 µg/ml
puromycin for 24–48 h at 37°C.
Patient samples
A total of 24 GBC samples were collected from
patients at the Department of General Surgery, Changzheng Hospital,
Second Military Medical University (Shanghai, China) between
January 2005 and January 2012. In the same period, 30 cases of
chronic cholecystitis specimens were collected from patients who
underwent cholecystectomy, and 8 normal gallbladder samples were
collected from patients who underwent the Whipple procedure as the
control group. All samples were stored at −70°C immediately
following resection until performance of subsequent enzyme-linked
immunosorbent assay (ELISA) experiments. Permission was granted by
the Ethical Committee of Changzheng Hospital, Second Military
Medical University to take biopsies from patients with GBC or
chronic cholecystitis. All patients consented to participate in the
present study.
Cell proliferation assays
Proliferation of cells cultured in the presence or
absence of exogenous IL-1β was measured using the water-soluble
tetrazolium salts (WST)-1 cell proliferation assay (Roche
Diagnostics GmbH, Mannheim, Germany). A total of 2×105
cells per well were seeded into a 96-well microplate. Exogenous
IL-1β was added to the wells of the microplates at the following
concentrations: 0, 1.25, 2.5, 5, 10, 20, 40, 80 and 160 ng/ml, and
incubated for 72 h at 37°C. Following aspiration of the growth
medium, the cells were washed once with 100 µl of PBS. DMEM (100
µl) containing 10% FBS was placed into the wells, followed by 10 µl
of WST-1 reagent. The cells were cultured in a CO2
incubator at 37°C for 1 h, and the absorbance at 450 nm, minus the
absorbance at 630 nm, was measured for each well using a microplate
reader.
Mouse xenografts
A total of 36 four-week-old immunodeficient nude
female mice were purchased from Shanghai Laboratory Animal Center,
Co., Ltd. (Shanghai, China). The mice were housed in a specific
pathogen-free environment, where temperature and humidity were
maintained at 21°C and 55%, respectively. Standard food and water
were available ad libitum. GBC-SD and SGC996 cells
(1×107 cells per mouse) were injected subcutaneously
into the right flanks of the mice (12 mice per group). When tumors
reached a volume of ~0.3 cm3, the mice were randomized
into two groups and injected intratumorally with 200 ng IL-1β or
PBS every two days for a 5-week period. Tumor formation was
monitored twice a week, and tumor volume based on caliper
measurements was calculated by the modified ellipsoidal formula
[tumor volume=1/2(length × width2)]. All animal
procedures were approved by the Ethical Committee of the Changzheng
Hospital, Second Military Medical University, and they were
performed in accordance with institutional guidelines.
Cell migration assays
For migration and invasion assays, 5×104
cells treated with IL-1β were plated in the top chamber of
Transwell inserts (EMD Millipore), with a membrane containing 8-mm
diameter pores in 200 µl serum-free media, in triplicate. The
inserts were subsequently placed into the bottom chamber wells of a
24-well plate containing media, with 10% FBS as a chemoattractant.
Following 24 h of incubation at 37°C, cells remaining on the
insert's top layers were removed with a cotton swab. Cells on the
lower surface of the membrane were fixed in 100% methanol for 20
min, followed by staining with Giemsa stain. The cell numbers in
five random fields (magnification, ×200) were counted under a light
microscope for each chamber, and the mean value was calculated.
Measuring IL-1β by ELISA
IL-1β secreted by cells or tissues was confirmed by
sandwich ELISA (R&D Systems, Inc., Minneapolis, MN, USA). For
detecting IL-1β content secreted by cells, cell culture medium was
transferred into a sterile tube and centrifuged for 20 min (500 ×
g) at 4°C, and the supernatant was carefully collected. For
detecting IL-1β content in tissues, 100 mg of tissue was fully
homogenized with PBS and protease inhibitors, and subsequently
centrifuged at 500 × g for 20 min at 4°C; the supernatant
was collected for subsequent experiments. All assays were performed
according to the manufacturer's protocol. The absorbance of the
supernatant was measured at 450 nm using a microplate reader.
Western blotting
Subconfluent cells were lysed in SDS Lysis Buffer
(Beyotime Institute of Biotechnology, Shanghai, China) and the
protein concentration was determined by the bicinchoninic acid
protein assay (Pierce Biotechnology; Thermo Fisher Scientific,
Inc.). A total of 30 µg protein samples were separated on a 12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and
transferred to a polyvinylidene difluoride membrane (Immobilon-P;
EMD Millipore). The membrane was blocked in 5% nonfat milk (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) in Tris-buffered saline and
Tween 20 (TBST; 10 mM Tris, 150 mM NaCl, pH 8.0 and 0.1% Tween 20)
for 1 h at room temperature. Membranes were probed with anti-Twist
(cat. no. sc-134136; 1:1,000; Santa Cruz Biotechnology, Inc.) and
anti-β-actin (cat. no. sc-47778; 1:1,000; Santa Cruz Biotechnology,
Inc.) primary antibodies overnight at 4°C, washed three times in
TBST, incubated with horseradish peroxidase-conjugated anti-mouse
(cat. no. sc-2005; 1:2,000; Santa Cruz Biotechnology, Inc.) and
anti-rabbit (cat. no. sc-2004; 1:5,000) secondary antibodies for 1
h at 25°C and then washed three times in TBST. The signal was
visualized using an enhanced chemiluminescence solution (ECL Plus;
GE Healthcare Life Sciences, Chalfont, UK) and was exposed to
Carestream® Kodak® Co. X-Omat LS film
(Sigma-Aldrich; EMD Millipore). Band intensities were quantified
using ImageJ 1.11 software (National Institutes of Health,
Bethesda, MD, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), and RT-PCR was
performed using the PrimeScript™ RT Master Mix for RT-PCR
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. PCR was performed using gene-specific
primers, as follows: Twist forward, 5′-TTCAAAGAAACAGGGCGTGG-3′ and
reverse, 5′-ATGCCTTTCCTTTCAGTGGC-3′; IL-1β forward,
5′-GGAGAATGACCTGAGCACCT-3′ and reverse, 5′-GGAGGTGGAGAGCTTTCAGT-3′;
glyceraldehyde-3-phosphate dehydrogenase forward,
5′-CACATCGCTCAGACACCATG-3′ and reverse, 5′-TGACGGTGCCATGGAATTTG-3′.
A total of 35 amplification cycles were performed as follows:
Denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec and
elongation at 72°C for 30 sec. A final extension step was performed
at 72°C for 5 min and then sustained at 4°C. PCR products were
resolved by 2% agarose gel electrophoresis and stained with
ethidium bromide (Sigma-Aldrich; EMD Millipore) for
visualization.
Statistical analysis
All experiments reported in the present study were
performed independently at least three times and data (expressed as
the mean ± standard deviation) from a representative experiment are
shown. Statistical significance was assessed by one-way analysis of
variance using SPSS 17.0 software (. P<0.05 was considered to
represent a statistically significant difference.
Results
IL-1 β is highly expressed in GBC
tissues and cell lines
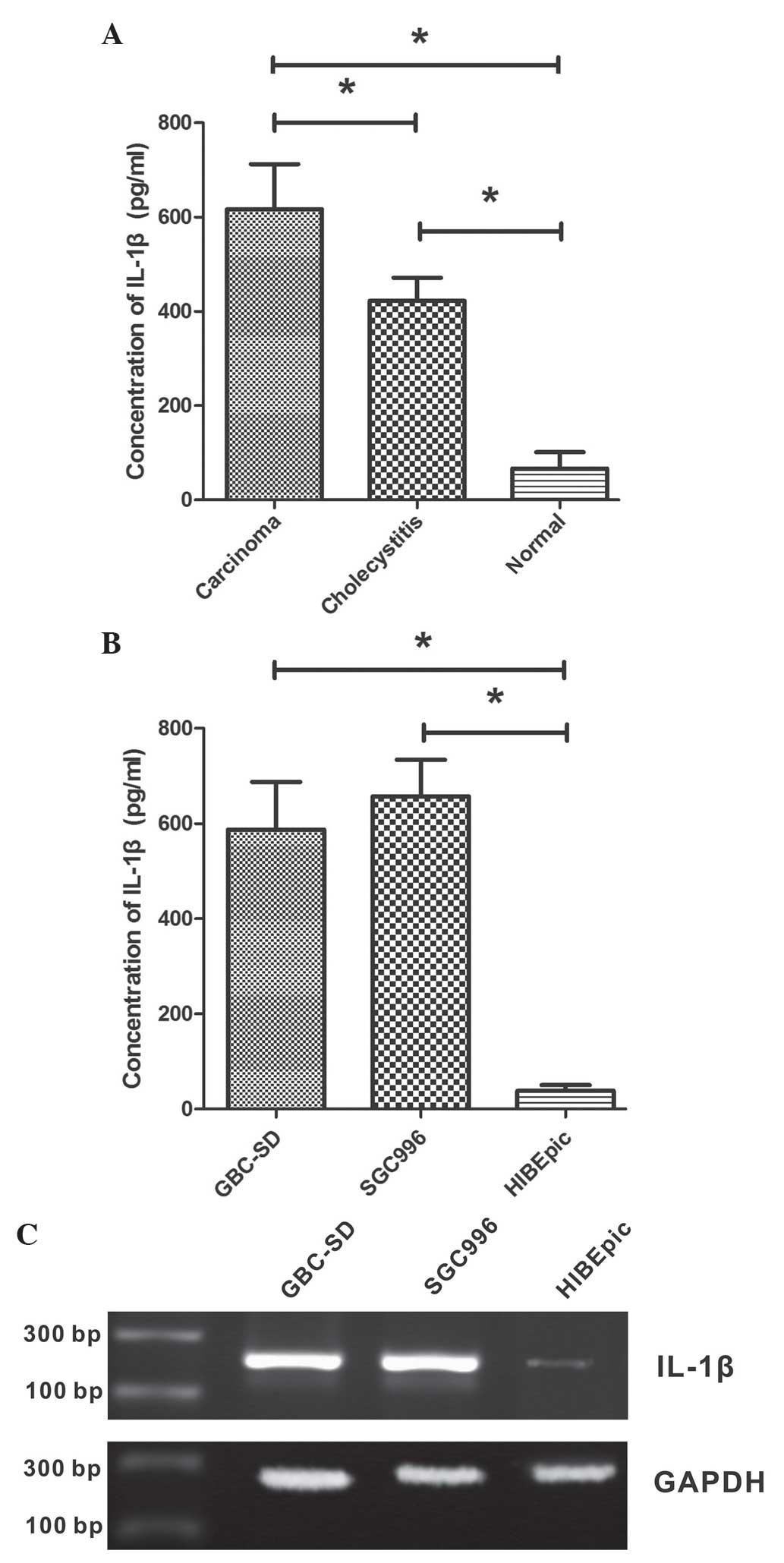
To investigate the secretion of IL-1β in tissues of
GBC, chronic cholecystitis and normal gallbladder, biopsies were
obtained from patients and ELISA was performed on these tissue
samples. It was observed that the level of IL-1β protein in normal
gallbladder tissue was low, while it was significantly increased in
GBC and chronic cholecystitis tissues (P<0.001; Fig. 1A). The IL-1β concentration was
422.3±48.9 ng/ml in chronic cholecystitis tissue and 616.4±95.7
ng/ml in GBC tissue, which was significantly increased compared
with that of the normal gallbladder tissue (66.4±35.0 ng/ml). The
present study also examined the IL-1β concentrations in GBC cell
lines GBC-SD and SGC996, as well as the non-malignant gallbladder
epithelial cell line HIBEpiC. As shown in Fig. 1B, GBC cell lines secreted
significantly increased levels of IL-1β compared with HIBEpiC cells
(P<0.001). The IL-1β concentrations in the growth medium of
GBC-SD, SGC996 and HIBEpiC cells were 587.4±99.8, 657.2±76.6 and
38.4±12.1 ng/ml, respectively.
The expression of IL-1β mRNA in GBC cell lines
GBC-SD and SGC996, as well as the non-malignant gallbladder
epithelial cell line HIBEpiC, was analyzed using RT-PCR. Expression
levels of IL-1β mRNA were increased in GBC cell lines compared with
HIBEpiC cells (Fig. 1C).
Exogenous IL-1β promotes the
proliferation of GBC-SD and SGC996 cells in vitro and in vivo
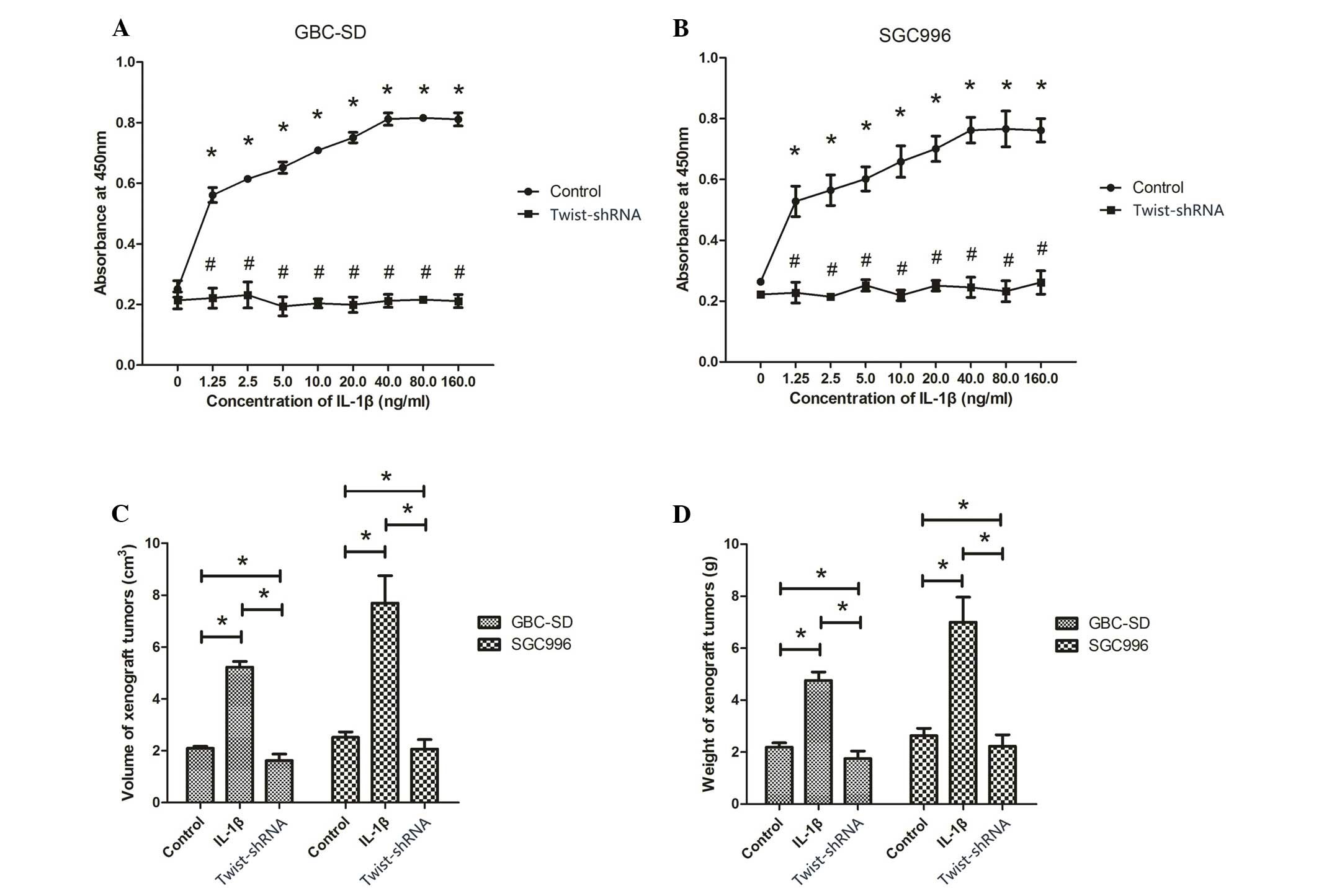
To study the effect of IL-1β on proliferation of
human GBC cell lines, WST-1 assays were performed to measure the
proliferation of GBC-SD and SGC996 cells exposed to 0, 1.25, 2.5,
5, 10, 20, 40, 80 and 160 ng/ml IL-1β. The WST-1 results revealed a
concentration-dependent increase in cell proliferation caused by
IL-1β (P<0.001; Fig. 2A and B).
Between the range of 1.25–40 ng/ml, the proliferation of GBS-SD and
SGC996 cells increased along with the concentration of IL-1β, while
the proliferation of cells did not continue to increase
significantly following treatment with 80 and 160 ng/ml IL-1β
(P>0.05; Fig. 2A and B).
Therefore, 40 ng/ml, the optimal concentration of IL-1β, was used
in subsequent experiments. The treatment of the cells with shRNA
targeting Twist significantly decreased the proliferation-promoting
effect of IL-1β.
To investigate whether IL-1β promotes tumor growth
in vivo, GBC xenograft tumor models were established by
subcutaneous injection of GBS-SD or SGC996 cells into the flanks of
immunodeficient nude mice. Following tumor formation, the mice were
randomized into two groups and injected intratumorally with 200 ng
IL-1β. As shown in Fig. 2C and D,
tumor injection with IL-1β induced a significant increase in tumor
growth compared to tumors without IL-1β injection (P<0.05).
However, following Twist silencing, the volume and weight of tumors
injected with IL-1β was significantly reduced compared with those
not injected with IL-1β (P<0.05).
Exogenous IL-1β promotes the migration
of GBC-SD and SGC996 cells
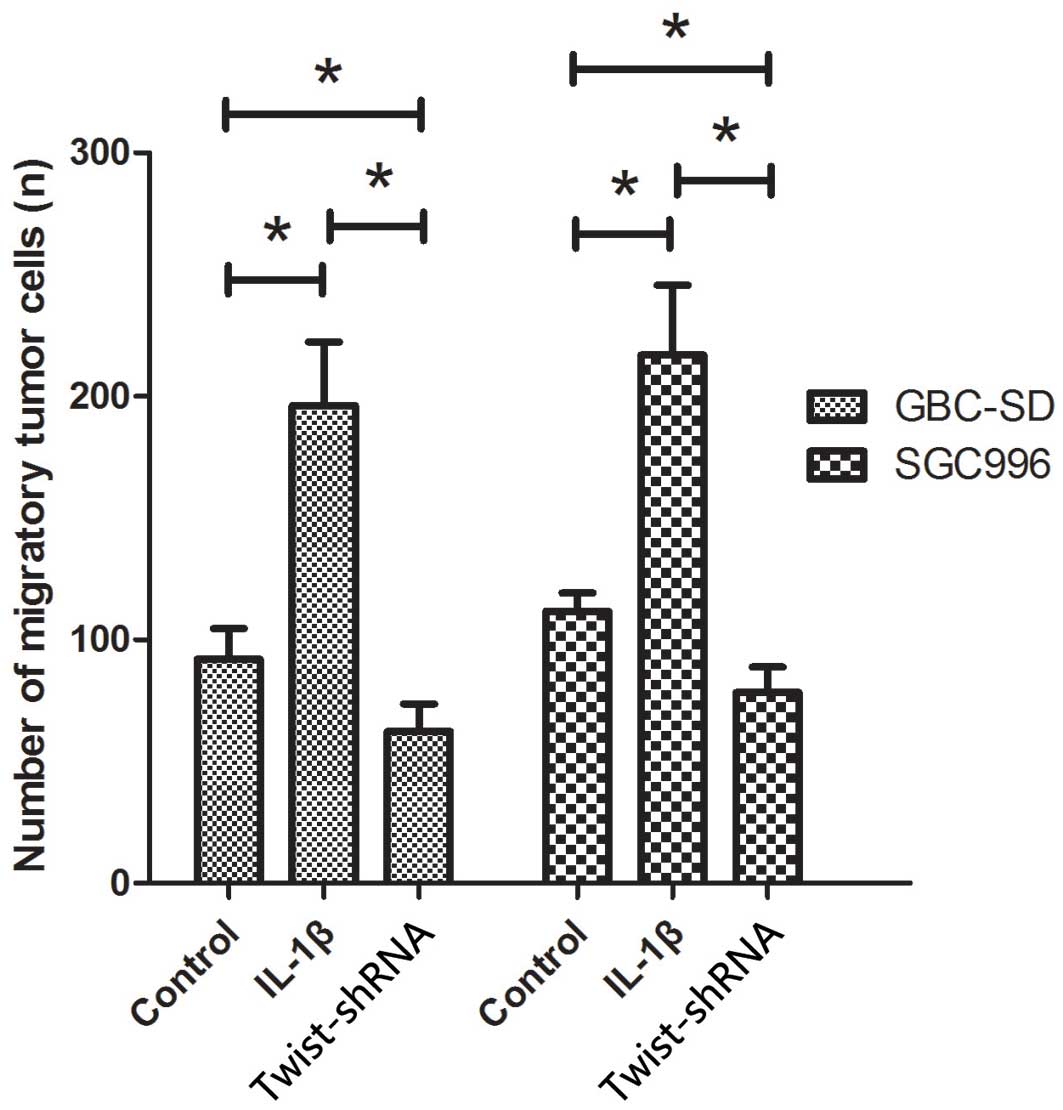
The present study additionally analyzed the effects
of IL-1β on the migratory behavior of GBC cell lines. As shown in
Fig. 3, cells treated with IL-1β
exhibited significantly increased migration capacity compared with
those not treated with IL-1β (P<0.05). The number of migrated
cells of GBC-SD and SGC996 lines treated with IL-1β was 196.3±26.1
and 231.3±48.2, respectively, while the number of migrated cells
not treated with IL-1β was 92±12.5 and 139.7±27.6, respectively.
GBC-SD and SGC996 cells deficient in Twist showed a significant
reduction of migration capacity following treatment with IL-1β,
compared to normal GBC-SD and SGC996 cells (P<0.05; Fig. 3).
IL-1β promotes proliferation and
migration of GBC cells via Twist activation
To investigate the potential regulatory pathway of
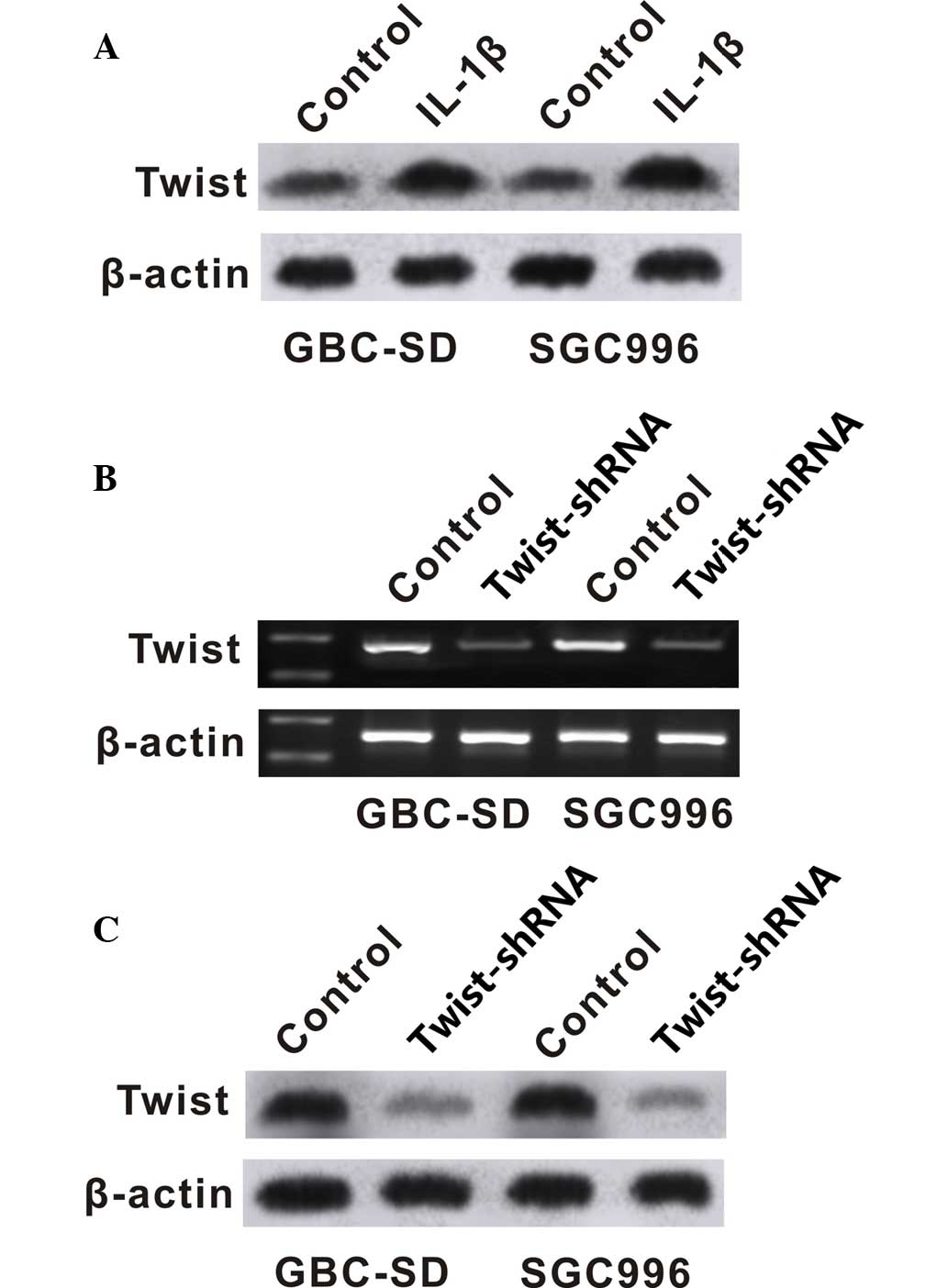
IL-1β in GBC cells, the present study analyzed the effect of IL-1β
on the expression of Twist. Western blot analysis was performed to
determine the protein level of Twist in GBC cells. GBC-SD and
SGC996 cells exposed to IL-1β showed increased protein and mRNA
levels of Twist (Fig. 4A and B).
Twist-shRNA significantly downregulated the expression of Twist
(Fig. 4B and C), and downregulation
of Twist significantly inhibited the proliferation and migration of
GBC-SD and SGC996 cells (Figs. 2 and
3).
Discussion
IL-1β is a multifunctional and proinflammatory
cytokine that has crucial roles in human physiological and
pathological activities (14). IL-1β,
which is widely produced by numerous types of cell, is mainly
secreted by immune cells including macrophages and monocytes
(15). Furthermore, non-phagocytic
cells have also been observed to be involved in secretion of IL-1β,
including epithelial and tumor cells (16,17). Cells
produce and secrete high levels of IL-1β following activation by
acute or chronic inflammation or other environmental stimuli; the
secretion of IL-1β under normal physiological conditions occurs in
a very limited fashion (18). As the
core mediator involved in immune regulation and inflammation
responses, IL-1β has significant roles in acute and chronic
inflammation (19). Previous studies
have demonstrated that IL-1β is one of the proinflammatory
cytokines released by the tumor microenvironment, and involved in
the process of tumor formation (19–24). The
aberrant expression of IL-1β is thought to promote tumor growth and
metastasis.
However, to the best of our knowledge, an
association between IL-1β and GBC has not previously been reported.
Previous studies have documented highly constitutive IL-1β protein
production in breast, lung and colon cancer (25–27), which
was consistent with the present study, in which IL-1β was
upregulated in GBC tissues. The IL-1β expression level was low in
normal gallbladder tissue, and was significantly increased in
chronic cholecystitis and GBC tissues. These data suggest that
IL-1β may participate in the process of chronic cholecystitis and
GBC, and its overexpression may contribute to the formation of GBC.
The results of the present study also indicate direct evidence of a
correlation between chronic cholecystitis and GBC. Additionally,
the secretion of IL-1β by GBC cell lines GBC-SD and SGC996 was
detected, and the level of secretion was significantly increased
compared to the normal gallbladder epithelial cell line HIBEpiC.
These results demonstrate that IL-1β secretion is not only induced
by inflammation responses, but IL-1β may also be secreted by the
gallbladder tumor itself, elevating the overall IL-1β
expression.
It is commonly accepted that overexpression of IL-1β
promotes tumor growth and metastasis, which is consistent with the
results of the present study; exogenous IL-1β promoted the
proliferation and migration of GBC cells in vitro and in
vivo. Due to its multifunctional role, the underlying mechanism
allowing IL-1β to promote tumor growth and metastasis may consist
of various signaling pathways, including inducing the expression of
p38, c-Jun N-terminal kinase, matrix metalloproteinases, vascular
endothelial growth factor, basic fibroblast growth factor, IL-8 and
transforming growth factor-β that are required for tumorigenesis
and metastasis (28–34). Previous studies have reported that
IL-1β is able to regulate the epithelial-to-mesenchymal (EMT)
transition by activating zinc finger E-box-binding homeobox 1 or
stabilizing Snail expression to promote colon cancer formation
(35,36).
Twist, another EMT activator, was investigated in
the present study. As a basic transcription factor, Twist has been
identified as an important factor during the promotion of EMT
involved in cancer progression and metastasis (37). Overexpression of Twist has been
observed in a number of types of tumor, including lung, stomach,
liver, colon, breast and prostate cancer (38–43). In
the present study, exposure of GBC-SD and SGC996 cells to IL-1β
markedly increased Twist expression, suggesting IL-1β may promote
the proliferation of GBC cells via Twist activation. To conform
this hypothesis, shRNA was used to knock down the expression of
Twist in GBC-SD and SGC996 cells. The results of the present study
revealed that gene silencing of Twist blocked IL-1β-induced
proliferation and migration of GBC-SD and SGC996 cells. Cells
deficient in Twist treated with IL-1β exhibited reduced migration
capacity compared with normal controls, which may be associated
with the inhibition of downstream signaling pathways of Twist. A
previous study reported that Twist downregulation may induce
migration inhibition and apoptosis (44). Taken together, the results of the
present study demonstrated that Twist may act as a key regulation
factor participating in IL-1β-driven proliferation and migration of
GBC cells.
In conclusion, the present study demonstrated that
abnormal overexpression of IL-1β contributes to GBC tumorigenesis.
IL-1β may promote proliferation and migration of GBC cells via
Twist activation. The correlation between chronic inflammation and
gallbladder carcinogenesis has been described previously (45,46),
although the exact inflammatory mediators involved has yet to be
elucidated. To the best of our knowledge, the present study is the
first to implicate IL-1β in the tumorigenesis of GBC. Future
studies should investigate the molecular mechanisms underlying
IL-1β-induced proliferation. Particularly, the expression of IL-1R,
which acts as the receptor of IL-1β, and the downstream regulators,
should be examined in GBC.
Acknowledgements
The present study was supported by the Fund of the
Shanghai Institute of Health Sciences (grant no., 2014zr008),
Shanghai Municipal Medical Health Cultivation Planning of
Outstanding Youth (grant no., AB83190002012023) and Shanghai
Medical Key Specialist Construction Plans (grant no.,
ZK2012A15).
References
|
1
|
Misra S, Chaturvedi A and Misra NC:
Gallbladder cancer. Curr Treat Options Gastroenterol. 9:95–106.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stinton LM and Shaffer EA: Epidemiology of
gallbladder disease: Cholelithiasis and cancer. Gut Liver.
6:172–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eslick GD: Epidemiology of gallbladder
cancer. Gastroenterol Clin North Am. 39:307–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hundal R and Shaffer EA: Gallbladder
cancer: Epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.PubMed/NCBI
|
|
5
|
Misra S, Chaturvedi A, Misra NC and Sharma
ID: Carcinoma of the gallbladder. Lancet Oncol. 4:167–176. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levy AD, Murakata LA and Rohrmann CA Jr:
Gallbladder carcinoma: Radiologic-pathologic correlation.
Radiographics. 21:295–314. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sikora SS and Singh RK: Surgical
strategies in patients with gallbladder cancer: Nihilism to
optimism. J Surg Oncol. 93:670–681. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bonet Beltrán M, Allal AS, Gich I, Solé JM
and Carrió I: Is adjuvant radiotherapy needed after curative
resection of extrahepatic biliary tract cancers? A systematic
review with a meta-analysis of observational studies. Cancer Treat
Rev. 38:111–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wistuba II, Sugio K, Hung J, Kishimoto Y,
Virmani AK, Roa I, Albores-Saavedra J and Gazdar AF:
Allele-specific mutations involved in the pathogenesis of endemic
gallbladder carcinoma in Chile. Cancer Res. 55:2511–2515.
1995.PubMed/NCBI
|
|
10
|
Landskron G, De la Fuente M, Thuwajit P,
Thuwajit C and Hermoso MA: Chronic inflammation and cytokines in
the tumor microenvironment. J Immunol Res. 2014:1491852014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh RK and Lokeshwar BL: The
IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling
to promote prostate cancer growth. Cancer Res. 71:3268–3277. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bai Z, Tai Y, Li W, Zhen C, Gu W, Jian Z,
Wang Q, Lin JE, Zhao Q, Gong W, et al: Gankyrin activates IL-8 to
promote hepatic metastasis of colorectal cancer. Cancer Res.
73:4548–4558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rashid A, Ueki T, Gao YT, Houlihan PS,
Wallace C, Wang BS, Shen MC, Deng J and Hsing AW: K-ras mutation,
p53 overexpression, and microsatellite instability in biliary tract
cancers: A population-based study in China. Clin Cancer Res.
8:3156–3163. 2002.PubMed/NCBI
|
|
14
|
Wang H, Ding W, Yang D, Gu T, Yang S and
Bai Z: Different concentrations of 17β-estradiol modulates
apoptosis induced by interleukin-1β in rat annulus fibrosus cells.
Mol Med Rep. 10:2745–2751. 2014.PubMed/NCBI
|
|
15
|
Suzuki H and Ikeda K: Mode of action of
long-term low-dose macrolide therapy for chronic sinusitis in the
light of neutrophil recruitment. Curr Drug Targets Inflamm Allergy.
1:117–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang HY, Wang F, Chen HM and Yan XJ:
κ-carrageenan induces the disruption of intestinal epithelial
Caco-2 monolayers by promoting the interaction between intestinal
epithelial cells and immune cells. Mol Med Rep. 8:1635–1642.
2013.PubMed/NCBI
|
|
17
|
Giovanni Germano, Paola Allavena and
Mantovani A: Cytokines as a key component of cancer-related
inflammation. Cytokine. 43:374–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Herman AP, Krawczyńska A, Bochenek J,
Dobek E, Herman A and Tomaszewska-Zaremba D: LPS-induced
inflammation potentiates the IL-1β-mediated reduction of LH
secretion from the anterior pituitary explants. Clin Dev Immunol.
2013:9269372013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Voronov E, Carmi Y and Apte RN: Role of
IL-1-mediated inflammation in tumor angiogenesis. Adv Exp Med Biol.
601:265–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lewis AM, Varghese S, Xu H and Alexander
HR: Interleukin-1 and cancer progression: The emerging role of
interleukin-1 receptor antagonist as a novel therapeutic agent in
cancer treatment. J Transl Med. 4:482006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tu S, Bhagat G, Cui G, Takaishi S,
Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl
O, Fox JG and Wang TC: Overexpression of interleukin-1beta induces
gastric inflammation and cancer and mobilizes myeloid-derived
suppressor cells in mice. Cancer Cell. 14:408–419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miki C, Konishi N, Ojima E, Hatada T,
Inoue Y and Kusunoki M: C-reactive protein as a prognostic variable
that reflects uncontrolled up-regulation of the IL-1-IL-6 network
system in colorectal carcinoma. Dig Dis Sci. 49:970–976. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krelin Y, Voronov E, Dotan S, Elkabets M,
Reich E, Fogel M, Huszar M, Iwakura Y, Segal S, Dinarello CA and
Apte RN: Interleukin-1beta-driven inflammation promotes the
development and invasiveness of chemical carcinogen-induced tumors.
Cancer Res. 67:1062–1071. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Voronov E, Shouval DS, Krelin Y, Cagnano
E, Benharroch D, Iwakura Y, Dinarello CA and Apte RN: IL-1 is
required for tumor invasiveness and angiogenesis. Proc Natl Acad
Sci USA. 100:2645–2650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soria G, Ofri-Shahak M, Haas I,
Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T, Weitzenfeld P,
Meshel T, Shabtai E, Gutman M and Ben-Baruch A: Inflammatory
mediators in breast cancer: Coordinated expression of TNFα &
IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal
transition. BMC Cancer. 11:1302011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Colasante A, Mascetra N, Brunetti M,
Lattanzio G, Diodoro M, Caltagirone S, Musiani P and Aiello FB:
Transforming growth factor beta 1, interleukin-8 and interleukin-1,
in non-small-cell lung tumors. Am J Respir Crit Care Med.
156:968–973. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Apte RN and Voronov E: Interleukin-1 - a
major pleiotropic cytokine in tumor-host interactions. Semin Cancer
Biol. 12:277–290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang Q, Lan F, Wang X, Yu Y, Ouyang X,
Zheng F, Han J, Lin Y, Xie Y, Xie F, et al: IL-1β-induced
activation of p38 promotes metastasis in gastric adenocarcinoma via
upregulation of AP-1/c-fos, MMP2 and MMP9. Mol Cancer. 13:182014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Petrella BL and Vincenti MP:
Interleukin-1β mediates metalloproteinase-dependent renal cell
carcinoma tumor cell invasion through the activation of CCAAT
enhancer binding protein β. Cancer Med. 1:17–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stetler-Stevenson WG and Yu AE: Proteases
in invasion: Matrix metalloproteinases. Semin Cancer Biol.
11:143–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mantovani A and Dejana E: Cytokines as
communication signals between leukocytes and endothelial cells.
Immunol Today. 10:370–375. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Strieter RM, Polverini PJ, Arenberg DA and
Kunkel SL: The role of CXC chemokines as regulators of
angiogenesis. Shock. 4:155–160. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Folkman J and D'Amore PA: Blood vessel
formation: What is its molecular basis? Cell. 87:1153–1155. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dinarello CA: Biologic basis for
interleukin-1 in disease. Blood. 87:2095–2147. 1996.PubMed/NCBI
|
|
35
|
Li Y, Wang L, Pappan L, Galliher-Beckley A
and Shi J: IL-1β promotes stemness and invasiveness of colon cancer
cells through Zeb1 activation. Mol Cancer. 11:872012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaler P, Galea V, Augenlicht L and
Klampfer L: Tumor associated macrophages protect colon cancer cells
from TRAIL-induced apoptosis through IL-1beta-dependent
stabilization of Snail in tumor cells. PLoS One. 5:e117002010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martin A and Cano A: Tumorigenesis: Twist1
links EMT to self-renewal. Nat Cell Biol. 12:924–925. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li LZ, Zhang CZ, Liu LL, Yi C, Lu SX, Zhou
X, Zhang ZJ, Peng YH, Yang YZ and Yun JP: miR-720 inhibits tumor
invasion and migration in breast cancer by targeting TWIST1.
Carcinogenesis. 35:469–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qian J, Luo Y, Gu X, Zhan W and Wang X:
Twist1 promotes gastric cancer cell proliferation through
up-regulation of FoxM1. PLoS One. 8:e776252013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang TM and Hung WC: Transcriptional
repression of TWIST1 gene by Prospero-related homeobox 1 inhibits
invasiveness of hepatocellular carcinoma cells. FEBS Lett.
586:3746–3752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chang LH, Chen CH, Huang DY, Pai HC, Pan
SL and Teng CM: Thrombin induces expression of twist and cell
motility via the hypoxia-inducible factor-1α translational pathway
in colorectal cancer cells. J Cell Physiol. 226:1060–1068. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
van Nes JG, de Kruijf EM, Putter H,
Faratian D, Munro A, Campbell F, Smit VT, Liefers GJ, Kuppen PJ,
van de Velde CJ and Bartlett JM: Co-expression of SNAIL and TWIST
determines prognosis in estrogen receptor-positive early breast
cancer patients. Breast Cancer Res Treat. 133:49–59. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gajula RP, Chettiar ST, Williams RD,
Thiyagarajan S, Kato Y, Aziz K, Wang R, Gandhi N, Wild AT, Vesuna
F, et al: The twist box domain is required for Twist1-induced
prostate cancer metastasis. Mol Cancer Res. 11:1387–1400. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Feng MY, Wang K, Song HT, Yu HW, Qin Y,
Shi QT and Geng JS: Metastasis-induction and apoptosis-protection
by TWIST in gastric cancer cells. Clin Exp Metastasis.
26:1013–1023. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Espinoza JA, Bizama C, García P, Ferreccio
C, Javle M, Miquel JF, Koshiol J and Roa JC: The inflammatory
inception of gallbladder cancer. Biochim Biophys Acta. 1865:245–54.
2016.PubMed/NCBI
|
|
46
|
Kumar S and Kumar S and Kumar S: Infection
as a risk factor for gallbladder cancer. J Surg Oncol. 93:633–639.
2006. View Article : Google Scholar : PubMed/NCBI
|