Introduction
Gastric cancer is one of the most common types of
cancer and ranks as the second leading cause of cancer-associated
mortality worldwide (1–3), with the majority of patients residing in
China. Due to the non-specific symptoms of gastric cancer at early
stages, the majority of patients present at an advanced stage at
initial diagnosis, making cancer therapy challenging. At present,
traditional methods, including surgery, chemotherapy and radiation
therapy, are the major approaches used to treat gastric cancer
(4,5).
However, the drugs in current use have limited efficiency due to
the advanced stages of cancer. There is an urgent requirement to
identify suitable targets for cancer therapy and to develop an
efficient drug.
Dopamine receptors are a class of G-protein-coupled
receptors and are important in the nervous system. Dopamine
receptors are involved in various neurological processes, with
dopamine as their ligand. Disordered dopamine secretion or
inactivation of dopamine receptors may lead to various neurological
diseases, including Parkinson's disease, schizophrenia and social
phobia (6–8). In this class of receptors, dopamine
receptor D2 (DRD2) is an important member.
In previous years, in addition to its traditional
function, DRD2 has been reported to be correlated with pituitary
tumors (9,10). A study investigating gene
polymorphisms in DRD2 found that DRD2 alleles are associated with
tobacco use and lung cancer (11,12).
Genome-wide short hairpin RNA screens have supported the importance
of DRD2 in glioblastoma. The mRNA and protein expression levels of
DRD2 were found to be elevated in clinical glioblastoma specimens,
compared with matched non-tumor tissues, and the inhibition of DRD2
and epidermal growth factor receptor induced synergistic antitumor
activity (11). A correlation between
the expression of DRD2 and tumors has also been found in bladder
cancer and leukemia (13,14). These findings suggest an association
between DRD2 and cancer, and its potential for use as a potential
target for cancer therapy. A small molecule screen of targeting
cancer stem cells demonstrated that the DRD2 inhibitor,
thioridazine, showed potent antileukemic stem cell activity
(15). As cancer stem cells are a
subpopulation of cells responsible for cancer progression,
metastasis and chemoresistance (16–18), this
finding further supported the potential significant function of
DRD2 in cancer. Although the inhibition of DRD2 has been reported
in several studies, to the best of our knowledge, the correlation
between the expression of DRD2 in gastric cancer and survival
durations has not been reported. In the present study, 84 gastric
cancerous tissue samples and matched adjacent non-tumor tissue
samples were collected. The protein expression levels of DRD2 were
detected, and the correlation between the expression of DRD2 and
prognosis was analyzed. The aim of the present study aimed to
identify a marker for gastric cancer prognosis and develop a
potential target for gastric cancer therapy.
Materials and methods
Sample collection and tissue chip
processing
A total of 84 gastric cancerous tissues and
respective adjacent non-cancerous tissues were obtained from 84
patients who were diagnosed with cancer and underwent surgical
resection in the Department of General Surgery, Xinhua Hospital
affiliated with Shanghai Jiaotong University (Shanghai, China)
between February 2007 and May 2008. The tissue samples were from 64
men and 20 women, who were aged between 34 and 83 years. The
majority of the patients (81/84) were diagnosed without metastasis.
According to the World Health Organization classification, 34 cases
were defined as well- or moderately differentiated, and the other
50 cases were considered to be poorly differentiated. In terms of
tumor-node-metastasis (TNM) classification, 32 cases were
classified as early or median stage and 52 cases were classified as
late stage. Informed consent was obtained prior to surgery. All
experiments using human tissues were performed in accordance with
the Institutional Review Board of Shanghai Jiaotong University. The
samples were observed by pathologists, and those suitable for
experiments were selected and processed into a paraffin-embedded
tissue chip by Shanghai Superchip Biotechnology Corporation
(Shanghai, China).
Immunohistochemical assay
The expression levels of DRD2 in the gastric cancer
tissues and adjacent non-cancerous tissues were detected using an
immunohistochemical assay. The paraffin-embedded tissues were
preheated at 63°C for 1 h, and then deparaffinized and rehydrated
using dimethylbenzene and degrading ethanol solution. Following
rinsing with water three times, the tissues were incubated in
citrate buffer (prepared by mixing 82 ml 0.1 M sodium citrate, 18
ml 0.1 M citric acid and 900 ml distilled water), heated in the
autoclave for 5 min and cooled to room temperature for 30 min.
Endogenous peroxidase activity was quenched using methanol and
hydrogen peroxidase solution (38.4 ml methanol, 12 ml 30% hydrogen
peroxidase and 9.6 ml distilled water) for 15 min at room
temperature. Following washing with phosphate-buffered saline (PBS)
three times, the tissues were incubated with mouse anti-DRD2
monoclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) at a dilution of 1:100 in a humidified atmosphere.
Following staining with the primary antibody at 4°C overnight, the
tissues were incubated with secondary antibody from the mouse
EnVision™+/HRP kit (Dako North America, Inc.,
Carpinteria, CA, USA) for 1 h at room temperature, following which
they were reacted with DAB solution (Dako North America, Inc.) for
5 min at room temperature. The tissues were then stained with
hematoxylin (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)
for 40 sec and subsequently with 0.25% hydrochloric acid alcohol
solution for 2 sec. The tissues were then dehydrated with graded
ethanol and dimethylbenzene, mounted and visualized under a
microscope (Leica BM E microscope; Leica Biosystems Inc., Buffalo
Grove, IL, USA).
The expression levels of DRD2 in the tissues were
evaluated by two independent examiners who were blinded to the
patient outcomes and classification of the tissues. The expression
levels of DRD2 were determined as the percentage of positive cells
and intensity of staining. The intensity of the staining was
classified between 0 and 3 (0, none; 1, weak; 2, moderate; 3,
strong). The level of DRD2 in each sample was calculated as the
labelling intensity × percentage of positive cells. The evaluations
of the two observers were identical for the majority of samples.
The remaining tissues were reevaluated and the final results were
analyzed.
Cell culture
The AGS gastric cancer cells were obtained from the
Cell Bank of the Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences (Shanghai, China). The AGS cells were
grown in Roswell Park Memorial Institute 1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (Sigma-Aldrich; Merck Millipore) and 1X
penicillin-streptomycin (Sigma-Aldrich; Merck Millipore). The cells
were incubated in a humidified atmosphere with 5% CO2 at
a temperature of 37°C.
Cell survival detection using a
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide
(MTT) assay
The AGS cells were seeded into 96-well dishes
(Corning Incorporated, Corning, NY, USA) at a density of
1×104 cells/well. They were treated with thioridazine
(Sigma-Aldrich; Merck Millipore) at indicated concentrations after
12 h.
To the cells, 10 µl MTT (4 mg/ml; Beyotime Institute
of Biotechnology, Haimen, China) was added 48 h following treatment
and incubated at 37°C for 4 h. The precipitate was dissolved in 100
µl DMSO following the removal of supernatant. The absorbance at
wavelengths of 595 and 630 nm were measured. The cell viability was
calculated as the optical density (OD)595 -
OD630. Experiments were repeated three times.
Statistical analysis
The overall survival durations of the patients with
gastric cancer were analyzed using Kaplan-Meier analysis. The
groups were compared using one-way analysis of variance using R
2.9.0 software. The correlation between the mRNA levels of DRD2 and
overall survival durations in patients with gastric cancer were
examined using online database resources and calculated using
Kaplan-Meier analysis (http://kmplot.com/analysis) (19).
Results
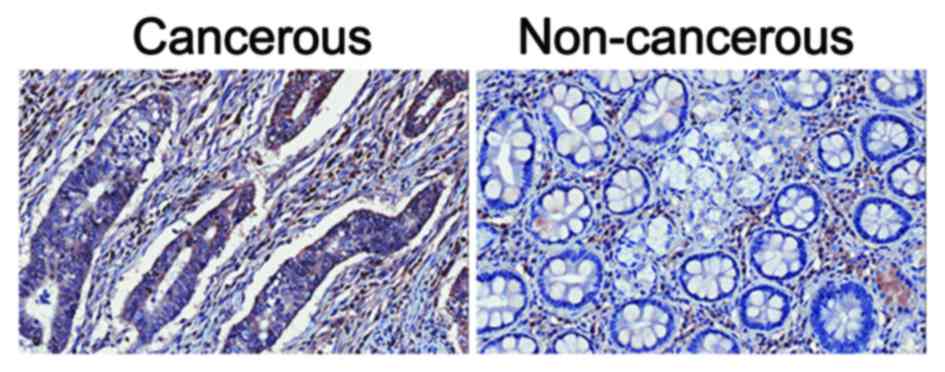
Gastric cancer tissues show higher
protein expression levels of DRD2, compared with adjacent
non-cancerous tissues
The expression levels of DRD2 in gastric cancer
tissues and respective adjacent non-cancerous tissues were detected
using an immunohistochemical assay. It was found that, in the 84
paired samples, 51.2% (43/84) of the cancerous tissues showed
higher expression of DRD2 (Fig. 1),
whereas the percentage of adjacent non-cancerous tissues showing
high protein expression levels of DRD2 was only 39.3% (33/84). The
remaining 9.5% (8/84) paired tissues showed similar levels of DRD2
in the cancerous and non-cancerous tissues.
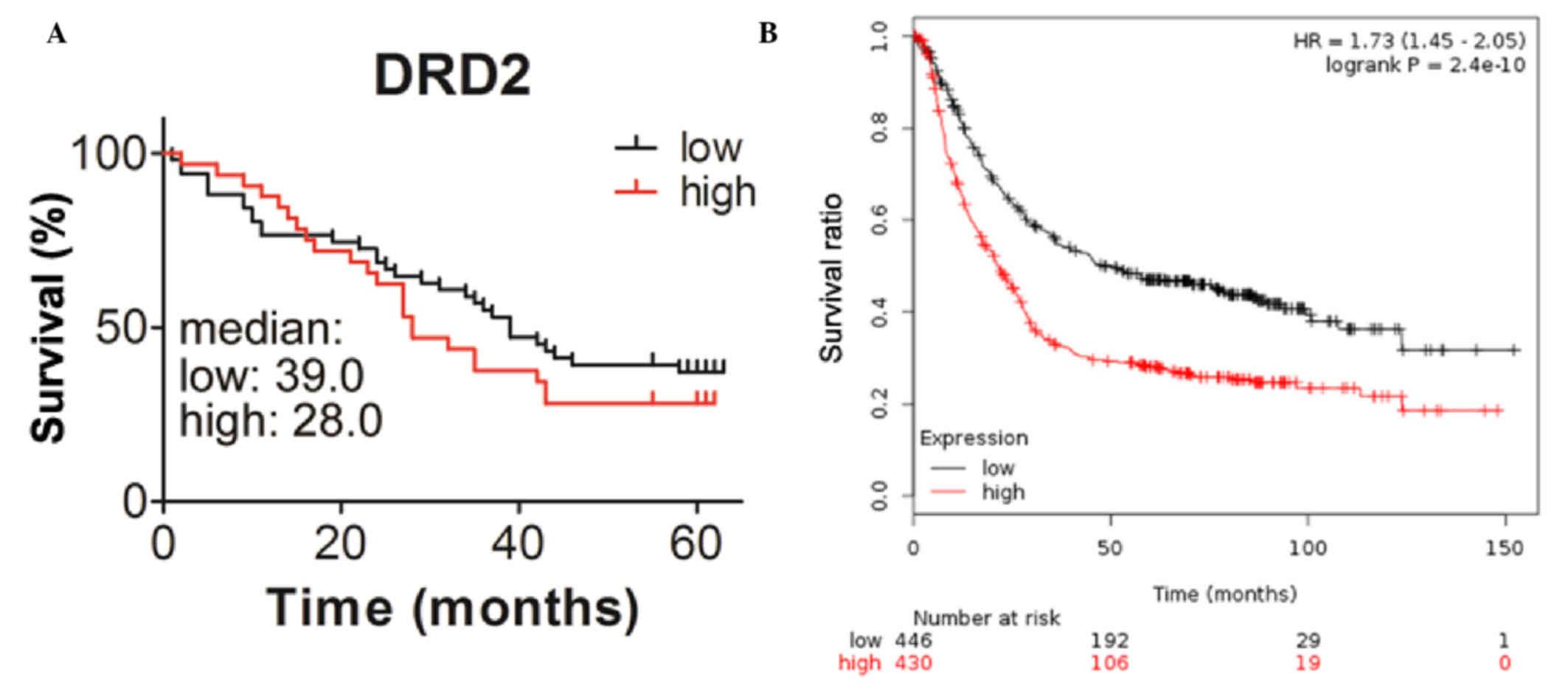
Patients with higher expression levels
of DRD2 exhibit shorter survival duration
To determine whether the expression of DRD2 is
correlated with the survival durations of the patients, the present
study analyzed the association between the level of DRD2 and
survival durations using Kaplan-Meier analysis with a log-rank
test. The median survival duration of patients with higher
expression levels of DRD2 was 28 months, which was shorter,
compared with that of the patients with lower expression levels of
DRD2, which was ~ 39 months (Fig.
2A). The correlation between the mRNA levels of DRD2 and
prognosis were also analyzed using online resources. Patients with
gastric cancer with higher transcription levels of DRD2 also had
reduced survival durations (Fig. 2B).
This further demonstrated the inverse correlation between the
expression of DRD2 and the survival durations of patients with
gastric cancer. The correlations between the expression of DRD2 and
other clinic pathological features were also analyzed in the
present study, however, no significant associations were found
between the expression of DRD2 and age, gender, tumor volume,
differentiation, metastasis, node positivity or TNM stage in the
patients with gastric cancer (Table
I).
 | Table I.Correlation between pathological
factors and expression of DRD2 in tumor tissues. |
Table I.
Correlation between pathological
factors and expression of DRD2 in tumor tissues.
|
| DRD2 (n) |
|
|---|
|
|
|
|
|---|
| Variable | Low | High | P-value |
|---|
| Age |
|
| 0.578 |
| ≥65
years | 26 | 18 |
|
| <65
years | 26 | 14 |
|
| Gender |
|
| 0.466 |
|
Female | 11 | 9 |
|
| Male | 41 | 23 |
|
| Tumor volume |
|
| 0.623 |
| ≥35
cm3 | 23 | 16 |
|
| <35
cm3 | 27 | 15 |
|
| Tumor
differentiation |
|
| 0.163 |
| I, II,
II–III | 18 | 16 |
|
| III | 34 | 16 |
|
| Metastasis |
|
| 0.299 |
| Yes | 1 | 2 |
|
| No | 51 | 30 |
|
| Node positive |
|
| 0.587 |
| ≥5 | 25 | 13 |
|
|
<5 | 27 | 18 |
|
| TNM stage |
|
| 0.311 |
|
1A-2B | 22 | 10 |
|
| 3A-4 | 30 | 22 |
|
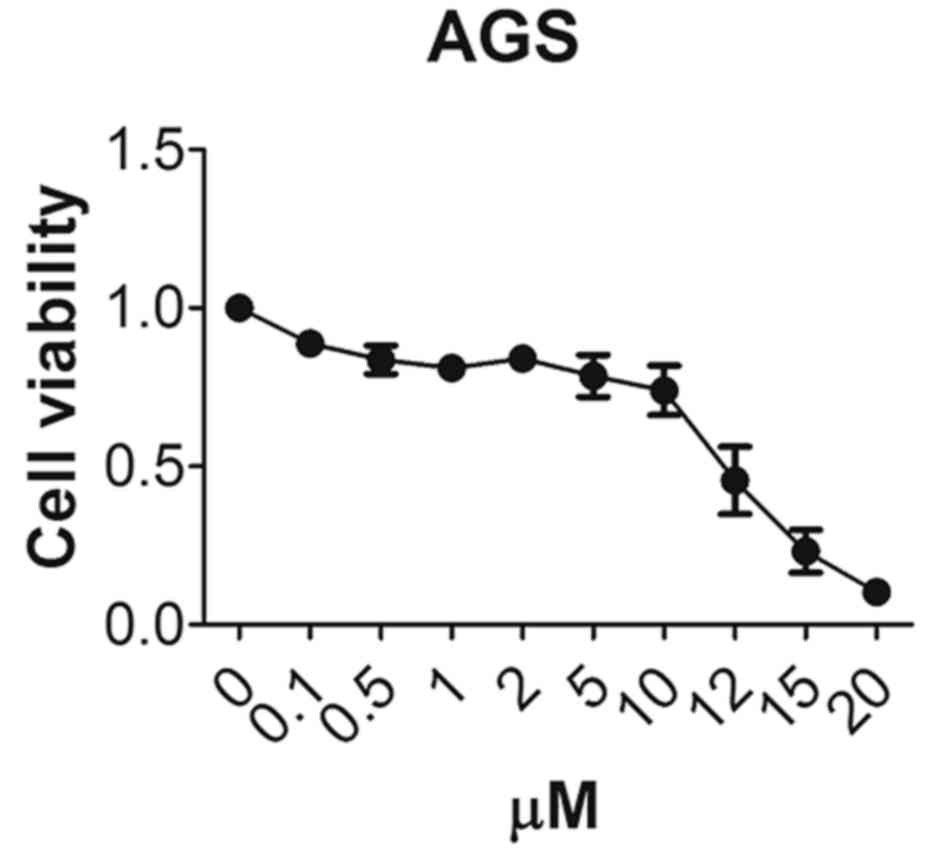
DRD2 inhibitor, thioridazine,
decreases gastric cancer cell survival
To assess whether the DRD2 inhibitor, thioridazine,
has any inhibitory effect on gastric cancer cells, the AGS cells
were treated with different concentrations of thioridazine and the
cell viability was measured 48 h later. Only 45% of the AGS cells
were viable following treatment with 12 µM thioridazine and 10% of
the cells were viable following treatment with 20 µM thioridazine
(Fig. 3). This demonstrated that the
DRD2 inhibitor inhibited the growth of the gastric cancer
cells.
Discussion
The dopamine receptor pathway is of vital importance
in the neurological system. Disorder of the dopamine pathway may
result in severe neurological disease. Previously, the DRD2
inhibitor thioridazine was predominantly used for patients with
psychosis, however, it has been found to have an antagonist effect
in tumors. Thioridazine has shown anti-angiogenic effects and
apoptosis-inducing abilities in breast and ovarian cancer (20,21). It
also induced cell death in cervical and gastric cancer (22,23). These
findings indicate that DRD2 may be involved in cancer progression,
and that its expression may be correlated with cancer
prognosis.
In the present study, 84 pairs of tumor and adjacent
non-tumor tissues were collected. Immunochemical analysis was used
to detect the expression levels of DRD2 in the tissues. DRD2 was
expressed at a higher level in tumors, compared with adjacent
matched non-tumor tissues (Fig. 1).
Patients with higher expression levels of DRD2 had lower survival
durations (Fig. 2). These results
demonstrated that the expression of DRD2 was negatively associated
with survival durations of patients with gastric cancer. The
inhibition of DRD2 by thioridazine in the gastric cancer cell line
markedly inhibited cell growth (Fig.
3). As the expression of DRD2 was correlated with the survival
of patients with gastric cancer, it may function in cancer cell
survival. Developing small molecule drugs to target DRD2 may
provide antitumor effects. Sachlos et al (15) found the anticancer stem cell effects
of thioridazine through drug screening. The inhibition of DRD2 by
thioridazine showed potent anticancer and cancer stem cell
properties. In addition to thioriazine, other DRD2 inhibitors may
have inhibitory effects on cancer cells, even cancer stem cells,
and require further investigation.
Gastric cancer is one of the most common types of
cancer. It is important to identify specific markers, develop
efficient drugs and use drug combinations to cure gastric cancer.
In addition, it is important to prevent the development of gastric
cancer by improving lifestyle factors. Maintaining the freshness of
food in a refrigerator, and decreasing the prevalence of
Helicobacter pylori by improving hygiene and reducing salt
intake may assist in preventing the occurrence of gastric tumors
(2,24). Regular detection of Helicobacter
pylori may assist in further decreasing the occurrence of
gastric cancer. It is hoped that, in the future, the incidence of
gastric cancer can be decreased through improved lifestyle. For
individuals with gastric cancer, the detection of DRD2 may assist
in evaluating prognosis, and the use of DRD2 inhibitors alone or
together with other drugs may have potent inhibitory effects on
tumors.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81172026, 81272402,
81301816 and 81172029), the Foundation of Shanghai Outstanding
Academic Leaders (grant no.11XD1403800), the National High
Technology Research and Development Program (863 Program; grant no.
2012AA022606), the Post-doctoral Research Foundation of China
(grant no. 2012M511107), the Foundation for Interdisciplinary
research of Shanghai Jiao Tong University (grant no. YG2011ZD07),
the Shanghai Science and Technology Commission Inter-governmental
International Cooperation Project (grant no. 12410705900), the
Shanghai Science and Technology Commission Medical-guiding Project
(grant no. 12401905800) and the Program for Changjiang Scholars and
Post-doctoral Research Program of Shanghai (grant no.
12R21415300).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi T, Saikawa Y and Kitagawa Y:
Gastric cancer: Current status of diagnosis and treatment. Cancers
(Basel). 5:48–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berardi R, Scartozzi M, Romagnoli E,
Antognoli S and Cascinu S: Gastric cancer treatment: A systematic
review. Oncol Rep. 11:911–916. 2004.PubMed/NCBI
|
|
6
|
Kienast T and Heinz A: Dopamine and the
diseased brain. CNS Neurol Disord Drug Targets. 5:109–131. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fuxe K, Manger P, Genedani S and Agnati L:
The nigrostriatal DA pathway and Parkinson's disease. J Neural
Transm Suppl. 71–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schneier FR, Liebowitz MR, Abi-Dargham A,
Zea-Ponce Y, Lin SH and Laruelle M: Low dopamine D(2) receptor
binding potential in social phobia. Am J Psychiatry. 157:457–459.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hentges ST and Low MJ: Ovarian dependence
for pituitary tumorigenesis in D2 dopamine receptor-deficient mice.
Endocrinology. 143:4536–4543. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wood DF, Johnston JM and Johnston DG:
Dopamine, the dopamine D2 receptor and pituitary tumours. Clin
Endocrinol (Oxf). 35:455–466. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Zhu S, Kozono D, Ng K, Futalan D,
Shen Y, Akers JC, Steed T, Kushwaha D, Schlabach M, et al:
Genome-wide shRNA screen revealed integrated mitogenic signaling
between dopamine receptor D2 (DRD2) and epidermal growth factor
receptor (EGFR) in glioblastoma. Oncotarget. 5:882–893. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Senogles SE: D2 dopamine receptor-mediated
antiproliferation in a small cell lung cancer cell line, NCI-H69.
Anticancer Drugs. 18:801–807. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clague J, Cinciripini P, Blalock J, Wu X
and Hudmon KS: The D2 dopamine receptor gene and nicotine
dependence among bladder cancer patients and controls. Behav Genet.
40:49–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Basu B, Sarkar C, Chakroborty D, Ganguly
S, Shome S, Dasgupta PS and Basu S: D1 and D2 dopamine
receptor-mediated inhibition of activated normal T cell
proliferation is lost in jurkat T leukemic cells. J Biol Chem.
285:27026–27032. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sachlos E, Risueno RM, Laronde S,
Shapovalova Z, Lee JH, Russell J, Malig M, McNicol JD, Fiebig-Comyn
A, Graham M, et al: Identification of drugs including a dopamine
receptor antagonist that selectively target cancer stem cells.
Cell. 149:1284–1297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li K, Dan Z and Nie YQ: Gastric cancer
stem cells in gastric carcinogenesis, progression, prevention and
treatment. World J Gastroenterol. 20:5420–5426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abdullah LN and Chow EK: Mechanisms of
chemoresistance in cancer stem cells. Clin Transl Med. 2:32013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li F, Tiede B, Massagué J and Kang Y:
Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Res.
17:3–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szász AM, Lánczky A, Nagy Á, Förster S,
Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A and Győrffy B:
Cross-validation of survival associated biomarkers in gastric
cancer using transcriptomic data of 1,065 patients. Oncotarget. Jun
30–2016.(Epub ahead of print).
|
|
20
|
Byun HJ, Lee JH, Kim BR, Kang S, Dong SM,
Park MS, Lee SH, Park SH and Rho SB: Anti-angiogenic effects of
thioridazine involving the FAK-mTOR pathway. Microvasc Res.
84:227–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rho SB, Kim BR and Kang S: A gene
signature-based approach identifies thioridazine as an inhibitor of
phosphatidylinositol-3′-kinase (PI3K)/AKT pathway in ovarian cancer
cells. Gynecol Oncol. 120:121–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mu J, Xu H, Yang Y, Huang W, Xiao J, Li M,
Tan Z, Ding Q, Zhang L, Lu J, et al: Thioridazine, an antipsychotic
drug, elicits potent antitumor effects in gastric cancer. Oncol
Rep. 31:2107–2114. 2014.PubMed/NCBI
|
|
23
|
Kang S, Dong SM, Kim BR, Park MS, Trink B,
Byun HJ and Rho SB: Thioridazine induces apoptosis by targeting the
PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells.
Apoptosis. 17:989–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Levi E, Sochacki P, Khoury N, Patel BB and
Majumdar AP: Cancer stem cells in Helicobacter pylori infection and
aging: Implications for gastric carcinogenesis. World J
Gastrointest Pathophysiol. 5:366–372. 2014.PubMed/NCBI
|