Introduction
Acute promyelocytic leukemia (APL), a distinct
subtype of acute myeloid leukemia, is defined by a specific
balanced translocation, t(15;17), leading to the fusion of the
promyelocytic leukemia (PML) and retinoic acid receptor-α (RARA)
genes (1,2). The PML-RARA gene is supposed to play a
vital role in the pathophysiological process of APL (3), and patients with the fusion gene could
benefit from treatment with all-trans retinoic acid (ATRA) and
AS2O3.
As supported by fluorescence in situ
hybridization (FISH) assessment, complex variant translocations of
15;17 have been increasingly reported in APL, while the majority of
complex variants of APL have revealed three-way translocations
(4,5).
The current study presents the fifth three-way translocation
involving chromosomes 3;17;15 (6–8), which may
be the second breakpoint involving the long arm of chromosome 3
reported thus far. Written informed consent was obtained from the
patient for the publication of this study.
Case report
A 33-year-old male with no significant previous
medical history was admitted to Yantai Yuhuangding Hospital
(Yantai, Shandong, China) in July 2014 due to bleeding gums. A
peripheral blood examination showed the following: Hemoglobin, 10.2
g/dl (normal, 13.0–17.5 g/dl); white blood cell count,
2.14×109/l (normal, 3.5–9.5×109/l), with 46%
atypical promyelocytes packed with numerous azurophilic granules;
and platelet count, 28×109/l (normal,
125–350×109/l). Coagulation tests revealed a prothrombin
time of 19.4 sec (normal, 15 sec), an activated partial
thromboplastin time of 41.8 sec (normal, 40 sec) and a fibrinogen
level of 67 mg/dl. Bone marrow was markedly hypercellular, with
84.5% atypical promyelocytes. The promyelocytes were stained by
allophycocyanin (APC)-conjugated cluster of differentiation (CD)33
(dilution, 1:2; catalog no. 340474), phycoerythrin (PE)-conjugated
CD123 (dilution, 1:10; catalog no. 340545), APC-conjugated CD38
(dilution, 1:2; catalog no. 345807), PE-conjugated CD13 (dilution,
1:5; catalog no. 347837) and PerCP-conjugated CD45 (dilution, 1:5;
catalog no. 347464). All of the antibodies were monoclonal,
composed of mouse immunoglobulin G1 heavy chains and κ light
chains, diluted with phosphate-buffered saline and purchased from
BD Biosciences (Franklin Lakes, NJ, USA). The immunophenotype was
CD33+, CD123+, CD117+,
CD38+ and CD13+. The PML-RARA (L-form)
rearrangement was confirmed by reverse transcription polymerase
chain reaction (RT-PCR), and a diagnosis of APL [M3 in the
French-American-British classification (9)] accompanying disseminated intravascular
coagulation (DIC) was formed. Due to ATRA (20 mg/m2/day)
intolerance, the patient started induction therapy with arsenic
trioxide alone at a daily dose of 10 mg for 35 days, to induce
remission through PML/RARA degradation, and cryoprecipitate
transfusion to control the DIC (accumulated dose, 83 units). At 15
days after therapy initiation, the atypical promyelocytes started
to differentiate. However, 27 days after therapy, the white blood
cells reached a level of 144×109/l with no evidence of
APL differentiation syndrome. Coinciding with this, symptoms of
high cerebrospinal fluid pressure emerged. The patient subsequently
received daunorubicin (20 mg for 3 days) and dexamethasone (10 mg
for 3 days) treatment, as well as intrathecal methotrexate (12 mg),
following which the white blood cell counts gradually decreased to
normal. Molecular remission was achieved 10 days after the
administration of the chemotherapy. The treatment was continued
with 3 courses of medial-dose cytosine arabinoside. The patient
currently remains in complete remission.
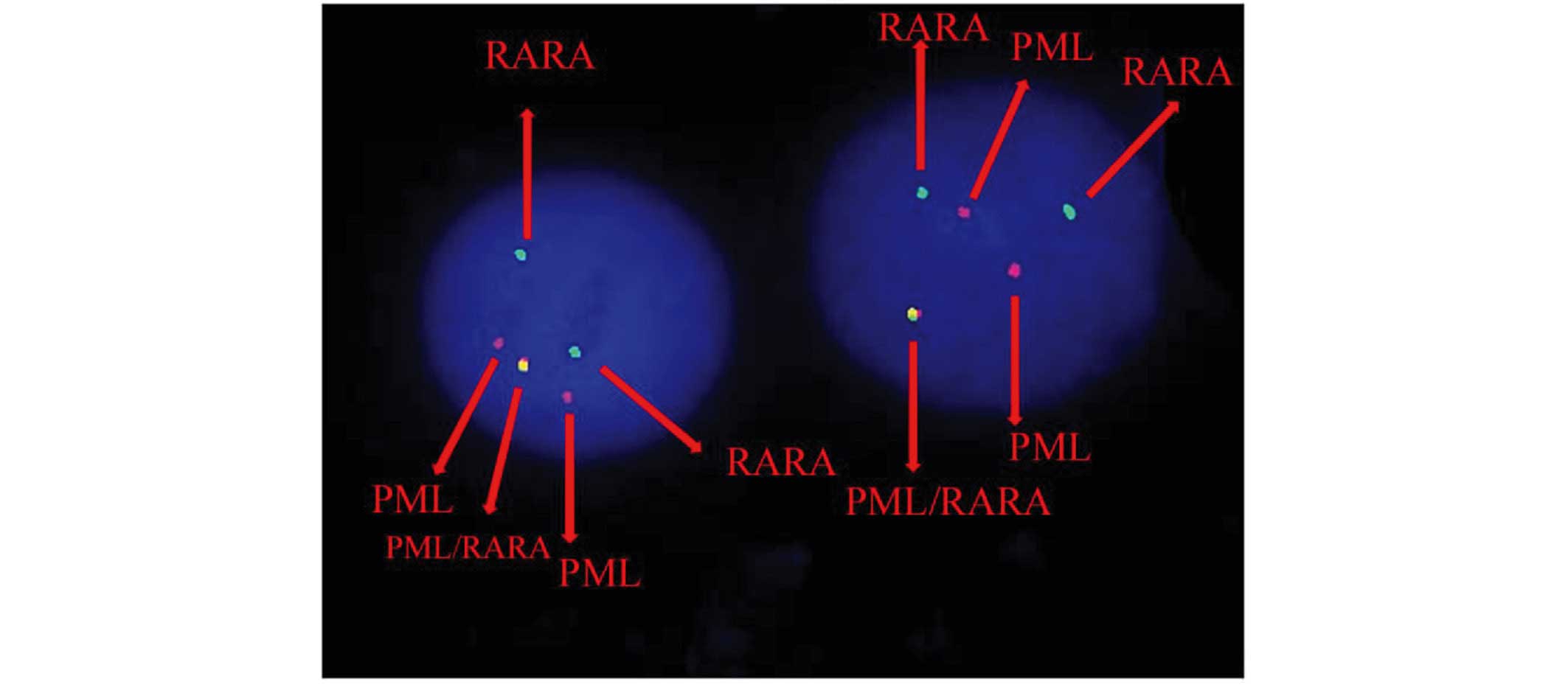
Karyotypic analysis and FISH
Cytogenetic analysis was performed on Giemsa-banded
chromosome preparations. The karyotype was presented according to
the 2005 International System for Human Cytogenetic Nomenclature
(10). As the metaphases from
abnormal cells were often of poor quality and resolution, it was
difficult to cytogenetically identify the characteristic PML/RARA
fusion gene. FISH was performed using the Vysis dual-color,
dual-fusion probe (Abbott Molecular, Des Plaines, IL, USA)
following the manufacturer's instructions. Fluorescent signals were
visualized and images were captured using a fluorescence
microscope. The FISH analysis revealed the 46, XY, der(3)t(3;17;15)(q25;q21;q24), der(15)t(3;17;15), der(17)der(17)(q11q12) karyotype (Fig. 1).
RT-PCR analysis
Total RNA was extracted from mononuclear cells in a
bone marrow sample obtained from the patient using TRIzol reagent
(Gibco; Thermo Fisher Scientific, Inc.), and reversed transcription
was performed. PCR for the PML-RARA fusion gene was performed as
previously described (6). An initial
denaturation at 94°C for 5 min was followed by 40 cycles at 94°C
for 15 sec and 60°C for 60 sec. The sequences of the PCR primers
were as follows: PML-RARA forward, 5′-CCGTCATAGGAAGTGAGGTCT-3′ and
reverse, 5′-GGCTGGGCACTATCTCTTCA-3′; and GAPDH forward,
5′-TGGAGATAACACTCTAAGCATAACTAAAGGT-3′ and reverse,
5′-TGGAGATAACACTCTAAGCATAACTAAAGGT-3′. The results demonstrated
that only the L-type chimeric transcription was expressed, while
the RARA/PML was not expressed.
Discussion
To date, >35 cases with three-way complex
translocations have been reported (6–8,11–30). Among
these translocations, 9 recurrent breakpoints have been identified,
including 2q21 (5,14,26), 19p13
(5,24), 3p21 (7,8), 4q21
(13,15), 11q13 (18,21), 1p36
(16,19,22), 20p13
(23,31), 6p21 (27) and Xq13 (11,25). As a
supplement, the present study reported another case of an APL
patient harboring a three-way translocation involving chromosomes
3;17;15. This case provided a similar survival outcome of this
complex translocation compared with the previous 4 cases (6,8).
According to FISH analysis, the complex
translocation can be assessed as follows: 15q24-qter translocated
to 3q25; a small piece of chromosome 17 (17q11-q22) to 15q24; and
3q25-qter connected to 17q22 that has been located in der(15)(q24).
To date, 4 cases of three-way translocation
regarding t(3;17;15) have been reported (6,8). However,
the breakpoint in the present case occurred in a novel area of the
long arm of chromosome 3. The previous 3 cases harboring 3;17;15,
reported in 2009, 1980 and 1994 (6–8) reported
survival times of 6 days, 33 months and 14 months, respectively. In
the present study, the patient presented with the typical
morphological and clinical features of APL, and exhibited a good
response to (arsenic trioxide) and chemotherapy treatment, as
observed in typical APL. Comigrating RARA/PML rearrangements were
present in 2 cases from previous reports (8), which differed from the present case. On
the basis of RT-PCR analysis, there were only PML-RARA fusion
transcripts and the RARA/PML fusion gene was not expressed in this
case. This was inconsistent with the opinion that the majority of
cases of APL lacking the t(15;17) are still associated with the
formation of the RARA/PML fusion gene (27).
The poor prognosis of the previously reported 3
cases with the three-way t(3;17;15) appears to be associated with
early mortality, which may due to the absence of targeted therapy
and the comigrating RARA/PML rearrangements. The inference is that
the involvement of the 3q25 region has no clear effect on disease
outcome, further supporting the aforementioned notions. It remains
unclear if the aberration can affect clinical consequences. A close
clinical follow-up will be important in the present patient and in
other cases of APL with a three-way t(3;17;15).
References
|
1
|
de Thé H, Chomienne C, Lanotte M, Degos L
and Dejean A: The t(15;17) translocation of acute promyelocytic
leukaemia fuses the retinoic acid receptor alpha gene to a novel
transcribed locus. Nature. 347:558–561. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Thé H, Lavau C, Marchio A, Chomienne C,
Degos L and Dejean A: The PML-RAR alpha fusion mRNA generated by
the t(15;17) translocation in acute promyelocytic leukemia encodes
a functionally altered RAR. Cell. 66:675–684. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang ZY and Chen Z: Acute promyelocytic
leukemia: From highly fatal to highly curable. Blood.
111:2505–2515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu S, Li Q, Pang W, Bo L, Qin S, Liu X,
Teng Q, Qian L and Wang J: A new complex variant t(4;15;17) in
acute promyelocytic leukemia: Fluorescence in situ hybridization
confirmation and literature review. Cancer Genet Cytogenet.
130:33–37. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brunel V, Lafage-Pochitaloff M, Alcalay M,
Pelicci PG and Birg F: Variant and masked translocations in acute
promyelocytic leukemia. Leuk Lymphoma. 22:221–228. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Freeman CE, Mercer DD, Ye Y, Van Brunt J
III and Li MM: Cytogenetic and molecular characterization of
complex three-way translocations in acute promyelocytic leukemia.
Beijing Da Xue Xue Bao. 41:477–479. 2009.PubMed/NCBI
|
|
7
|
Bernstein R, Mendelow B, Pinto MR, Morcom
G and Bezwoda W: Complex translocations involving chromosomes 15
and 17 in acute promyelocytic leukaemia. Br J Haematol. 46:311–314.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McKinney CD, Golden WL, Gemma NW, Swerdlow
SH and Williams ME: RARA and PML gene rearrangements in acute
promyelocytic leukemia with complex translocations and atypical
features. Genes Chromosomes Cancer. 9:49–56. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DAG, Gralnick HT and Sultan C: Proposal for the
classification of the acute leukemias French-American-British (FAB)
co-operative group. Br J Haematol. 33:451–458. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shaffer LG and Tommerup N: ISCN 2005: An
International System For Human Cytogenetic Nomenclature. S. Karger;
Basel: 2005
|
|
11
|
Callen DFDB, Sage RE and Ford JH: A
complex translocation in acute promyelocytic leukemia. Cancer Genet
Cytogenet. 16:45–48. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Misawa S, Lee E, Schiffer CA, Liu Z and
Testa JR: Association of the translocation (15;17) with malignant
proliferation of promyelocytes in acute leukemia and chronic
myelogenous leukemia at blastic crisis. Blood. 67:270–274.
1986.PubMed/NCBI
|
|
13
|
Berger R, Flandrin G, Bernheim A, Le
Coniat M, Vecchione D, Pacot A, Derré J, Daniel MT, Valensi F,
Sigaux F, et al: Cytogenetic studies on 519 consecutive de novo
acute nonlymphocytic leukemias. Cancer Genet Cytogenet. 29:9–21.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bjerrum OW, Philip P, Pressler T and
Tygstrup I: Acute promyelocytic leukemia with t(15;17) and
t(2;17;15). Cancer Genet Cytogenet. 28:107–111. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huret JL, Couet D, Guilhot F, Brizard A
and Tanzer J: A two-step t(4;der(15)) t(15;17) complex
translocation in an acute promyelocytic leukaemia and review of the
literature. Leuk Res. 11:761–765. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Osella P, Wyandt H, Vosburgh E and
Milunsky A: Report of a variant t(1;15;17)(p36;q22;q21.1) in a
patient with acute promyelocytic leukemia. Cancer Genet Cytogenet.
57:201–207. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogawa S, Mitani K, Sato Y, Sugimoto K,
Toyoshima H, Mano H, Takaku F, Yazaki Y and Hirai H: Detection of
the PML/RAR alpha fusion gene in acute promyelocytic leukemia with
a complex translocation involving chromosomes 15, 17 and 18. Cancer
Genet Cytogenet. 69:113–117. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Z, Morgan R, Stone JF and Sandberg
AA: Identification of complex t(15;17) in APL by FISH. Cancer Genet
Cytogenet. 72:73–74. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JP and Fairweather RB: Complex
t(1;15;17) in acute promyelocytic leukemia with duplication of RAR
alpha and PML sequences. Cancer Genet Cytogenet. 89:52–56. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Calabrese G, Min T, Stuppia L, Powles R,
Swansbury JG, Morizio E, Peila R, Donti E, Fioritoni G and Palka G:
Complex chromosome translocations of standard t(8;21) and t(15;17)
arise from a two-step mechanism as evidenced by fluorescence in
situ hybridization analysis. Cancer Genet Cytogenet. 91:40–45.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Casula L, Archidiacono N, Pau M Grazia,
Addis M, Mura R, Galanello R, Biddau P, Cao A and Nucaro A:
Cytogenetic and molecular characterization of a variant
translocation associated with acute promyelocytic leukemia and
involving chromosomes 11, 15 and 17. Leukemia. 10:1655–1657.
1996.PubMed/NCBI
|
|
22
|
Galieni P, Marotta G, Vessichelli F,
Diverio D, Minoletti F, Bucalossi A, Lo Coco F and Lauria F:
Variant t(1;15;17)(q23;q22;q23) in a case of acute promyelocytic
leukemia. Leukemia. 10:1658–1661. 1996.PubMed/NCBI
|
|
23
|
Yamamoto K, Hamaguchi H, Nagata K,
Kobayashi M, Takashima T and Taniwaki M: A new complex
translocation (15;20;17)(q22;p13;q21) in acute promyelocytic
leukemia. Cancer Genet Cytogenet. 101:89–94. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saitoh K, Miura I, Kobayashi Y, Kume M,
Utsumi S, Takahashi N, Hatano Y, Nimura T, Hashimoto K, Takahashi S
and Miura AB: A new variant translocation of t(15;17) in a patient
with acute promyelocytic leukemia (M3): t(15;19;17)(q22;p13;q12).
Cancer Genet Cytogenet. 102:15–18. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wan TS, Chim CS, So CK, Chan LC and Ma SK:
Complex variant 15;17 translocations in acute promyelocytic
leukemia. A case report and review of three-way translocations.
Cancer Genet Cytogenet. 111:139–143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujishima M, Takahashi N, Miura I,
Kobayashi Y, Kume M, Nishinari T and Miura AB: A PML/RARA chimeric
gene on chromosome 2 in a patient with acute promyelocytic leukemia
(M3) associated with a new variant translocation:
t(2;15;17)(q21;q22;q21). Cancer Genet Cytogenet. 120:80–82. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grimwade D, Biondi A, Mozziconacci MJ,
Hagemeijer A, Berger R, Neat M, Howe K, Dastugue N, Jansen J,
Radford-Weiss I, et al: Characterization of acute promyelocytic
leukemia cases lacking the classic t(15;17): Results of the
European Working Party. Groupe Français de Cytogénétique
Hématologique, Groupe de Français d'Hematologie Cellulaire, UK
Cancer Cytogenetics Group and BIOMED 1 European Community-Concerted
Action ‘Molecular Cytogenetic Diagnosis in Haematological
Malignancies’. Blood. 96:1297–1308. 2000.PubMed/NCBI
|
|
28
|
Xu L, Zhao WL, Xiong SM, Su XY, Zhao M,
Wang C, Gao YR, Niu C, Cao Q, Gu BW, et al: Molecular cytogenetic
characterization and clinical relevance of additional, complex
and/or variant chromosome abnormalities in acute promyelocytic
leukemia. Leukemia. 15:1359–1368. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tirado CA, Golembiewski-Ruiz V,
Horvatinovich J, Moore JO, Buckley PJ, Stenzel TT and Goodman BK:
Cytogenetic and molecular analysis of an unusual case of acute
promyelocytic leukemia with a t(15;17;17)(q22;q23;q21). Cancer
Genet Cytogenet. 145:31–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eclache V, Viguie F, Frocrain C, Cassinat
B, Chomienne C, Cymbalista F and Fenaux P: A new variant
t(6;15;17)(q25;q22;q21) in acute promyelocytic leukemia:
Fluorescence in situ hybridization confirmation. Cancer Genet
Cytogenet. 159:69–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zaccaria A, Testoni M, Martinelli G,
Pelliconi S, Buzzi M, Farbegoli P, Naldi S, Salvucci M and Tura S:
Four-chromosomes complex translocations in acute promyelocytic
leukemia: Description of two cases. Eur J Haematol. 52:129–133.
1994. View Article : Google Scholar : PubMed/NCBI
|