Introduction
In 1901, Emile Zuckerkandl first described the
abdominal para-aortic paraganglia in fetal and newborn humans as a
paired retroperitoneal organ located laterally to the abdominal
aorta at the level of the inferior mesenteric aorta (1). This paraganglionic complex, known as the
organ of Zuckerkandl (OZ), also includes smaller accessory
paraganglia located anteriorly to the aorta between the lateral
organs or below the aortic bifurcation (2). In 1903, Alfred Kohn established that the
OZ commonly originated from chromaffin cells of the adrenal medulla
(3), and it has later been
established that it constitutes the largest accumulation of
extradrenal chromaffin cells in mammals. In humans, the OZ reaches
its maximal size at the age of ~3 years and subsequently regresses
after reaching its peak by autophagy (4). The OZ is considered to be most important
physiologically throughout the early gestational period, during
which it secretes catecholamines into the fetal circulation,
functioning as a homeostatic regulator of blood pressure (5). The OZ represents a site of origin for
paragangliomas (PGLs) that preferentially secrete norepinephrine
and induce symptoms of catecholamine excess (6). OZ-PGLs are rare tumors typically located
close to the origin of the inferior mesenteric artery or between
the proximal common iliac arteries (1). These lesions may occur sporadically or,
in ~70% of cases, in association with succinate dehydrogenase
complex iron sulfur subunit B (SDHB) or, less commonly,
SDHD gene mutations (7). In
addition, OZ-PGLs are particularly aggressive with high rates of
metastatic spread (8). At least 150
cases of OZ-PGLs have been reported in the literature. They are
strongly associated with an aggressive behavior, likely associated
with the SDHB mutation status (7).
Due to the rarity of this disease, not much is known about its
natural history. A single-center retrospective study of 371
patients with either pheochromocytoma or sympathetic paraganglioma
revealed only 21 cases of OZ-PGLs, 14 of which (66%) had metastases
at diagnosis (9). To the best of our
knowledge, the current case demonstrates that somatic HIF2α
[also known as endothelial PAS domain-containing protein 1
(EPAS1)] mutations may be associated with OZ-PGL for the
first time.
Case report
In September 2014, a 32-year-old African woman
native to Burkina Faso was referred to the hypertension unit of La
Timone University Hospital (Marseille, France) for screening for
secondary hypertension. Hypertension was initially noted during the
first trimester of pregnancy. The patient went into premature labor
at 22 weeks and a cesarean delivery was performed 15 days later;
the baby did not survive and succumbed a few minutes after birth.
Following delivery, the patient experienced persistent and
uncontrolled hypertension despite taking nicardipine (60 mg/day)
and labetalol (400 mg/day) for 3 months. A diagnostic hysteroscopy
was performed 3 months later, precipitating a hypertensive crisis
[systolic blood pressure (BP), 300 mmHg; normal, <140 mmHg].
Thereafter, the patient was referred to the hypertension unit of La
Timone University Hospital for secondary hypertension screening in
September 2014. There was no known family history of tumors,
syncope or sudden death. At admission (weight, 51 kg; height, 163
cm; and body mass index, 19.2), the patient presented with
headaches, recurring episodes of palpitations and sweating, chest
tightness, and polyuria. Treatment with nicardipine and labetalol
was replaced with verapamil (240 mg/day). Ambulatory 24-h BP
monitoring was performed during treatment with verapamil and
demonstrated that the patient maintained a BP of 155/96 mmHg.
Prazosin (2.5 mg once per day) was subsequently administered to
reduce blood pressure further until surgical intervention.
Additional laboratory tests identified highly
elevated 24 h urinary normetanephrine levels [20,140 nmol/24 h;
upper reference limit (URL), <1900 nmol/24 h] and normal
metanephrine levels (380 nmol/24 h; URL, <1600 nmol/24 h). In
addition, serum chromogranin A was observed to be elevated (223
µg/l; URL, <100 µg/l). A complete blood count revealed mild
normocytic anemia (hemoglobin count, 108.0 g/l; normal hemoglobin
count for female adults, 11.5–15.0 g/dl; mean corpuscular volume,
83.4 fl; normal mean corpuscular volume of adults, 80–100 fl) of an
inflammatory origin with normal platelets and leukocytes.
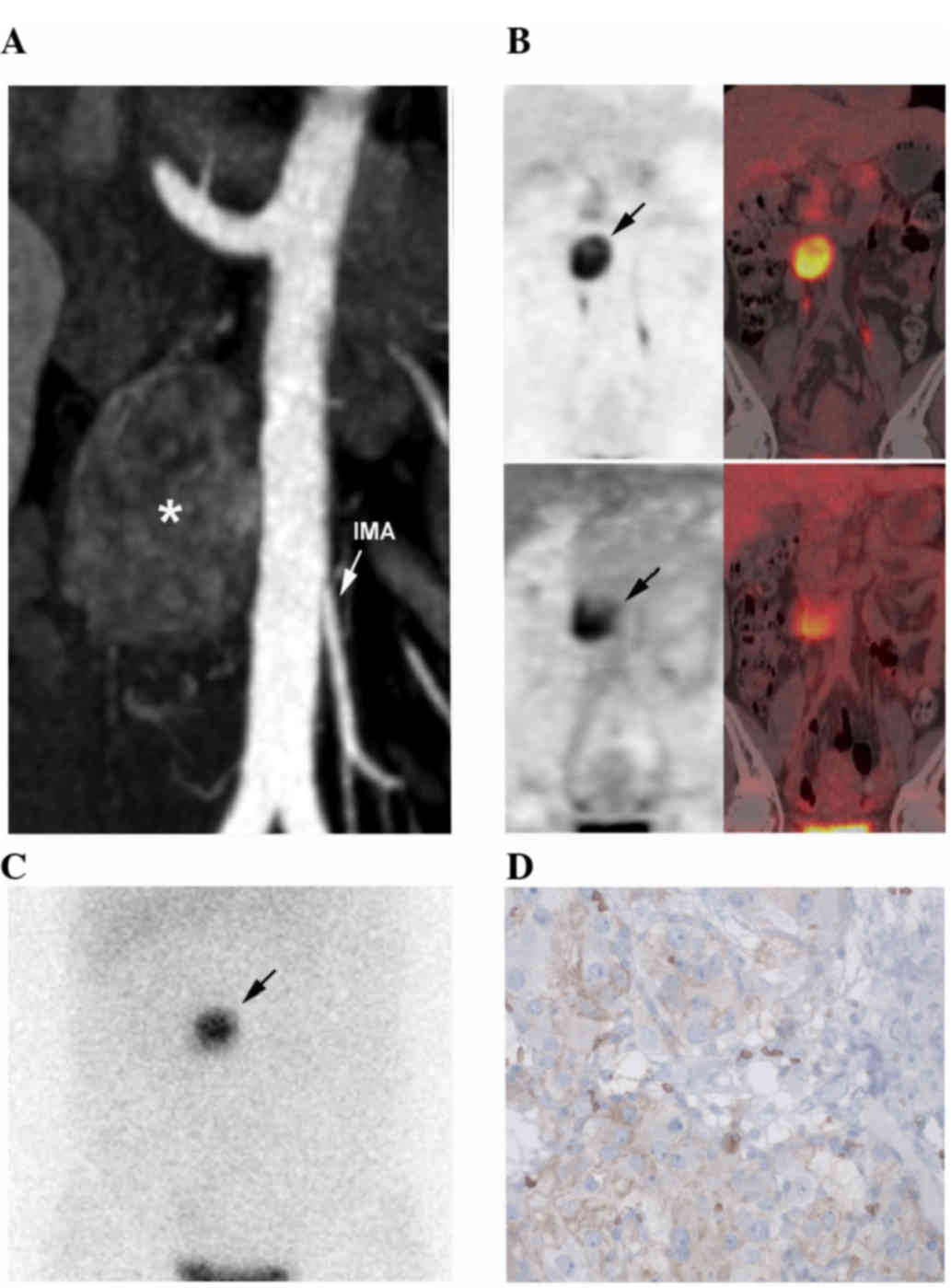
Diagnostic computed tomography (CT) revealed a 40-mm hypervascular,
heterogeneous, left para-aortic mass located at the level of the
inferior mesenteric artery (Fig. 1A).
18Fluorine-L-dihydroxyphenylalanine positron emission
tomography/CT (Fig. 1B) and
iodine-123-metaiodobenzylguanidine scintigraphy (Fig. 1C) confirmed the diagnosis of OZ-PGL
without multifocal disease. The tumor also exhibited moderate
heterogeneous 18F-fluorodeoxyglucose uptake (Fig. 1B). In October 2014, complete surgical
resection was performed. Histopathological analysis of the tumor
tissue revealed typical PGL features, including a low Ki-67 index
(<1%) (monoclonal mouse antibody; clone, MIB-1; catalogue no.,
M7240; dilution, 1:100: Dako, Glostrup, Denmark). Genetic testing
for germline mutations (including large deletions) in the von
Hippel-Lindau tumor suppressor (VHL), succinate
dehydrogenase complex iron sulfur subunit B (SDHB),
SDHC and SDHD genes was normal. Immunostaining
demonstrated that the tumor cells were positive for SDHB. Further
genetic testing revealed a heterozygous cysteine to tyrosine
substitution at base 1589 (c.1589Cys>Tyr) in the HIF2α
coding sequence of the OZ-PGL, resulting in the replacement of
alanine with valine at amino acid position 530 (Ala530Val). This
leads to HIF2α stabilization as described by a previous in
vitro experiment (10). A
germline HIF2α mutation was excluded based on the negative
results of blood DNA testing.
In order to assess the metabolic properties of the
tumor, the present study performed 1H-high-resolution
magic-angle-spinning (HRMAS) nuclear magnetic resonance
spectroscopy-based global metabolomic profiling on tumor samples. A
one-dimensional proton spectrum (1.5–7.2 ppm) using a
Carr-Purcell-Meiboom-Gill pulse sequence with water presaturation
was acquired from each intact tissue sample Low levels of succinate
were detected, and according to our previous study (11) this excludes a SDH deficiency. Notably,
the tumor also exhibited abnormally high levels of α- and β-glucose
isomers as identified by HRMAS (Fig.
2). The patient is currently in remission, and regular clinical
follow up occured every 6 months with normal metanephrines.
Conventional radiological imaging (MRI) and functional imaging
(PET-FDOPA) at 1 year post-intervention were also normal.
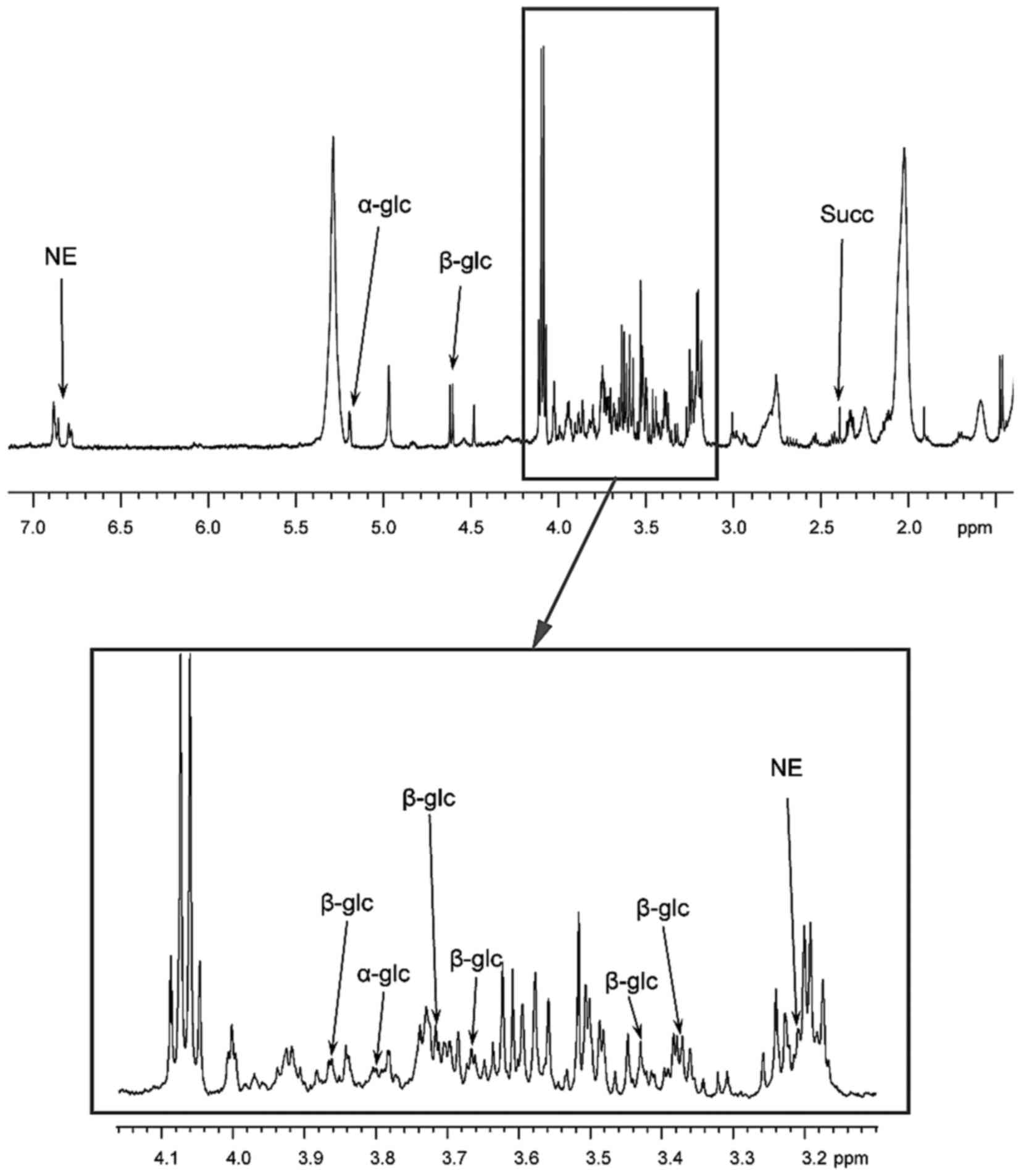 | Figure 2.Results of HRMAS NMR spectroscopy (500
MHz) performed on tumor samples from the organ of Zuckerkandl
paraganglioma. Partial metabolite assignment is indicated. The
metabolic content may be directly compared as the spectrum
intensity was normalized with respect to the weight of each
examined sample. For display purposes, the amplitude of the lactate
peak at 4.09 ppm has been cut out. The top image shows a
representative spectrum with highly elevated levels of α-glc and
β-glc. Spectrum regions ranging from 3.10–4.15 ppm are magnified in
the lower box. Amongst catecholamines, only an NE signal was
detected in all the examined tissue samples. Finally, the level of
Succ was low, which confirmed the absence of a succinate
dehydrogenase complex deficiency. HRMAS,
1H-high-resolution magic-angle-spinning; NMR, nuclear
magnetic resonance; α-glc, α-glucose; β-glc, β-glucose; NE,
norepinephrine; Succ, succinate. |
Written informed consent was obtained from the
patient for publication of the present case report and any
accompanying images.
Discussion
To the best of our knowledge, the present case
demonstrates, for the first time, that patients with somatic
HIF2α mutations may present with OZ-PGL.
Germline mutations in the HIF2α/EPAS1
gene have been previously associated with congenital polycythemia
(12). A syndromic association has
been reported between somatic gain-of-function mutations in
HIF2α and congenital polycythemia, multiple PGL, duodenal
somatostatinoma and ocular vascular abnormalities (for example,
Pacak-Zhuang syndrome) (10,13–16).
Mutations in HIF2α have also been observed in apparently
sporadic pheochromocytomas (PHEOs)/PGLs without polycythemia
(17–19). In one study, mutations (exon 12) were
identified in 2 cases of solitary PHEO and 1 para-adrenal PGL
(18). In an additional study, 6/42
cases of apparently sporadic PHEOs were identified to have
HIF2α mutations (3 in exon 9 and 3 in exon 12) (17). HIF2α protein stability is dependent on
the hydroxylation of two specific proline residues (Pro405 and
Pro531) located in the O2-dependent degradation domain
(10). Until present, all mutations
described were known to be located in hot spots adjacent to
hydroxylation sites (16). These
specific mutations disturb HIF2α prolyl hydroxylation and
subsequent recognition by the VHL protein, resulting in the failure
of HIF2α degradation via ubiquitination (16). As mutant HIF2α protein has a longer
half-life compared with the wild-type protein, it has a targeted
effect downstream of HIF2α (10).
The mutation identified in the present study had
previously been reported in a case of apparently sporadic PHEO/PGL
(18). The mutation involved Ala530,
which is located in close proximity to the second hydroxylation
site (Pro531) and at the interface with VHL and Egl-9 family
hypoxia-inducible factor 1 (EGLN1) client-proteins. Homology
modeling was performed to outline the biological properties of the
Val530 mutant (Fig. 3). These
three-dimensional models were generated with IBM SPSS Modeler v14
(IBM SPSS, Armonk, NY, USA) using the crystal structures of HIF1α
in interaction with EGLN1 or VHL as templates. HIF1α and HIF2α
exhibit a sequence identity of 65% in the region modeled, which
guarantees (>50% identity) that the models are of a high
quality. The model anticipates that valine, a larger residue than
alanine, increases steric hindrance at Pro531, leading to: i) A
reduction in its accessibility to EGLN1 by inhibition of Pro531
hydroxylation; and ii) impairment of HIF2α/VHL interaction with
decreased HIF2α ubiquitination. The present study also identified a
novel metabolomic pattern with low succinate and high glucose
levels associated with HIF2α mutation. Abnormally high
levels of glucose may be explained by increased glucose uptake
induced by HIF2α stabilization (20).
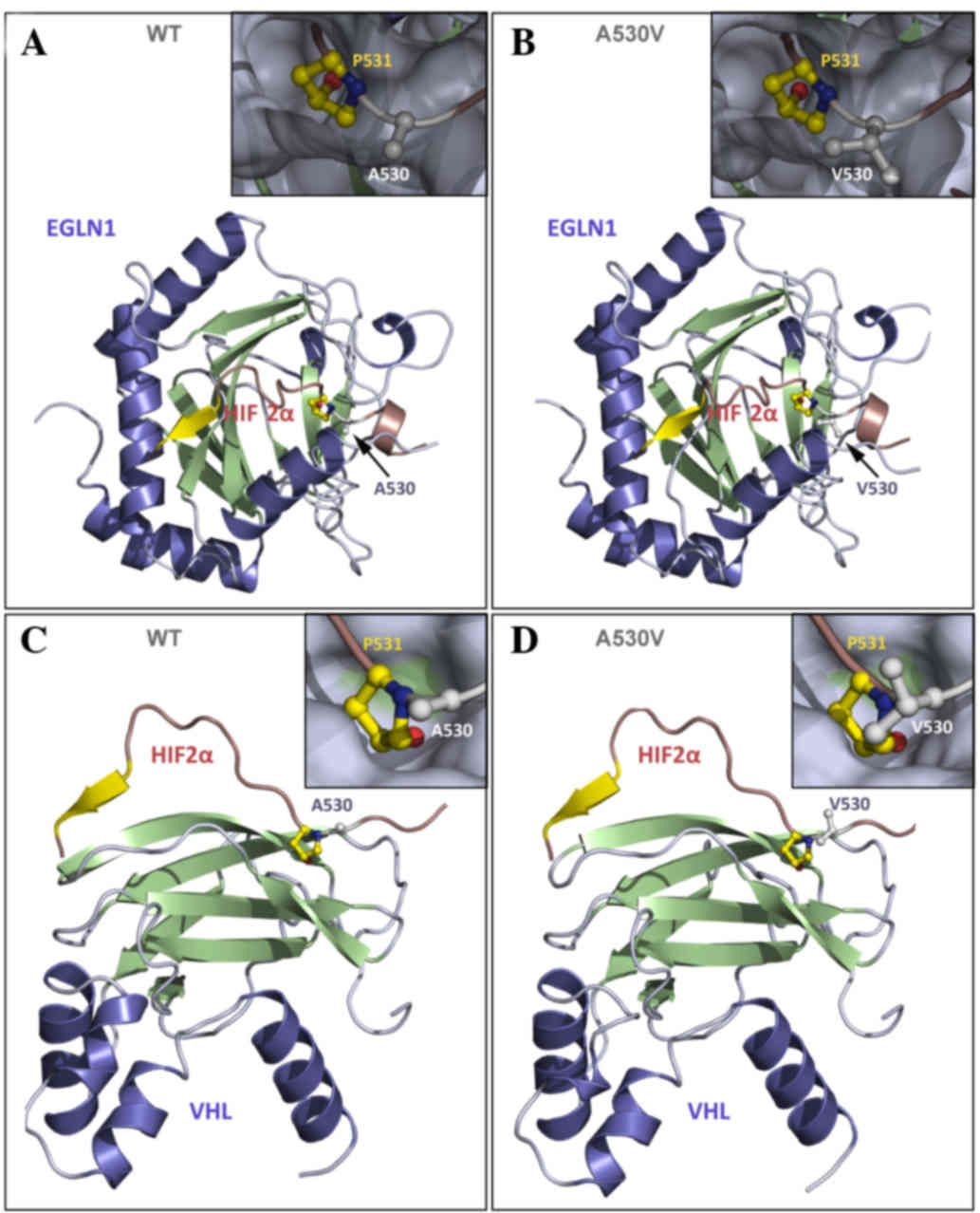 | Figure 3.Representation of human HIF2α in the
presence of its binding partners EGLN1 and VHL. (A) WT HIF2α (A560)
interacting with EGLN1; (B) mutant Val560 interacting with EGLN1;
(C) WT HIF2α interacting with VHL; and (D) mutant Val560
interacting with VHL. HIF2α (16 residues) is represented in red and
yellow, and the interactive partners (EGLN1 or VHL) are represented
in blue and green. (B and D) The inserts present a closer view of
Pro531 and Ala530 (or Val530) from HIF2α in the ball-and-stick
representation to show the atomic details, while HIF-2α partners,
(A and B) EGLN1 or (C and D) VHL are shown as a grey surface
showing that residues 530 and 531 bind to small pockets at the
surface of the protein partner. Residue Ala530 is located in close
proximity to residue Pro531, which is hydroxylated by EGLN1 and at
the interface with the binding partners EGLN1 and VHL.
Hydroxylation of Pro531 is required for interaction with VHL. It is
anticipated that valine, which is a larger residue than alanine,
increases steric hindrance at Pro531, resulting in a reduction in
its accessibility to EGLN1 by inhibition of Pro531 hydroxylation;
therefore, interaction with VHL and subsequent ubiquitination is
prevented. Panel D is presented as a model, but VHL interaction
should not occur in the Val530 mutant. HIF2α, hypoxia inducible
factor 2α; EGLN1, Egl-9 family hypoxia-inducible factor 1; VHL, von
Hippel-Lindau tumor suppressor; WT, wild-type. |
In conclusion, to the best of our knowledge, the
current study identified, for the first time, an association
between somatic HIF2α mutations and OZ-PGL. It is therefore
recommended that patients with OZ-PGL in the absence of germline
SDHx mutations should undergo testing for HIF2α
mutations.
References
|
1
|
Zuckerkandl E: About sympathetic
paraganglions in the retroperitoneal space of man. Verh Anat Ges.
15:95–107. 1901.(In German).
|
|
2
|
Unsicker K, Huber K, Schütz G and Kalcheim
C: The chromaffin cell and its development. Neurochem Res.
30:921–925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kohn A: The paraganglia. Arch Mikrosk
Anat. 52:262–265. 1903.(In German).
|
|
4
|
Schober A, Parlato R, Huber K, Kinscherf
R, Hartleben B, Huber TB, Schütz G and Unsicker K: Cell loss and
autophagy in the extra-adrenal chromaffin organ of Zuckerkandl are
regulated by glucocorticoid signalling. J Neuroendocrinol.
25:34–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
West GB, Shepherd DM, Hunter RB and
McGregor AR: The function of the organs of Zuckerkandl. Clin Sci.
12:317–325. 1953.PubMed/NCBI
|
|
6
|
Martucci VL and Pacak K: Pheochromocytoma
and paraganglioma: Diagnosis, genetics, management, and treatment.
Curr Probl Cancer. 38:7–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lodish MB, Adams KT, Huynh TT, Prodanov T,
Ling A, Chen C, Shusterman S, Jimenez C, Merino M, Hughes M, et al:
Succinate dehydrogenase gene mutations are strongly associated with
paraganglioma of the organ of Zuckerkandl. Endocr Relat Cancer.
17:581–588. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Subramanian A and Maker VK: Organs of
Zuckerkandl: Their surgical significance and a review of a century
of literature. Am J Surg. 192:224–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ayala-Ramirez M, Feng L, Johnson MM, Ejaz
S, Habra MA, Rich T, Busaidy N, Cote GJ, Perrier N, Phan A, et al:
Clinical risk factors for malignancy and overall survival in
patients with pheochromocytomas and sympathetic paragangliomas:
Primary tumor size and primary tumor location as prognostic
indicators. J Clin Endocrinol Metab. 96:717–725. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhuang Z, Yang C, Lorenzo F, Merino M,
Fojo T, Kebebew E, Popovic V, Stratakis CA, Prchal JT and Pacak K:
Somatic HIF2A gain-of-function mutations in paraganglioma with
polycythemia. N Engl J Med. 367:922–930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imperiale A, Moussallieh FM, Sebag F,
Brunaud L, Barlier A, Elbayed K, Bachellier P, Goichot B, Pacak K,
Namer IJ and Taïeb D: A new specific succinate-glutamate
metabolomic hallmark in SDHx-related paragangliomas. PLoS One.
8:e805392013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Percy MJ, Furlow PW, Lucas GS, Li X,
Lappin TR, McMullin MF and Lee FS: A gain-of-function mutation in
the HIF2A gene in familial erythrocytosis. N Engl J Med.
358:162–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taïeb D, Yang C, Delenne B, Zhuang Z,
Barlier A, Sebag F and Pacak K: First report of bilateral
pheochromocytoma in the clinical spectrum of HIF2A-related
polycythemia-paraganglioma syndrome. J Clin Endocrinol Metab.
98:E908–E913. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang C, Sun MG, Matro J, Huynh TT,
Rahimpour S, Prchal JT, Lechan R, Lonser R, Pacak K and Zhuang Z:
Novel HIF2A mutations disrupt oxygen sensing, leading to
polycythemia, paragangliomas, and somatostatinomas. Blood.
121:2563–2566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pacak K, Chew EY, Pappo AS, Yang C,
Lorenzo FR, Wilson MW, Aronow MB, Young JA, Popovic V and Zhuang Z:
Ocular manifestations of hypoxia-inducible factor-2α
paraganglioma-somatostatinoma-polycythemia syndrome. Ophthalmology.
121:2291–2293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pacak K, Jochmanova I, Prodanov T, Yang C,
Merino MJ, Fojo T, Prchal JT, Tischler AS, Lechan RM and Zhuang Z:
New syndrome of paraganglioma and somatostatinoma associated with
polycythemia. J Clin Oncol. 31:1690–1698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Welander J, Andreasson A, Brauckhoff M,
Bäckdahl M, Larsson C, Gimm O and Söderkvist P: Frequent
EPAS1/HIF2α exons 9 and 12 mutations in non-familial
pheochromocytoma. Endocr Relat Cancer. 21:495–504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Comino-Méndez I, de Cubas AA, Bernal C,
Álvarez-Escolá C, Sánchez-Malo C, Ramírez-Tortosa CL, Pedrinaci S,
Rapizzi E, Ercolino T, Bernini G, et al: Tumoral EPAS1 (HIF2A)
mutations explain sporadic pheochromocytoma and paraganglioma in
the absence of erythrocytosis. Hum Mol Genet. 22:2169–2176. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Favier J, Buffet A and Gimenez-Roqueplo
AP: HIF2A mutations in paraganglioma with polycythemia. N Engl J
Med. 367:2161–2162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Keith B, Johnson RS and Simon MC: HIF1α
and HIF2α: Sibling rivalry in hypoxic tumour growth and
progression. Nat Rev Cancer. 12:9–22. 2011.PubMed/NCBI
|