Introduction
Colon cancer is a common malignant tumor of the
digestive tract, mostly occurring in the boundary of rectum and
sigmoid colon (1,2). Clinically, it is more prevalent in males
as compared to females. It occurs in the 50-60-year age group, and
is the third most prevalent of digestive tract malignant tumors.
For many years, the treatment of colon cancer included surgical
treatment, chemotherapy and radiotherapy. However, all these
methods have some limitations. At present, the biological treatment
has become a method of choice secondary to the three conventional
treatment methods. It is also one of the most popular research
areas. Biological therapy includes many methods such as target
therapy, gene therapy and tumor vaccine, of which gene therapy is
the most recent research topic (3).
Evidence has shown that the occurrence and development of malignant
tumor is closely related to gene alteration (3). Gene therapy may change the biological
behavior of the tumor cells by correcting or repairing the
malignant genes.
Cytokines are a class of small molecule proteins
with a variety of biological effects. The members of this family
play an important role in the occurrence and development of many
tumors, therefore cytokine therapy is commonly used for tumor
immunotherapy (3,4). In previous studies the focus was on the
tumor itself, whereas increasingly investigations have focused on
the tumor microenvironment. The tumor microenvironment is a
comprehensive system composed of tumor cells, endothelial cells,
fibroblasts, extracellular matrix, and all types of cells
associated with immunity and inflammation (5,6). It is the
internal environment in which the tumor lies during its occurrence
and development (7). The various
immune cells and related cytokines in the tumor microenvironment
play an important role in the occurrence and development of
tumor.
Interleukin (IL)-17 is the characteristic cytokine
produced by Th17 cells. IL-17 is associated with various immune
responses (8). There are varied
theories on its mechanism, ranging from the fact that IL-17 can
promote tumor metastasis by promoting angiogenesis and enhance the
ability of tumor metastasis and invasion in order that IL-17 can
inhibit tumor growth by enhancing the immune activity of cytotoxic
T cells. The results of our previous studies showed that IL-17 is
capable of significantly reducing the size of transplanted tumors
in tumor-bearing mice, but has no influence on their survival time.
In the present study, a mouse model was established and
successfully transfected with IL-17 genes. By investigating
the effect of IL-17 in the number and distribution of lymphocyte
infiltration in tumor tissues, the expression of cytokines and
transcription factors associated with the subsets of
CD4+ T cells in tumor tissues, the distribution of
subsets of spleen lymphocytes in tumor-bearing mice, a preliminary
discussion on the possible antitumor mechanism of IL-17 was
conducted, providing sufficient evidence for targeting tumor
therapy in the future and providing a new method for tumor
therapy.
Materials and methods
Cells and tumor tissues
The cells and tumor tissues used in the present
study were all obtained from our previous study (9). In brief, 60 BALB/c nu/nu mice (4- to
5-week-old male and female mice; n=30 per group) were randomly
divided into the control, vector control and experimental groups,
n=20 mice per group. C26, C26/pcDNA3.1, and C26/IL-17 cells were
injected subcutaneously into the mice of the control group (C26),
vector control group (C26/pcDNA3.1) and experimental group
(C26/IL-17), respectively. The mice from each group were sacrificed
after 5 weeks of the inoculation. The animal experiments were
approved by the Animal Experiment and Welfare committee at Hebei
Medical University. Tumor tissues were stocked into liquid nitrogen
(−196°C). The spleen lymphocytes and tumor-infiltrating lymphocytes
from each group were obtained using density gradient centrifugation
and stocked in liquid nitrogen (−196°C).
Quantitative polymerase chain reaction
(qPCR)
Extracted RNA from tumor tissues and spleen cells
was obtained from a previous study (9). The expression of cytokines and
transcription factors associated with T-cell subset differentiation
or effect [i.e., interferon (IFN)-γ, IL-4, GATA-3 and
retinoid-related orphan receptor (ROR)-γt] were detected. The
primer sequence pairs were designed using Primer5 and NCBI online
Primer-BLAST software.
The primer sequence pairs used were: β-actin sense:
5′-TCACCAGGCATTGCTGACAGG-3′ and antisense:
5′-ACTTGCGGTGCACGATGGA-3′; IFN-γ sense: 5′-AGCTCATCCGAGTGGTCCAC-3′
and antisense: 5′-AAAATTCAAATAGTGCTGGCAGAA-3′; IL-4 sense:
5′-GGGTCTCAACCCCCAGCTA-3′ and antisense:
5′-CGAGCTCACTCTCTGTGGTGTT-3′; IL-12 sense:
5′-TACTAGAGAGACTTCTTCCACAACAAGAG-3′ and antisense:
5′-TCTGGACACTCTTCAAGTCCTCATAGA-3′; IL-5 sense:
5′-CCCATGAGCACAGTGGTGAA-3′ and antisense:
5′-CTCATCGTCTCATTGCTTGTCAA-3′; IL-10 sense:
5′-GCCAAGCCTTATCGGAAATG-3′ and antisense:
5′-CTTGATTTCTGGGCCATGCT-3′; IL-13 sense: 5′-CCTGGATTCCCTGACCAACA-3′
and antisense: 5′-GGGCCTTGCGGTTACAGA-3′; IL-17 sense:
5′-AAGCTCAGCGTGTCCAAACA-3′ and antisense:
5′-TGCGCCAAGGGAGTTAAAGA-3′; transforming growth factor-β sense:
5′-TGACGTCACTGGAGTTGTACGG-3′ and antisense:
5′-GGTTCATGTCATGGATGGTGC-3′; IL-23 sense:
5′-AATAATGTGCCCCGTATCCA-3′ and antisense:
5′-CTGGAGGAGTTGGCTGAGTC-3′; GATA-3 sense:
5′-CCTACCGGGTTCGGATGTAAGT-3′ and antisense:
5′-AGTTCGCGCAGGATGTCC-3′; ROR-γt sense: 5′-TCCAGACAGCCACTGCATTC-3′
and antisense: 5′-GTGCGCTGCCGTAGAAGGT-3′; Foxp3 sense:
5′-CTGCTCCTCCTATTCCCGTAAC-3′ and antisense:
5′-AGCTAGAGGCTTTGCCTTCG-3′; T-bet sense: 5′-CAACAACCCCTTTGCCAAAG-3′
and antisense: 5′-TCCCCCAAGCAGTTGACAGT-3′.
Western blot analysis
Extracted total proteins from tumor tissues were
obtained from a previous study (9).
Protein concentrations were determined by Nanodrop ND-1000 (Gene
Co., Ltd., Hong Kong, China). Once protein concentrations were
determined, 50 µl of the protein specimens were denatured in 50 µl
of 2X sample buffer [125 mmol/l Tris-HCl, pH 6.8, 20% glycerol, 10%
β-mercaptoethanol, 0.02% bromophenol blue, and 4% sodium dodecyl
sulphate (SDS)]. Briefly, 30 µg of denatured protein were separated
using SDS-polyacrylamide gel electrophoresis and transferred to a
polyvinylidene fluoride membrane, which was blocked in 5% milk
proteins that were suspended in tris-buffered saline Tween-20
(TBST) for 2 h at room temperature. The membrane was rinsed three
times with TBST, followed by incubation with the primary antibody
rabbit anti-mouse IL-17 (polyclonal, bs-1183R) at a 1:500 dilution;
rabbit anti-mouse IL-13 (polyclonal, bs-0560R) (both from Beijing
Biosynthesis Biotechnology, Beijing, China) at a 1:500 dilution;
rabbit anti-mouse IFN-γ (polyclonal, 15365-1-AP) at a 1:500
dilution; rabbit anti-mouse IL-10 (polyclonal, 20850-1-AP) (both
from Proteintech, USA) at a 1:500 dilution, rabbit anti-mouse IL-12
(polyclonal, bs-0767R, Beijing Biosynthesis Biotechnology) at a
1:500 dilution; rabbit anti-mouse T-bet (polyclonal, 13700-1-AP) at
a 1:200 dilution; rabbit anti-mouse ROR-γt (polyclonal, 13205-1-AP)
at a 1:200 dilution; and rabbit anti-mouse Foxp-3 (polyclonal,
22228-1-AP) (all from Proteintech) at a 1:500 dilution overnight at
4°C. The membrane was then washed and treated with anti-rabbit
secondary antibodies (polyclonal, BS13278; Bioworld, China) that
were conjugated with horseradish peroxidase at a 1:5,000 dilution.
The immunoreactive proteins were visualized with a
chemiluminescence detection kit (PerkinElmer, Inc., Waltham, MA,
USA), and the expression of glyceraldehyde-3-phosphate
dehydrogenase protein was used as the loading control across all
the samples analyzed using western blotting.
Hematoxylin and eosin (H&E)
staining
The difference of the number of lymphocyte
infiltrations in tumor tissues of mice inoculated with C26/IL-17,
C26 and C26/pcDNA3.1 cells was investigated. In a previous study
(9), at 35 days after tumor cell
inoculation in mice, the tumor tissues of mice in each group, were
fixed in 10% formalin (pH 6.8–7.2) for ≥24 h. Paraffin-embedded
blocks were prepared, and 4-µm sections were cut. Sections (4 µm)
were dewaxed using xylene and washed with alcohol and water,
followed by hematoxylin staining for 5 min. The sections were
washed with tap water, differentiated with hydrochloric acid for 30
sec, soaked in tap water for 15 min, and stained with eosin for 2
min. The sections were then dehydrated, cleared and mounted. The
difference in the number of lymphocyte infiltrations in tumor
tissues was measured. Images of the sections were captured using a
Positive electric microscope (U-MCZ, Olympus, Tokyo, Japan) at a
high-power field magnification of ×400. The number of lymphocytes
under a single field of vision were counted. Subsequently, five
fields were selected for each section and the average value was
calculated.
Statistical analysis
Statistical analysis was performed using SPSS 21.0
software (IBM SPSS, Armonk, NY, USA). Normal distribution test
(Kolmogorov-Smirnov test) was used to assess the enumeration data
and the one-way ANOVA test was used to evaluate the data with equal
variance. The Kruskal-Wallis test was used for data without normal
distribution or equal variance. Measurement data were presented as
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of IL-17 gene transfection on
the distribution of subsets of splenic cells in tumor-bearing
mice
C26, C26/pcDNA3.1, and C26/IL-17 cells were
inoculated into the back of mice. The mice were sacrificed 35 days
thereafter. qPCR was applied to detect the expression of the
characteristic cytokine (IL-17) and transcription factor (ROR-γt)
of Th17 cells, characteristic cytokine (IFN-γ) and transcription
factor (T-bet) of Th1 cells, characteristic cytokine (IL-4) and
transcription factor (GATA-3) of Th2 cells, and the characteristic
cytokine (IL-10) and transcription factor (Foxp-3) of Treg cells in
the spleen lymphocyte of mice.
The results showed that compared with the mice
inoculated with C26 and C26/pcDNA3.1 cells, the spleen lymphocytes
of mice inoculated with C26/IL-17 cells had a higher expression of
ROR-γt, IFN-γ, IL-4, GATA-3 and IL-10 mRNA (Fig. 1, Table
I) but a lower expression of IL-17, T-bet and Foxp-3 mRNA
(P<0.05) (Fig. 2). Additionally,
the expression of mRNA of IL-17, ROR-γt, IFN-γ, T-bet, IL-4,
GATA-3, IL-10 and Foxp-3 in the spleen lymphocyte of mice
inoculated with C26 cells and C26/pcDNA3.1 cells had no significant
difference (P>0.05).
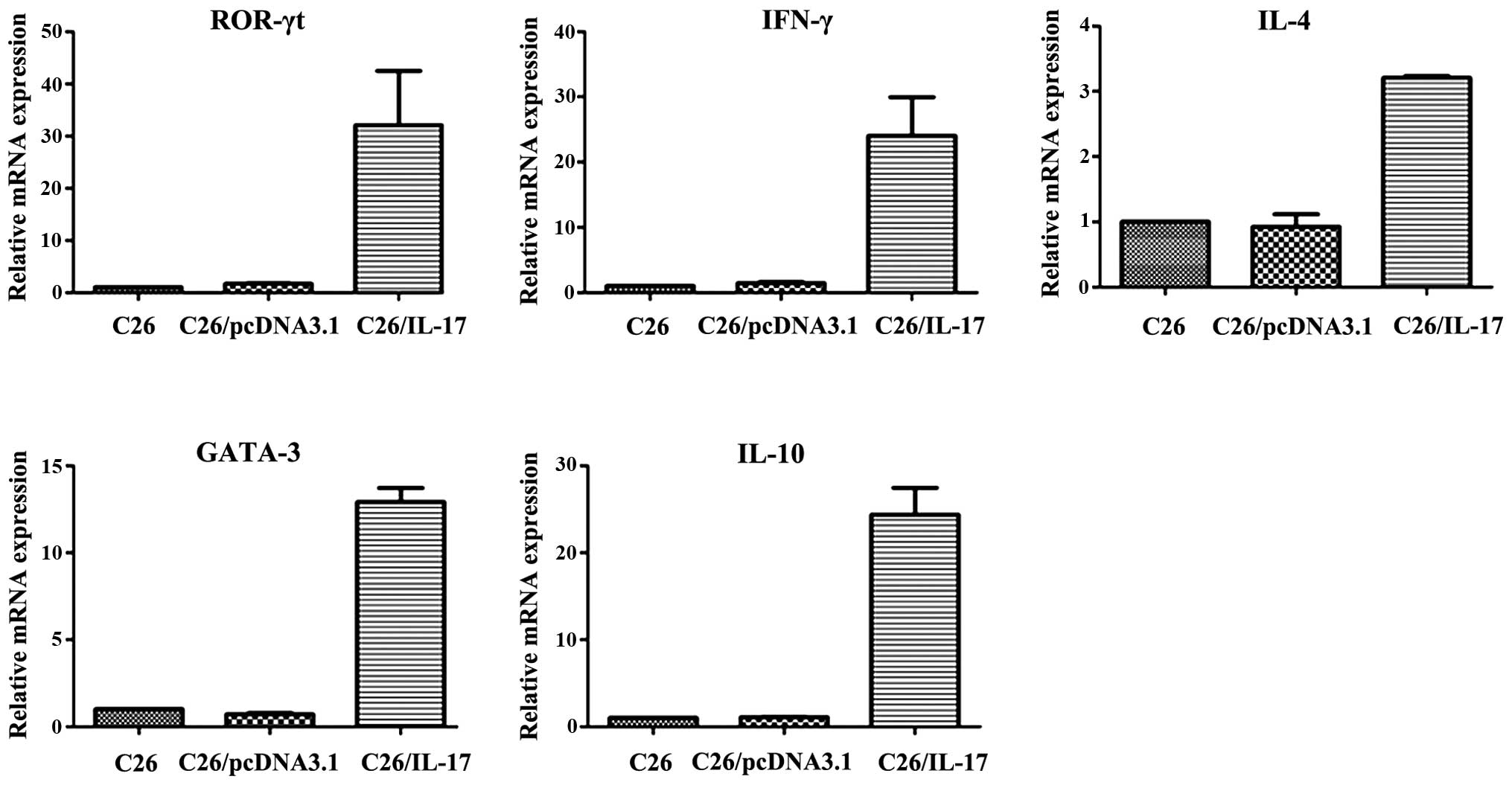 | Figure 1.High expression of cytokines and
transcription factors in splenocytes from the mice inoculated with
C26/interleukin (IL)-17 cells by quantitative polymerase chain
reaction (qPCR) (P<0.05). Inoculated C26 cells, C26/pcDNA3.1
cells, C26/IL-17 cells into the back of mice, and executed the mice
35 days later. Applied qPCR to detect the expression of cytokines
and transcription factors in the spleen lymphocyte of mice. Results
showed that compared with the mice inoculated with C26 cells and
C26/pcDNA3.1cells, the spleen lymphocyte of mice inoculated with
C26/IL-17 cells had higher expression of retinoid-related orphan
receptor (ROR)-γt, interferon (IFN)-γ, IL-4, GATA-3 and IL-10 mRNA.
The expression of mRNA of ROR-γt, IFN-γ, IL-4, GATA-3 and IL-10 in
spleen lymphocyte of mice inoculated with C26 cells and
C26/pcDNA3.1 cells had no significant difference (P>0.05). |
 | Table I.Expression of cytokines and
transcription factors in spleen lymphocytes from different mice
(mean ± standard deviation). |
Table I.
Expression of cytokines and
transcription factors in spleen lymphocytes from different mice
(mean ± standard deviation).
|
| Group |
|---|
|
| Group |
|---|
| Factors | C26 | C26/pcDNA3.1 | C26/IL-17 |
|---|
| ROR-γt | 1±0 | 1.690±0.096 |
32.076±10.399a |
| IL-17 | 1±0 | 1.066±0.193 |
0.560±0.248a |
| IFN-γ | 1±0 | 1.439±0.185 |
24.043±5.921a |
| T-bet | 1±0 | 1.066±0.119 |
0.297±0.018a |
| IL-4 | 1±0 | 0.923±0.193 |
3.207±0.028a |
| GATA-3 | 1±0 | 0.705±0.079 |
12.921±0.793a |
| IL-10 | 1±0 | 1.080±0.047 |
24.393±3.077a |
| Foxp-3 | 1±0 | 0.676±0.120 |
0.558±0.143a |
Effect of IL-17 gene transfection on
lymphocyte infiltration in tumor tissues
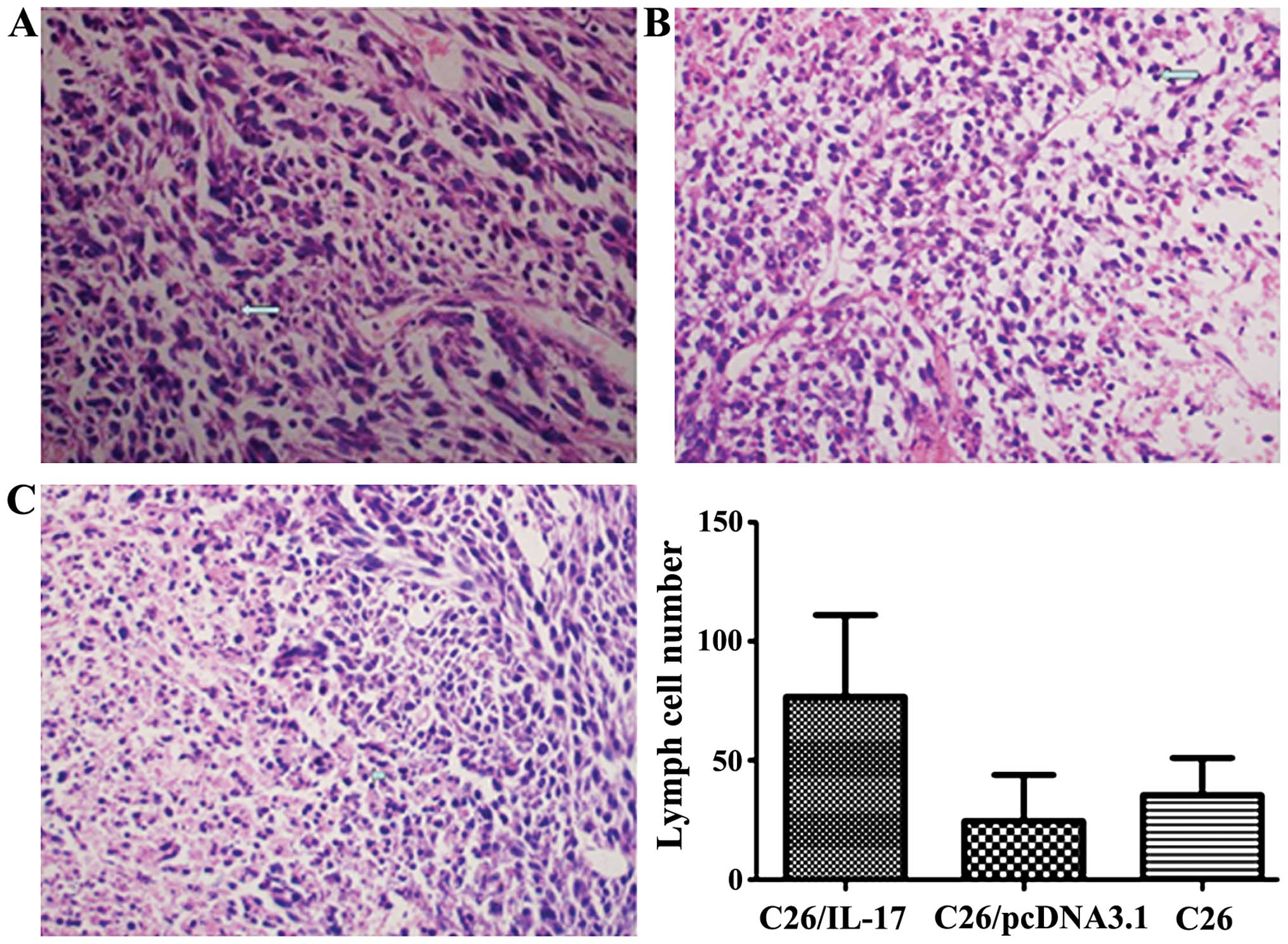
The H&E results showed that the number of
lymphocyte infiltration in tumor tissues of mice inoculated with
C26/IL-17 cells was significantly more than the other two groups
(P<0.05) (Fig. 3, Table II). The number of lymphocyte
infiltration in tumor tissues of mice inoculated with C26 and
C26/pcDNA3.1 cells had no significant difference (P>0.05).
 | Table II.The number of lymphocytes infiltrated
in tumor tissue of different mice. |
Table II.
The number of lymphocytes infiltrated
in tumor tissue of different mice.
| Group | No. of
lymphocytes |
|---|
| C26 | 20±12 |
| C26/pcDNA3.1 | 33±13 |
| C26/IL-17/male | 82±32a |
Effect of IL-17 gene transfection on
the expression of cytokines in the tumor tissues of mice
C26, C26/pcDNA3.1, and C26/IL-17 cells were
inoculated into the back of mice, and the mice were sacrificed 35
days later. The expression of cytokines IL-17 and IL-23 was
detected in Th17 cells, IFN-γ and IL-12 in Th1 cells, IL-4 and
IL-13 in Th2 cells, and IL-10 in Treg cells.
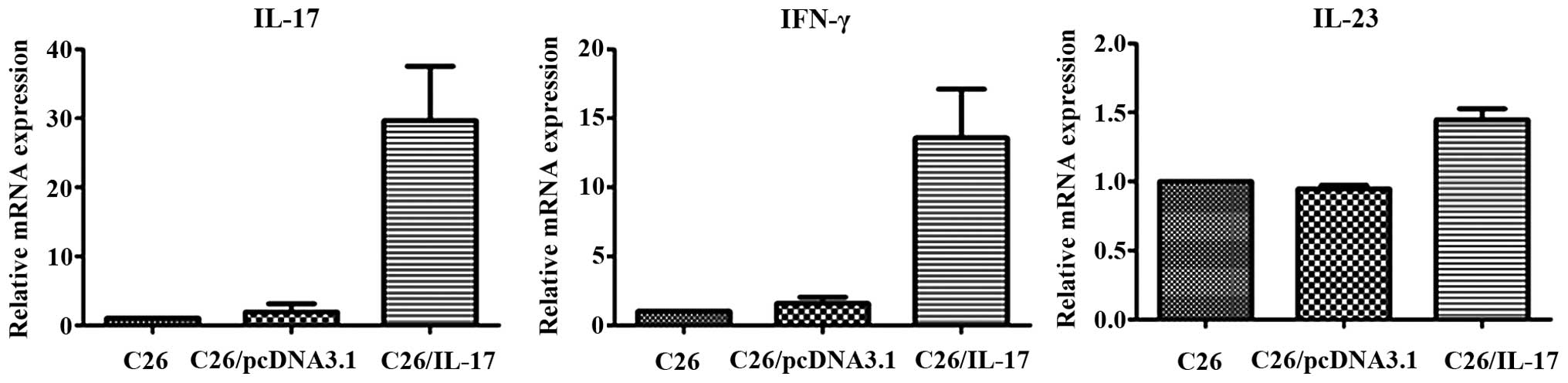
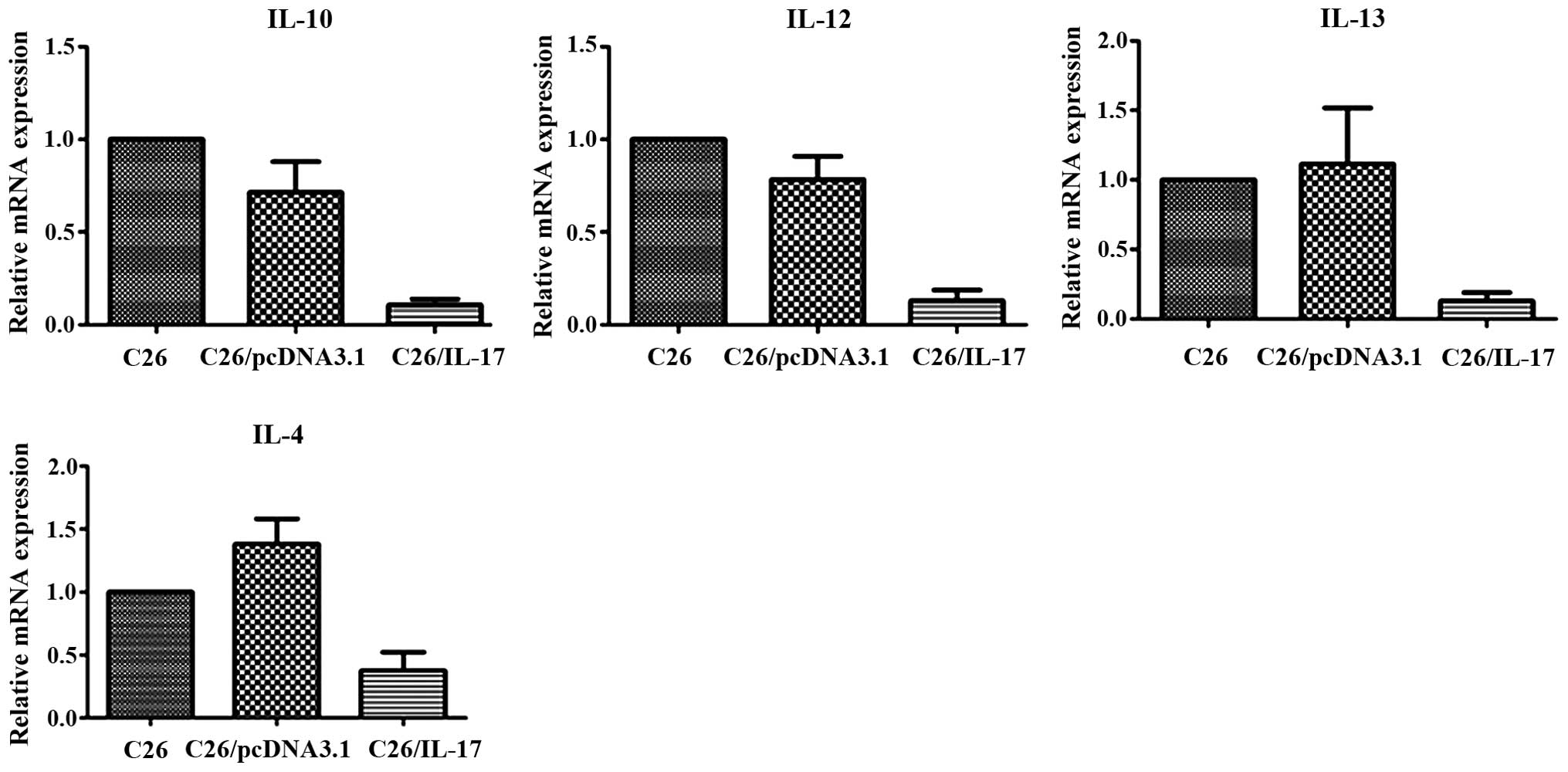
The qPCR results showed that, compared with the mice
inoculated with C26 and C26/pcDNA3.1 cells, the tumor tissues of
mice inoculated with C26/IL-17 cells had a higher expression of
IL-17, IFN-γ and IL-23 mRNA, and differences were statistically
significant (P<0.05) (Fig. 4,
Table III), whereas a lower
expression of IL-4, IL-10, IL-12 and IL-13 mRNA was identified
(P<0.05) (Fig. 5). The mRNA
expression of the above cytokines in the tumor tissues of mice
inoculated with C26 cells and C26/pcDNA3.1 cells had no significant
difference (P>0.05).
 | Table III.Expression of cytokines and
transcription factors in tumor tissue from different mice (mean ±
standard deviation). |
Table III.
Expression of cytokines and
transcription factors in tumor tissue from different mice (mean ±
standard deviation).
|
| Group |
|---|
|
|
|
|---|
| Factors | C26 | C26/pcDNA3.1 | C26/IL-17 |
|---|
| ROR-γt | 1±0 | 1.485±0.412 |
34.221±12.598a |
| IL-17 | 1±0 | 3.833±1.197 |
42.408±7.863a |
| IFN-γ | 1±0 | 1.572±0.449 |
13.573±3.529a |
| IL-23 | 1±0 | 0.945±0.028 |
1.448±0.080a |
| IL-4 | 1±0 | 1.491±1.414 |
0.360±0.116a |
| GATA-3 | 1±0 | 1.232±0.269 |
0.500±0.086a |
| IL-10 | 1±0 | 0.713±0.166 |
0.107±0.032a |
| Foxp-3 | 1±0 | 1.162±0.137 |
0.301±0.169a |
| IL-12 | 1±0 | 0.780±0.120 |
0.130±0.060a |
| IL-13 | 1±0 | 1.112±0.406 |
0.128±0.061a |
The western blot results revealed that, compared
with the mice inoculated with C26 and C26/pcDNA3.1 cells, the tumor
tissues of mice inoculated with C26/IL-17 cells had a higher
expression of IL-17, IFN-γ protein (Fig.
6) but a lower expression of IL-10, IL-12 and IL-13 protein.
The expression of IL-17, IFN-γ, IL-10, IL-12 and IL-13 proteins in
the tumor tissues of mice inoculated with C26 and C26/pcDNA3.1
cells had no significant difference (P>0.05).
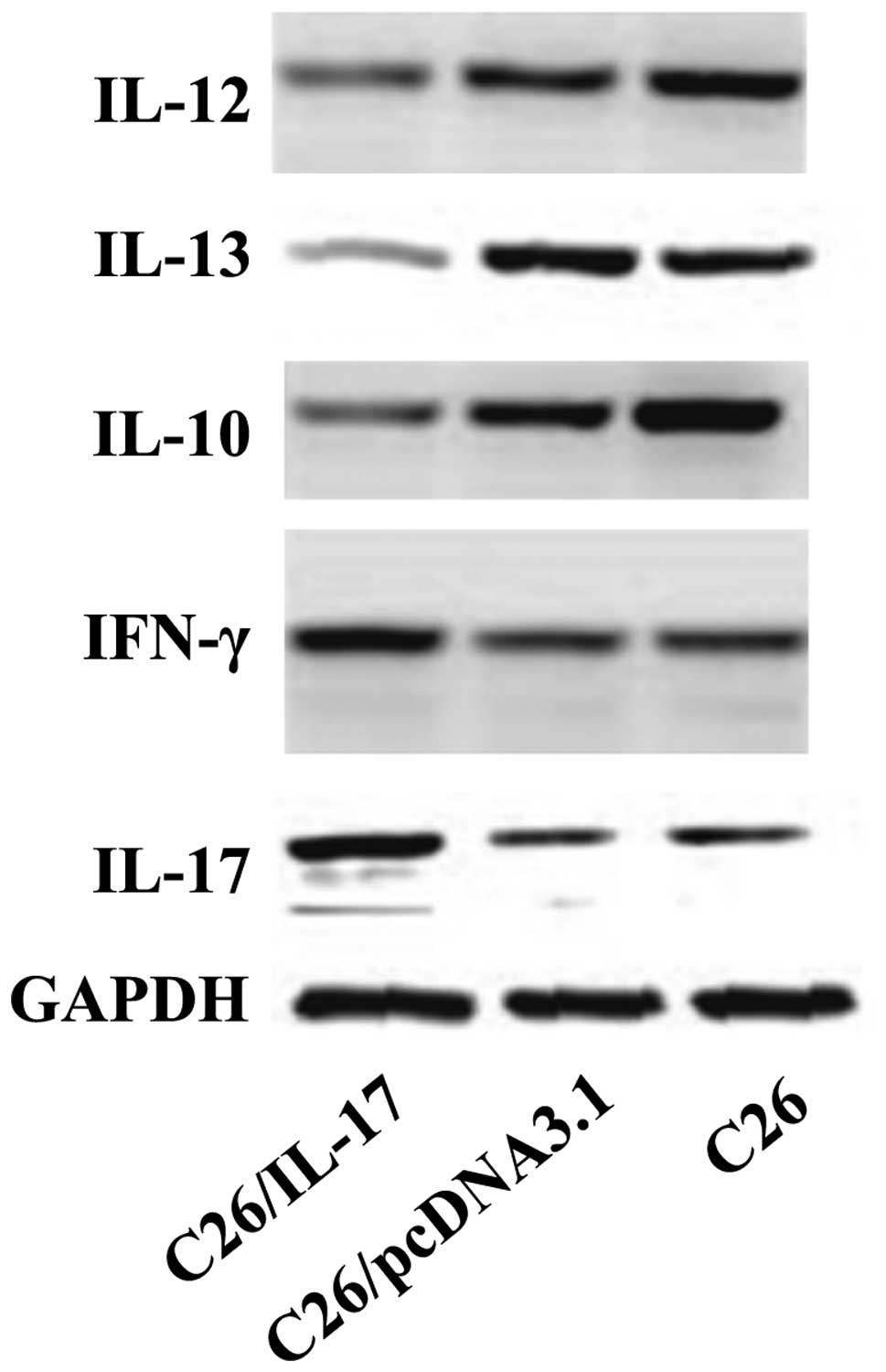 | Figure 6.The protein levels of interleukin
(IL)-17, interferon (IFN)-γ, IL-10, IL-12, IL-13 in different mouse
tumor tissue by western blotting. C26, C26/pcDNA3.1, and C26/IL-17
cells were inoculated into the back of mice, and the mice were
sacrificed 35 days later. The expression of cytokines of tumor
tissues was detected. Western blotting results show that, compared
with the mice inoculated with C26 and C26/pcDNA3.1 cells, the tumor
tissues of mice inoculated with C26/IL-17 cells had a higher
expression of IL-17, and IFN-γ protein, but a lower expression of
IL-10, IL-12 and IL-13 protein. The expression of IL-17, IFN-γ,
IL-10, IL-12 and IL-13 proteins in the tumor tissues of mice
inoculated with C26 and C26/pcDNA3.1 cells had no significant
difference (P>0.05). |
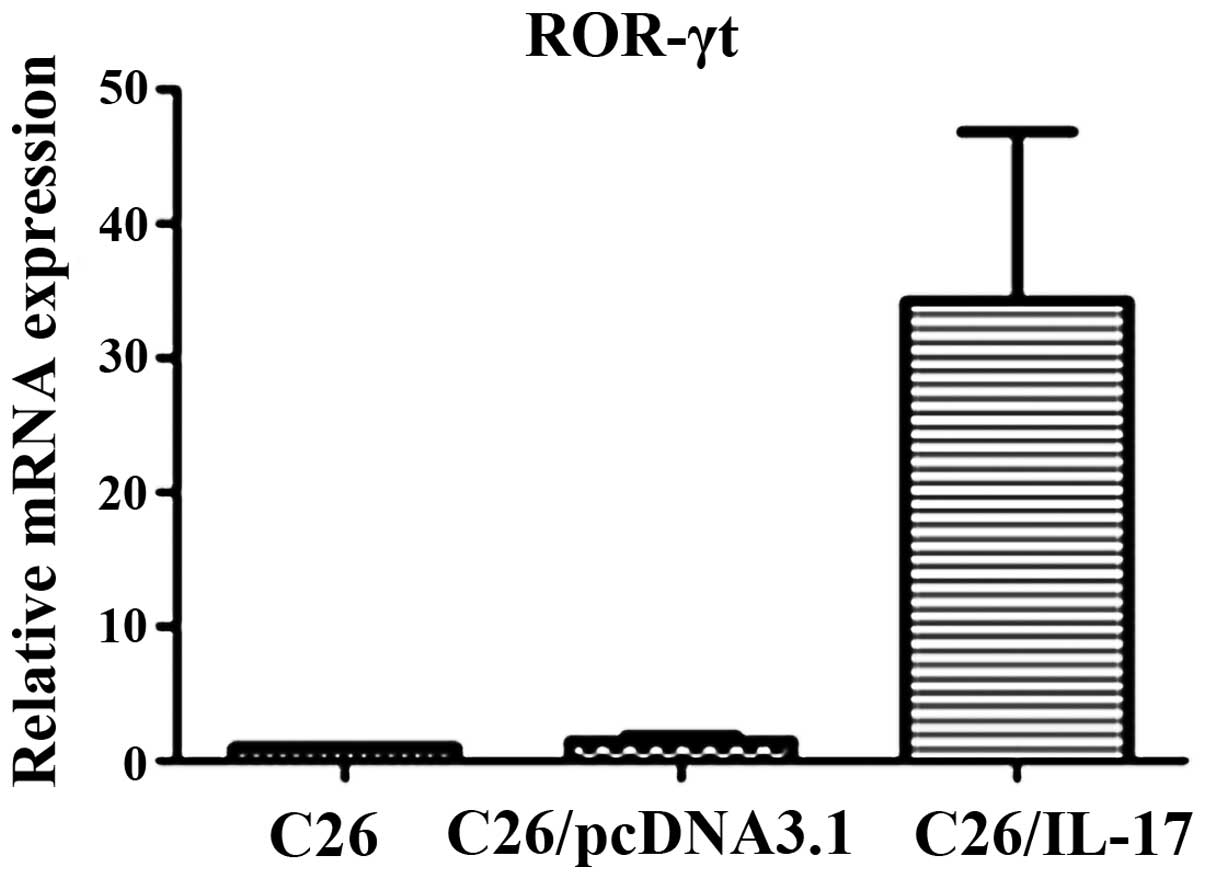
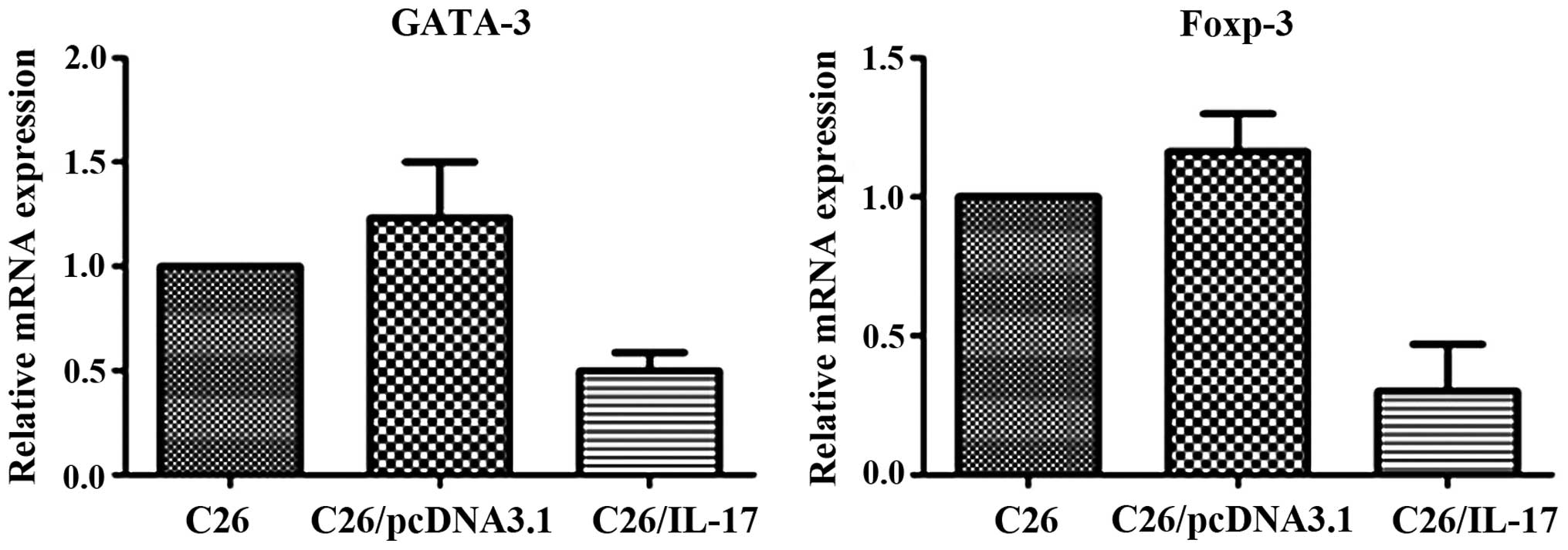
Expression of characteristic
transcription factors of Th1, Th2, Th17, and Treg cells in the
tumor tissues of tumor-bearing mice
C26, C26/pcDNA3.1, and C26/IL-17 cells were
inoculated into the back of mice, and the mice were sacrificed 35
days later. The expression of ROR-γt associated with Th17 cells,
T-bet with Th1 cells, GATA-3 with Th2 cells, and Foxp-3 with Treg
cells was detected.
The qPCR results showed that compared with the mice
inoculated with C26 and C26/pcDNA3.1 cells, the tumor tissues of
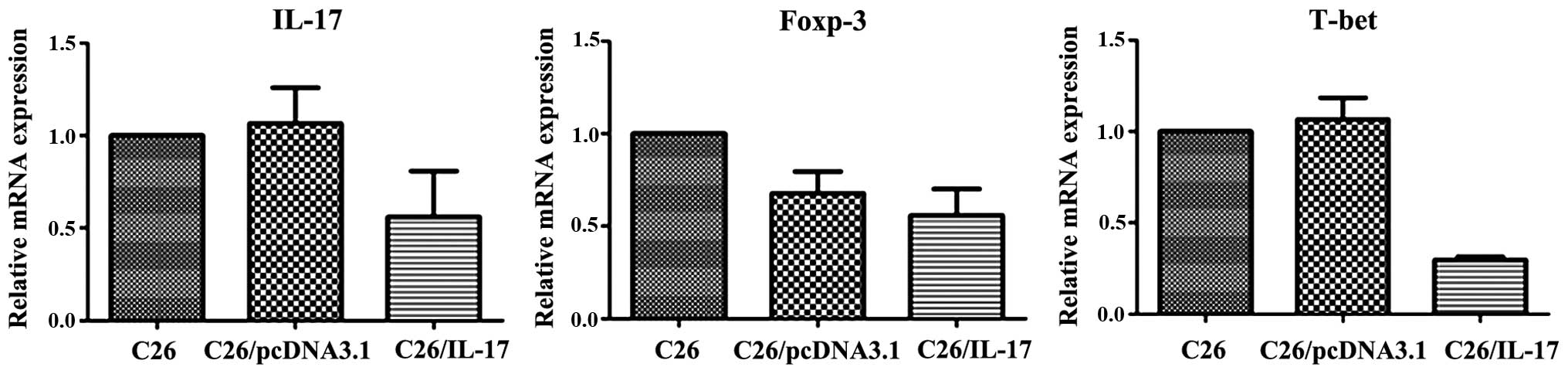
mice inoculated with C26/IL-17 cells had a higher expression of
ROR-γt mRNA (P<0.05) (Fig. 7) but
a lower expression of GATA-3 and Foxp-3 mRNA, and differences were
statistically significant (P<0.05) (Fig. 8). The mRNA expression of the above
transcription factors in the tumor tissues of mice inoculated with
C26 and C26/pcDNA3.1 cells had no significant difference
(P>0.05).
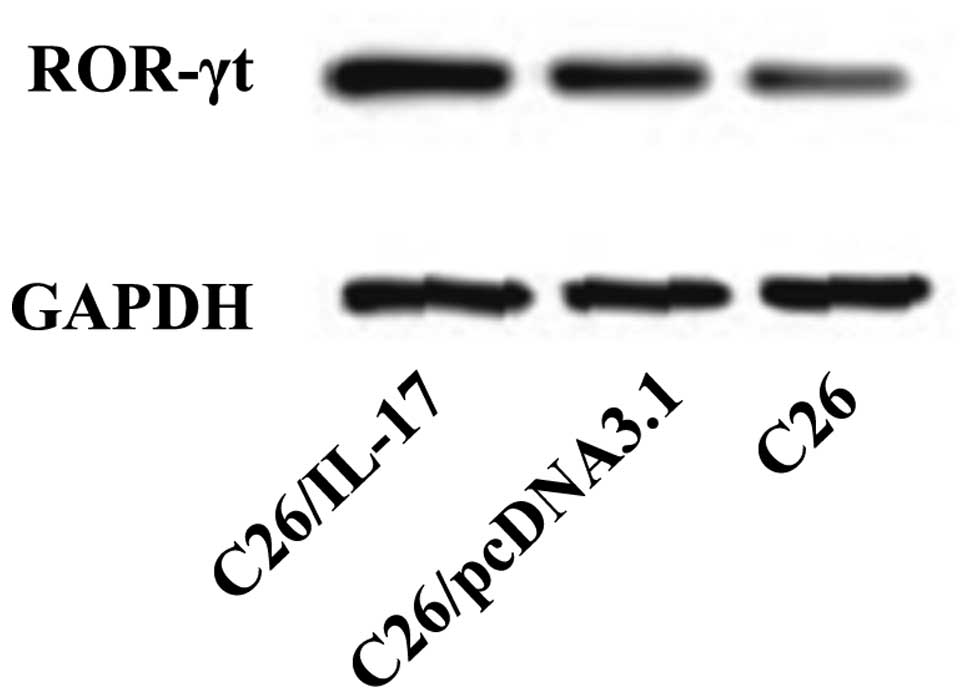
The western blotting results showed that, compared
with the mice inoculated with C26 and C26/pcDNA3.1 cells, the tumor
tissues of mice inoculated with C26/IL-17 cells had a higher
expression of ROR-γt protein (Fig.
9). The expression of ROR-γt protein in the tumor tissues of
mice inoculated with C26 and C26/pcDNA3.1 cells had no significant
difference (P>0.05).
Discussion
IL-17 is a cytokine with multiple biological
effects. It may be produced by NK T cells, CD8+ T cells,
γδT cells, dendritic cells, macrophages and other cells (9), but is mainly produced by Th17 cells
(10). Th17 is a newly identified
T-helper cell subset. Its appearance has challenged the traditional
classification of CD4+T-cell subsets. Previous findings
have shown that Th17 cell was characterized by secreting cytokine
IL-17 (9). Clinical data have shown
that IL-17 was involved in the occurrence of many types of
autoimmune diseases and inflammation, and also closely associated
with the occurrence and development of tumor. However, its role in
tumor is controversial. IL-17 is known to promote tumor by
promoting angiogenesis, inhibiting tumor cell apoptosis and
promoting tumor metastasis and invasion (11–14). By
contrast, IL-17 is considered to inhibit tumor by enhancing the
activity of NK cells, promoting the activation and production of
CTL cells and inhibiting the infiltration of tumor cells (15–24).
Radosavljevic et al (25) demonstrated that IL-17 is an important
indicator of the development of colon cancer. In a previous study,
we successfully established mouse models of colon cancer and
identified that IL-17 gene transfection may significantly
reduce the tumor size of tumor-bearing mice. This finding may be
associated with its antitumor effect. In the current study, we
investigated the antitumor mechanism of IL-17 in mice with colon
cancer.
It is known that the various subsets of
CD4+T cells are of great significance in inhibiting
tumors and Th1, Th2, Th17 and Treg cells are most closely
associated with tumors. Therefore, in the current study four types
of CD4+T cells were used. The results of qPCR showed
that compared with the mice inoculated with C26 and C26/pcDNA3.1
cells, the tumor tissues of mice inoculated with C26/IL-17 cells
had an increased expression of ROR-γt but a decreased expression of
IL-17 (P<0.05), indicating that IL-17 increases the number of
ROR-γt+ cells in the spleen of the tumor-bearing mice
but reduces the number of Th17 cells. In addition, compared with
the mice inoculated with C26 and C26/pcDNA3.1 cells, the tumor
tissues of mice inoculated with C26/IL-17 cells had an elevated
expression of IFN-γ but a reduced expression of T-bet (P<0.05),
indicating that IL-17 can increase the number of IFN-γ+
cells in spleen of tumor-bearing mice, but reduce the number of TH1
cells. Compared with the mice inoculated with C26 and C26/pcDNA3.1
cells, the tumor tissues of mice inoculated with C26/IL-17 cells
had a higher expression of IL-4 and GATA-3 (P<0.05), indicating
that IL-17 can increase the number of Th2 cells in tumor-bearing
mice. In addition, compared with the mice inoculated with C26 and
C26/pcDNA3.1 cells, the tumor tissues of mice inoculated with
C26/IL-17 cells had a higher expression of IL-10 but a lower
expression of Foxp-3 (P<0.05), indicating that IL-17 can
decrease the number of Foxp-3+ cells in spleen of
tumor-bearing mice. Thus, the increased IL-10 may be derived from
Th2 cells. mRNA expression in the various cytokines and
transcription factors in the splenocyte of tumor-bearing mice
inoculated with C26 and C26/pcDNA3.1 cells had no significant
difference (P>0.05). The above results indicated that
IL-17 gene may exert an antitumor effect by affecting the
distribution of the subsets of spleen cells. The specific phenotype
and characteristics of ROR-γt+ cells with a low
expression of IL-17 and the IFN-γ+ cells with a low
expression of T-bet remain to be investigated.
Tumor microenvironment is an essential internal
environment in the development of tumor. It is a comprehensive
system composed of tumor cells, endothelial cells, fibroblasts,
extracellular matrix, and cells associated with immunity and
inflammation (5,6). In this complex system, cytokines and
various immune cells interact with each other and cooperate to
regulate the occurrence, development and invasion and metastasis of
tumor. In this study, we conducted an in-depth investigation on the
cytokines and Th1, Th2, Th17 and Treg cells in tumor tissues of
tumor-bearing mice. Firstly, we applied H&E staining to count
the number of infiltrating lymphocytes in tumor tissues. The
results showed that IL-17 gene transfection is capable of
increasing the number of lymphocytes in the tissues of colon
cancer, and the data were statistically significant (P<0.05),
indicating that IL-17 gene may exert an antitumor effect by
increasing the infiltration of lymphocytes. We also detected the
transcription factors associated with Th subsets in the colon
cancer tissues and identified that mRNA and proteins of ROR-γt in
tumor tissues of mice inoculated with C26/IL-17 cells, were
significantly more than the mice inoculated with C26 and
C26/pcDNA3.1 cells, and differences were statistically significant
(P<0.05), indicating that IL-17 gene transfection can
increase the number of ROR-γt+ cells in tumor tissues.
Compared with the mice inoculated with C26 and C26/pcDNA3.1 cells,
the mice inoculated with C26/IL-17 cells had a lower expression of
Foxp-3 and GATA-3 mRNA (P<0.05), indicating that IL-17
gene transfection can reduce the number of Th2 and Treg cells in
tumor tissues of mice. The above results indicated that IL-17
could, not only increase the number of TIL, but also regulate the
distribution of Th subsets. The antitumor effect of IL-17 may be
associated with its effect in increasing the number of TIL and
ROR-γt+ cells, and reducing the number of Th2 and Treg
cells.
We also observed cytokines associated with the
differentiation and function of Th subsets in the colon cancer
tissues of mice. The results show that compared with the mice
inoculated with C26 and C26/pcDNA3.1 cells, the mRNA and proteins
of IL-17 in mice inoculated with C26/IL-17 cells were increased,
and differences were statistically significant (P<0.05),
indicating that we successfully established C26 cells that steadily
transfected IL-17 gene, and the successfully established
tumor cells effectively expressed IL-17 mRNA and protein. A number
of studies have shown that IFN-γ significantly enhanced immunity.
It has been previously shown that the growth ability of melanoma
and bladder cancer cells in mice with IFN-γ gene defect was
greatly intensified (14). The
current results showed that IL-17 gene transfection can
significantly increase the mRNA and protein of IFN-γ in colon
cancer tissues of mice, and the data were statistically significant
(P<0.05). The largely increased IFN-γ may be from the T or NK
cells of CD8+. Treg cells have been generally recognized
as a cell with immunosuppressive action and its inhibitory effect
was closely associated with the secretion of IL-10. The results of
the present study have shown that IL-17 gene transfection
can lower the mRNA and protein expression of IL-10 in tumor tissue
of tumor-bearing mice, which may be associated with the plasmid.
However, the IL-17 gene further reduced the number of Treg
cells. A previous study identified that the role of IL-13 in tumor
was the same as IL-10 (26,27). Both were able to promote the tumor
growth by inhibiting immunity. The results of our study showed that
IL-17 gene transfection can reduce the expression of IL-13
in colon cancer tissues. From the above analysis, we suggest that
IL-17 gene exerts an antitumor effect by increasing the
expression of IFN-γ and reducing the expression of IL-10 and
IL-13.
In conclusion, the antitumor effect of IL-17
gene transfection in the colon cancer of mice may be associated
with the following mechanisms: i) IL-17 gene transfection
can change the distribution of different subsets of spleen
lymphocytes in mice; ii) IL-17 gene transfection can
increase the number of lymphocyte infiltration in tumor tissues;
and iii) IL-17 gene transfection can promote the high
expression of IFN-γ in tumor tissue, while reducing the expression
of IL-10 and IL-13 factors, thus exerting an antitumor effect.
Overall, we predict that IL-17 directly or indirectly induces the
polarization of tumor tissue infiltration lymphocytes and exerts an
anti-tumor effect, which requires further investigation.
Acknowledgements
The present study was supported by grant nos.
10396106D, 13397703D, 14967719D, C2010000474 and 2011101.
References
|
1
|
Yang TM: Clinical pathological features
and survival rate of 116 cases of colorectal cancer. Chin J Med
Guide. 16:602–603. 2014.
|
|
2
|
Zhao S and Xue B: Clinical and
pathological features of rectal cancer in young patients. Mod
Oncol. 21:1296–1298. 2013.
|
|
3
|
Zhang C, Wang Q-T, Liu H, Zhang ZZ and
Huang WL: Advancement and prospects of tumor gene therapy. Chin J
Cancer. 30:182–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hou L-N, He X-H and Zhang Z-X: The present
situation and outlook of the treatment of tumor with cytokine gene.
J Clin Exp Med. 7:145–147. 2008.
|
|
5
|
Sung SY, Hsieh CL, Wu D, Chung LW and
Johnstone PA: Tumor microenvironment promotes cancer progression,
metastasis, and therapeutic resistance. Curr Probl Cancer.
31:36–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nelson D, Fisher S and Robinson B: The
‘Trojan Horse’ approach to tumor immunotherapy: Targeting the tumor
microenvironment. J Immunol Res. 2014:7890692014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Egeblad M, Nakasone ES and Werb Z: Tumors
as organs: Complex tissues that interface with the entire organism.
Dev Cell. 18:884–901. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jie L, Zhou X-H, Qian S, et al: The
function of IL-17 family and their roles in related diseases. Curr
Immunol. 34:262–265. 2014.
|
|
9
|
Korn T, Bettelli E, Oukka M and Kuchroo
VK: IL-17 and Th17 Cells. Annu Rev Immunol. 27:485–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fossiez F, Djossou O, Chomarat P,
Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E,
Saeland S, et al: T cell interleukin-17 induces stromal cells to
produce proinflammatory and hematopoietic cytokines. J Exp Med.
183:2593–2603. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wakita D, Sumida K, Iwakura Y, Nishikawa
H, Ohkuri T, Chamoto K, Kitamura H and Nishimura T:
Tumor-infiltrating IL-17-producing gammadelta T cells support the
progression of tumor by promoting angiogenesis. Eur J Immunol.
40:1927–1937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He D, Li H, Yusuf N, Elmets CA, Li J,
Mountz JD and Xu H: IL-17 promotes tumor development through the
induction of tumor promoting microenvironments at tumor sites and
myeloid-derived suppressor cells. J Immunol. 184:2281–2288. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dhodapkar KM, Barbuto S, Matthews P,
Kukreja A, Mazumder A, Vesole D, Jagannath S and Dhodapkar MV:
Dendritic cells mediate the induction of polyfunctional human
IL17-producing cells (Th17-1 cells) enriched in the bone marrow of
patients with myeloma. Blood. 112:2878–2885. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Yi T, Kortylewski M, Pardoll DM,
Zeng D and Yu H: IL-17 can promote tumor growth through an
IL-6-Stat3 signaling pathway. J Exp Med. 206:1457–1464. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hirahara N, Nio Y, Sasaki S, Minari Y,
Takamura M, Iguchi C, Dong M, Yamasawa K and Tamura K: Inoculation
of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells
induces T cell-dependent tumor-specific immunity in mice. Oncology.
61:79–89. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Benchetrit F, Ciree A, Vives V, Warnier G,
Gey A, Sautès-Fridman C, Fossiez F, Haicheur N, Fridman WH and
Tartour E: Interleukin-17 inhibits tumor cell growth by means of a
T-cell-dependent mechanism. Blood. 99:2114–2121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirahara N, Nio Y, Sasaki S, Takamura M,
Iguchi C, Dong M, Yamasawa K, Itakura M and Tamura K: Reduced
invasiveness and metastasis of Chinese hamster ovary cells
transfected with human interleukin-17 gene. Anticancer Res 20 (5A).
3137–3142. 2000.
|
|
18
|
Martin-Orozco N, Muranski P, Chung Y, Yang
XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW and Dong C: T
helper 17 cells promote cytotoxic T cell activation in tumor
immunity. Immunity. 31:787–798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE
and Ansell SM: Malignant B cells skew the balance of regulatory T
cells and TH17 cells in B-cell non-Hodgkin's lymphoma. Cancer Res.
69:5522–5530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Horlock C, Stott B, Dyson PJ, Morishita M,
Coombes RC, Savage P and Stebbing J: The effects of trastuzumab on
the CD4+CD25+FoxP3+ and CD4+IL17A+ T-cell axis in patients with
breast cancer. Br J Cancer. 100:1061–1067. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Muranski P, Boni A, Antony PA, Cassard L,
Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K,
et al: Tumor-specific Th17-polarized cells eradicate large
established melanoma. Blood. 112:362–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sfanos KS, Bruno TC, Maris CH, Xu L,
Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB and Drake CG:
Phenotypic analysis of prostate-infiltrating lymphocytes reveals
TH17 and Treg skewing. Clin Cancer Res. 14:3254–3261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dougan M and Dranoff G: Inciting
inflammation: The RAGE about tumor promotion. J Exp Med.
205:267–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kryczek I, Wei S, Szeliga W, Vatan L and
Zou W: Endogenous IL-17 contributes to reduced tumor growth and
metastasis. Blood. 114:357–359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Radosavljevic G, Ljujic B, Jovanovic I,
Srzentic Z, Pavlovic S, Zdravkovic N, Milovanovic M, Bankovic D,
Knezevic M, Acimovic LJ, et al: Interleukin-17 may be a valuable
serum tumor marker in patients with colorectal carcinoma.
Neoplasma. 57:135–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J-J and Zheng W-M: The relationship
of IL-13 and its receptor to malignant tumor. Foreign Med Sci Oncol
Sect. 32:736–739. 2005.
|
|
27
|
Terabe M, Park JM and Berzofsky JA: Role
of IL-13 in regulation of anti-tumor immunity and tumor growth.
Cancer Immunol Immunother. 53:79–85. 2004. View Article : Google Scholar : PubMed/NCBI
|