Introduction
The phenotypic diversity of immune cells in the
tumor microenvironment may exhibit a positive or negative influence
on tumor development and clinical outcome. The tumor
microenvironment is composed of stromal, endothelial and innate
cells, as well as lymphocytes, which interact to form a complex
system that surrounds tumor cells (1,2). The
recruitment of immune effector cells to the tumor site and the
intensity of specific responses are mediated by cytokines, which
are secreted by immune system and tumor cells (3). Additionally, these cytokines are
essential for the differentiation of all immune cell types in the
tumor milieu (4). For an appropriate
immune response against pathogens, adequate activation of naive T
lymphocytes is required. The stimuli received by the immune system
leads to the activation of a lymphocyte response, which is
different for each antigen (5). In
response to the stimulus provided by an antigen-presenting cell, a
precursor T helper (Th) 0 lymphocyte may differentiate into a T
helper type 1 cell (Th1), Th2, Th17 or a Foxp3+
regulatory T (Treg) cell, depending on the cytokines present in the
microenvironment (5,6). The Th1 profile, which promotes a
pro-inflammatory immune response, is induced by interleukin (IL)-12
and tumor necrosis factor α (TNF-α) (7), whereas the Th2 profile, which promotes a
humoral immune response, is initiated by IL-4 (8). Both Th1 and Th2 are involved in
intensifying anti-tumor immunity by inducing the expansion of
cytotoxic CD8+ T cell populations (6); however, Treg cells that are
predominantly induced by IL-2, promote antitumor suppressor
activity via the inhibition of CD8+ T cells (5,9). The Th17
phenotype presents a distinct strain of CD4+ T cells that secrete
important ILs, including IL-17A, IL-17F, IL-21 and IL-22.
Differentiation of Th17 cells occurs in the presence of IL-6, IL-21
and tumor growth factor-β (TGF-β), while their stability is
maintained by IL-1β and IL-23 (6,10).
Although the Th17 profile is involved with increased immunity and
host defenses, its involvement in neoplastic processes remains
controversial (6,10). Previous studies suggest that Th17
cells are involved in cancer promotion (11–15).
Notably, in the tumor microenvironment, Th17 cells have been
observed to positively correlate with immune effector cells,
including CD8+ T cells and natural killer cells, promoting an
antitumor response that is mediated by cytotoxic cells (11,12). As a
result of the production of pro-inflammatory cytokines by Th17
cells that accumulate in the tumor microenvironment, and the
association between the development and progression of cancer, a
positive correlation between these cells and disease progression
has been hypothesized (13,14). The presence of inflammatory cells and
soluble factors in the tumor microenvironment has been
demonstrated; however, predicting patient prognosis based on the
presence of immune system cells is difficult as various organs
react differently to certain cytokines (15). A number of studies have identified the
presence of numerous tumor-associated lymphocytes in Hashimoto's
thyroiditis, as well as in the absence of the typical symptoms of
autoimmune thyroiditis (16–19). The effect of the Th17 profile, which
includes immune cells, cytokines, chemokines and their receptors
may be protective or act as a trigger for thyroid tumors, depending
on the histological classification of the tumor (16). Notably, the presence of lymphocytic
infiltrates in patients with PTC is higher than that in patients
with benign lesions; however, PTC associated with thyroiditis
exhibits a better prognosis (17,18).
Thyroid carcinomas with a poor prognosis, such as
poorly-differentiated and anaplastic thyroid carcinomas, are
characterized by a marked reduction in lymphocyte cell infiltrates
compared with PTCs, which indicates that these cells may exhibit a
protective function in thyroid cancer (19). Thyroid neoplasms are the most common
type of endocrine tumor, worldwide, accounting for 1% of malignant
neoplasms (20). Notably, the
incidence rate of thyroid neoplasms has gradually increased in
recent decades in a number of countries (21). Our previous study investigated the
association between the immune system and thyroid tumors (22,23) to
determine whether certain molecules may be associated with
different tumor subtypes and patient prognoses. Thus, considering
the significant effect of IL-17 and the importance of IL-23 in
maintaining the cellular Th17 response, evaluating the expression
profile of these cytokines in lymphocytes and their distribution in
neoplastic and non-neoplastic thyroid tissue is essential to
understanding the complex association between the tumor
microenvironment and the Th17 profile.
Materials and methods
Specimens
A total of 131 thyroid biopsy specimens collected
between January 1999 and December 2012 were obtained from the
archives of the Pathology Department, Faculty of Medicine of
Ribeirão Preto (FMRP), University of São Paulo (São Paulo, Brazil).
The specimens included 61 cases of papillary thyroid carcinoma
(PTC), 19 cases of follicular thyroid carcinoma (FTC), 8 cases of
medullary thyroid carcinoma (MTC), 22 cases of follicular thyroid
adenoma (FTA) and 21 goiter biopsies, which represented
non-neoplastic lesions. In addition, 9 normal thyroid tissue
specimens, which were obtained from the Endocrinology Department of
the University of São Paulo, were included as the control. The
tissues corresponded to patients who had undergone surgery for
thyroid cancer, which implies total or partial removal of the
thyroid gland when the result of cytology is positive or
suggestive. However, paraffin-embedded tissue from the hospital
file or tumor bank were used in the present study. Tissues
exhibiting no autolysis, artifacts or signs of inadequate
processing during fixation were selected for the study. Biopsies
obtained from patients who were human immunodeficiency virus
positive, immunosuppressed or had previously undergone radiotherapy
for the treatment of cancer were excluded. The carcinomas were
staged based on the size of the tumor, lymph node metastases and
distant metastases, according to the criteria defined by the
American Joint Committee on Cancer (2002) (24) and reviewed by the American Thyroid
Association (25). Patient
clinicopathological information, including age, gender, recurrence,
metastases and mortality was obtained from the service records of
the FMRP University Hospital (Table
I). The study protocol was approved by the Brazilian
Institutional Ethics Committee on Human Experimentation (1286/2011)
(School of Medicine of Ribeirão Preto, University of São
Paulo).
 | Table I.Association between IL-17 and IL-23
expression and clinicopathological parameters in malignant
differentiated and medullary thyroid carcinoma patients. |
Table I.
Association between IL-17 and IL-23
expression and clinicopathological parameters in malignant
differentiated and medullary thyroid carcinoma patients.
|
| IL-17
expression |
| IL-23
expression |
|
|---|
|
|
|
|
|
|
|---|
| Parameter | Low, n | High, n | P-value | Low, n | High, n | P-value |
|---|
| Age, years |
|
| 0.8345 |
|
| 1.0000 |
| ≤
45 | 20 | 23 |
| 9 | 34 |
|
| >
45 | 22 | 23 |
| 9 | 36 |
|
| Gender |
|
| 0.6275 |
|
| 0.2197 |
|
Female | 33 | 34 |
| 16 | 51 |
|
|
Male | 9 | 12 |
| 2 | 19 |
|
| Tumor size, cm |
|
| 0.2832 |
|
| 0.1560 |
| ≤2 | 12 | 20 |
| 6 | 26 |
|
|
>2–4 | 22 | 17 |
| 11 | 28 |
|
| ≥4 | 8 | 9 |
| 1 | 16 |
|
| Metastasis |
|
| 0.4914 |
|
| 0.9971 |
|
Ganglionic | 10 | 10 |
| 4 | 16 |
|
|
Distant | 3 | 7 |
| 2 | 8 |
|
|
None | 29 | 29 |
| 12 | 46 |
|
| Histological
grade |
|
| 0.9443 |
|
| 0.9945 |
|
I–II | 25 | 29 |
| 11 | 43 |
|
|
III | 10 | 10 |
| 4 | 16 |
|
| IV | 7 | 7 |
| 3 | 11 |
|
|
Recurrence/Mortality |
|
| 0.0376 |
|
| 1.0000 |
| No | 38 | 31 |
| 14 | 55 |
|
|
Yes | 5 | 14 |
| 4 | 15 |
|
Immunohistochemistry
Tissue sections (5-µm) were cut, placed on slides
pretreated with organosilane and subjected to immunohistochemical
assay using the avidin-biotin-peroxidase method with a universal
Novostain Super ABC kit (Novocastra, Newcastle Upon Tyne, UK) to
analyze IL-17 and −23 expression. Polyclonal rabbit anti-mouse
IL-17 (1:200; cat. no. ab79056; Abcam, Cambridge, UK) and IL-23
(1:70; cat. no. ab45420; Abcam) were used as the primary
antibodies. The sections were deparaffinized in xylene, rehydrated
in alcohol with decreasing concentration and washed in water. For
antigen retrieval, the sections were immersed in 10 mM sodium
citrate buffer (pH 6.0) at 95°C for 35 min. Endogenous peroxidase
activity was blocked following incubation with 3% hydrogen peroxide
in phosphate-buffered saline (PBS) for 20 min, and nonspecific
binding was blocked following incubation with 1:50 horse serum
[included in the Polymer Detection system Novolink kit
(Novocastra)] in PBS for 30 min. The slides were washed with PBS
and incubated with IL-17 and IL-23 primary antibodies overnight in
a humidified chamber at 4°C overnight. The final step was
incubation for 15 min with probe (similar to a secondary antibody),
followed by signal amplification of the reaction with polymer
incubation for 30 min, according to the protocol provided by the
manufacturer of the Polymer Detection system Novolink kit. The
samples were then incubated with 3,3′-diaminobenzidine (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) diluted in 0.01%
H2O2 for 40 sec and lightly counterstained
with Harris' hematoxylin monohydrate (Merck Millipore, Darmstadt,
Germany) for 60 sec. The sections were then rehydrated in absolute
alcohol and xylene, and the slides were mounted using Permount
mounting medium (Merck Millipore) and visualized by microscopy with
Image Pro Plus software (Media Cybernetics, Inc., Rockville, MD,
USA).
Immunohistochemical evaluation
The semi-quantitative evaluation of IL-17 and IL-23
expression was based on the proportion of positive cells within the
tumors. To determine the proportion of positive cells in the tissue
specimens, a score was calculated based on the mean basal
expression observed in normal thyroid tissues (controls). The mean
basal expression of control tissues was performed by evaluating
with an optical microscope the staining of 10 different fields in
the same slide. Qualitative analysis was determined by the
intensity of staining. Samples without expression or with low
intensity of staining were considered low-expression specimens,
while samples with moderate or severe staining intensity were
considered as high-expression specimens. The mean basal expression
rates were 20 and 24% for IL-17 and IL-23, respectively. These
values were used as the cutoff points to define subgroups
exhibiting low and high expression of IL-17 and IL-23 in the tumor
tissues. The amount and distribution of positive lymphocytes
between tumor cells as well as around adjacent tumor were evaluated
from the mean intensity of immunostaining. Histological sections of
laryngeal tissues with epithelial invasive carcinoma served as the
positive controls.
Statistical analysis
For statistical analyses, the carcinomas were
grouped according to their histological characteristics.
Comparisons were performed between the following groups:
Differentiated thyroid carcinoma [(DTC) including PTCs and FTCs)
(malignant neoplasm), MTC (malignant neoplasm), FTA (benign
neoplasm) and goiter (non-neoplastic lesion) tissues. Fisher's
exact test was used to compare two groups of data and the
χ2 test was used to analyze differences between more
than three groups. Kruskal-Wallis test with Dunn's post-hoc test
was used to compare the mean expression of IL-17 and IL-23 in the
lymphocytes among thyroid lesions. Spearmen's rank correlation
analysis was used to assess the correlation between these
parameters. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using Graph Pad Prism 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA).
Results
IL-17 expression
The expression of IL-17 was compared between
different thyroid lesions. IL-17 positive cells were identified in
both tumor tissues and in adjacent non-tumoral lymphocytic tissues.
The expression of IL-17 expression was quantified according to the
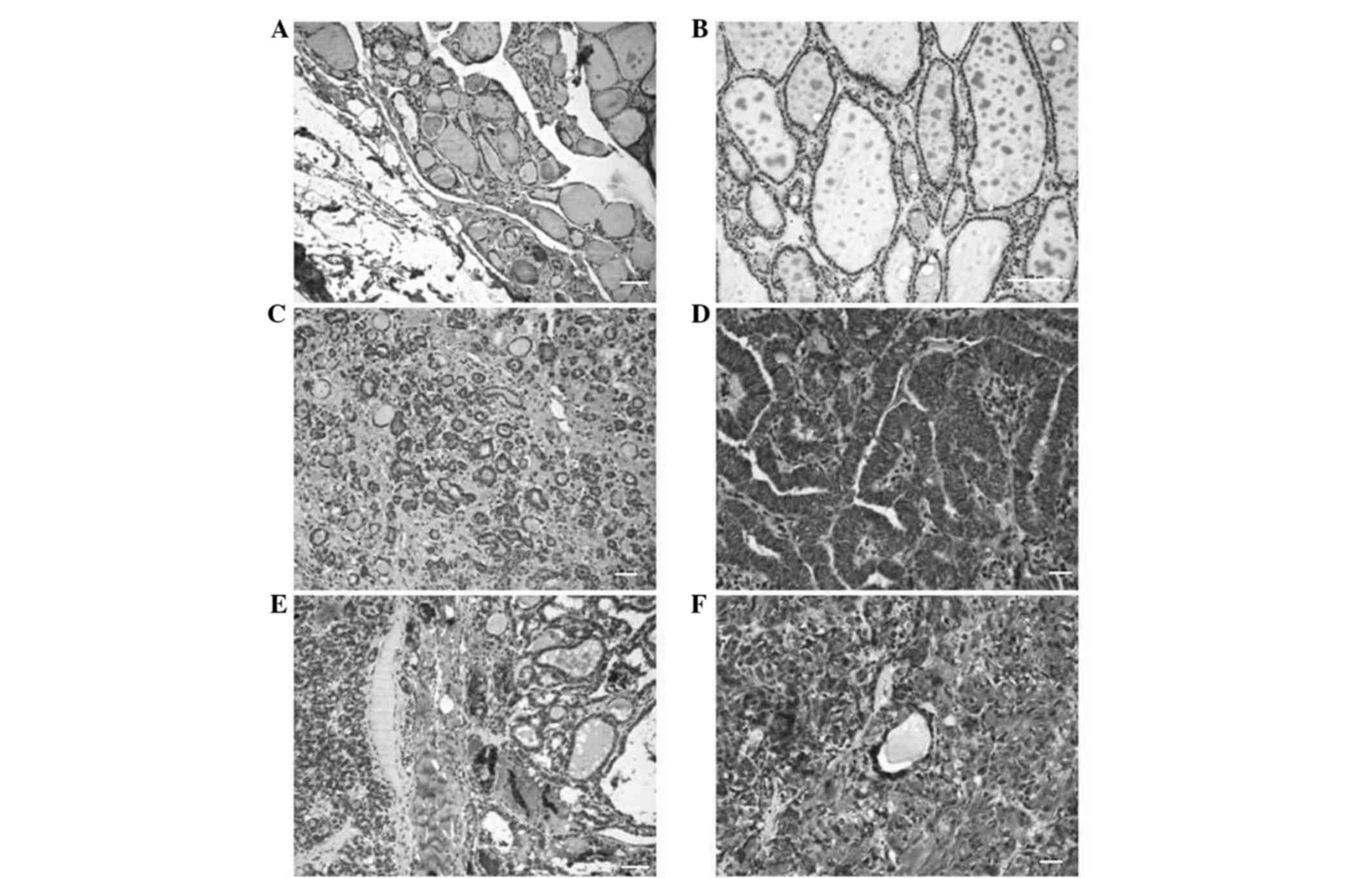
mean expression exhibited in normal thyroid tissue (Fig. 1). The mean [±standard deviation (SD)]
IL-17 expression rate of normal thyroid tissue was 20±9.3%. Thus,
tissues exhibiting IL-17 expression rates of <20 and ≥20% were
defined as low and high expression groups, respectively.
Semi-quantitative analyses revealed that neoplastic lesions
exhibited significantly increased IL-17 expression rates compared
with non-neoplastic lesions (Table
II). The results revealed a higher percentage of cells
expressed positivity for IL-17 in the DTC (100%), MTC (100%) and
FTA (95.4%) groups compared with the goiter group (52.4%). The mean
(±SD) IL-17 expression rates were 82.1±9.9, 75±20.7, 69.1±19 and
33.8±18.6% in DTC, MTC, FTA and goiter tissues, respectively. The
following mean (±SD) IL-17 expression rates of the lymphocytic
infiltrates of DTC, MTC and FTA tissues were 35.6±13.1, 43.8±15.1
and 27.7±10.7%, respectively. In the goiter group, the mean (±SD)
IL-17 expression rate of the lymphocytic infiltrate cells was
20.9±17%, which was significantly lower than that of the DTC
(P<0.0001), FTA (P=0.0015) and MTC (P=0.0265) groups. However,
no significant differences in the IL-17 expression rate of the
lymphocytic infiltrates were identified between the neoplastic
tissues. Kruskal-Wallis test with Dunn's post-hoc test revealed
significant differences in IL-17 expression of the labeled
lymphocytes between the malignant neoplasm groups and the goiter
group (DTC vs. goiter, P<0.001; MTC vs. goiter, P<0.01). A
significant correlation between the mean IL-17 expression rate was
identified between the tumor cells and adjacent lymphocytes in MTC
tissues (P=0.0072).
 | Table II.IL-17 expression in neoplastic and
non-neoplastic thyroid lesions (n=131). |
Table II.
IL-17 expression in neoplastic and
non-neoplastic thyroid lesions (n=131).
|
| Low IL-17
expression | High IL-17
expression |
|
|---|
|
|
|
|
|
|---|
| Thyroid tissue | n (%) | n (%) | P-value |
|---|
| DTC | 0
(0) | 80 (100) | 0.2157a/<0.0001b |
| MTC | 0
(0) | 8
(100) | 1.0000c/0.02650d |
| FTA | 1
(4.6) | 21 (95.4) | 0.0015e |
| Goiter | 10 (47.6) | 11 (52.4) | 0.0015e |
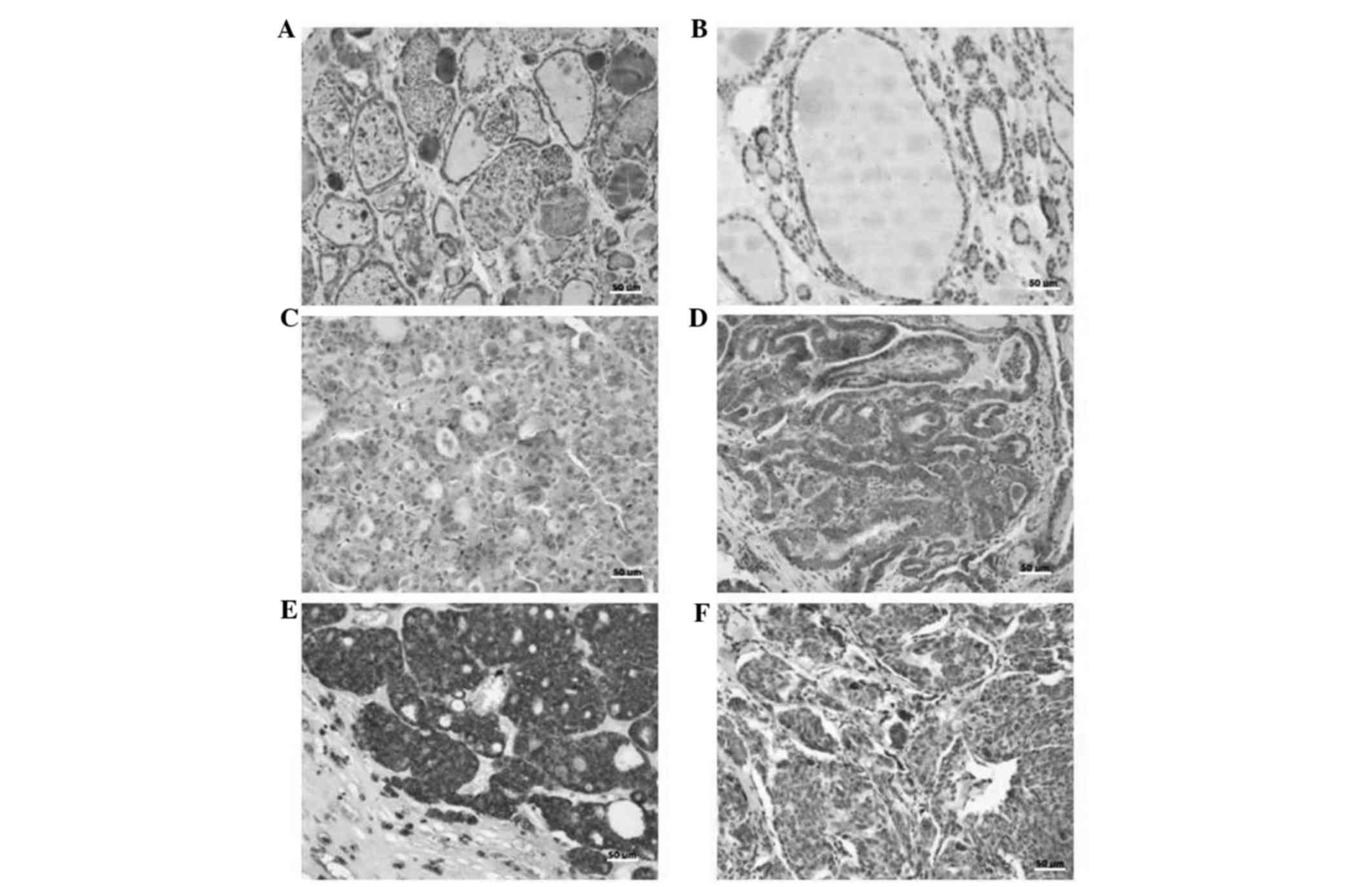
IL-23 expression
The expression of IL-23 was quantified according to
the mean expression exhibited in normal thyroid tissue (Fig. 2). The mean [±standard deviation (SD)]
IL-23 expression rate of normal thyroid tissue was 24±22.4%. Thus,
tissues exhibiting IL-23 expression rates of <24 and ≥24% were
defined as low and high expression groups, respectively. Analysis
reveled that IL-23 expression rates were significantly higher in
the neoplastic groups when compared with the goiter group. As shown
in Table III, a significant
difference in IL-23 expression was identified between the goiter
(benign lesion) group and the DTC (malignant neoplasm) group
(P<0.0001), as well as the FTA (benign neoplasm) group
(P=0.0212). These results also revealed that a higher percentage of
DTC (100%), MTC (100%) and FTA (95.4%) cases exhibited high IL-23
expression compared with the goiter group (66.7%). The mean (±SD)
IL-23 expression rates of the labeled cells were 89.2±11.8, 89±9.9,
76±19.6 and 44±26.4% in the DTC, MTC, FTA and goiter groups,
respectively. The mean staining of the tumor microenvironment
lymphocytes was more homogeneous in the tumors studied (DTC, 32±15
and MTC, 31±14). However, the mean of the labeled lymphocytes was
lowest (19±14) in the goiter group. Kruskal-Wallis test with Dunn's
post hoc test revealed significant differences in IL-23 expression
of the labeled lymphocytes between the malignant neoplasm group
(DTC) and the goiter group (DTC vs. goiter, P<0.001). A
significant positive correlation was identified between the mean
IL-23 expression rate in the thyroid lesion cells and their
microenvironment lymphocytes in the DTC (P=0.0252), FTA (P=0.0015)
and goiter (P=0.0002) groups.
 | Table III.IL-23 expression in neoplastic and
non-neoplastic thyroid lesions (n=131). |
Table III.
IL-23 expression in neoplastic and
non-neoplastic thyroid lesions (n=131).
|
| Low IL-23
expression | High IL-23
expression |
|
|---|
|
|
|
|
|
|---|
| Thyroid tissue | n (%) | n (%) | P-value |
|---|
| DTC | 0 (0) | 80 (100) | 0.2157a/<0.0001b |
| MTC | 0 (0) | 8
(100) | 1.0000c/0.1421d |
| FTA | 1 (4.6) | 21 (95.4) | 0.0212e |
| Goiter | 7 (33.3) | 14 (66.7) | 0.0212e |
IL-17 expression is associated with
poor outcomes in DTC and MTC
The associations between IL-17 and IL-23 expression
and clinicopathological parameters were evaluated (Table I). The results revealed that IL-17
expression was associated with recurrence/mortality (P=0.0376).
Notably, of the 69 patients that exhibited no recurrence or
mortality, 45% (31/69) exhibited high IL-17 expression, whereas in
the patient group that exhibited recurrence or mortality, 74% of
patients (14/19) exhibited high IL-17 expression. No significant
associations were identified between IL-23 expression and any of
the clinicopathological parameters evaluated.
IL-17 and IL-23 expression is
positively correlated in DTC tissues
A positive correlation between IL-17 and IL-23
expression was identified in the DTC group (P=0.0112). However, no
significant correlation was identified between IL-17 and IL-23
expression in the MTC, FTA or goiter groups.
Discussion
Increasing evidence suggests that tumorigenesis
results from chronic inflammation (26), which involves cytokine and chemokine
networks that have been extensively studied to provide novel
therapies against cancer (27). The
association between DTC and thyroiditis, which affects the thyroid
gland, is frequently cited as evidence of this theory (28). In this type of association, a mixture
of macrophages and lymphocytes is observed in the tumor
microenvironment, thus supporting the hypothesis that the immune
response can influence thyroid cancer progression (29). IL-17 is a cytokine produced by a
subset of lymphocytes known as Th17 cells. IL-17 is involved in the
initial activation of the immune system and exhibits an important
function in the interaction between the innate and adaptive immune
response. Since IL-17 is involved in the pathogenesis of chronic
inflammatory responses and is known to induce the production of
high concentrations of IL-1β, TNF-α, TGF-β, CC chemokine ligand
(CCL)2 and matrix metalloproteinase mediators that are widely found
in the tumor microenvironment (30),
this cytokine presents a potential target that requires
investigation.
In the present study, immunohistochemical analysis
revealed that all cells were positive for IL-17 and IL-23 in all
thyroid tumor types evaluated, as well as in the lymphocytes
surrounding the lesions. These findings indicate a significant
association between the Th17 profile and thyroid neoplasms. Due to
its strong presence in the tumor microenvironment, it is
hypothesized that IL-17 functions in the early stages of
tumorigenesis. An increase in IL-17 expression triggers the release
of IL-6 inflammatory cytokines and consequently the activation of
signal transducer and activator of transcription 3 (STAT3) and
necrosis factor kappa β, which are associated with the production
of pro-inflammatory cytokines. The tumor cells are capable of
perpetuating the local inflammatory response via the expression of
CCL2, which stimulates the monocyte secretory IL-17 input,
contributing to inflammation and tumor growth (31,32).
Additionally, STAT3 is regulated by IL-23, which promotes a
protumor activity cascade and the presence of IL-23 stabilizes Th17
cells (33). In the present study,
the expression of IL-17 protein was decreased in the biopsies of
benign lesions (FTA and goiter tissues) when compared to malignant
lesions (DTC and MTC). We hypothesize that the increased cytokine
expression in the tumor environment may be associated with tumor
progression, which is in agreement with the findings of Su et
al (34) who demonstrated that a
high number of CD4+ Th17 cells were present in melanoma, ovarian,
breast and colon carcinomas (34). In
the present study, the lymphocytes present in the tumor
microenvironment of the MTC group tumors expressed the highest
level of IL-17 expression compared with the DTC, FTA and goiter
groups. In addition, a positive correlation was identified between
IL-17 expression in tumor cells and adjacent lymphocytes in the MTC
group. Notably, MTC patient exhibited the worst disease outcome;
therefore, we postulate that the presence of IL-17-producing
lymphocytes may influence disease progression. Similar to the
results of IL-17, analysis of IL-23 expression revealed that a
significant number of neoplastic cells exhibited high IL-23 protein
expression, which was not observed in goiter tissues. IL-23 is a
heterodimeric cytokine belonging to the IL-12 family that consists
of 2 subunits, p40 (shared with IL-12) and p19 (35,36). This
cytokine signals via a specific receptor consisting of the IL-12
receptor (R) chain and the IL-23R chain (37). Notably, IL-23 is required for the
survival of Th17 cells (38). In the
present study, a positive correlation between the expression of
IL-17 and IL-23 was identified in the DTC group, which may indicate
explain the presence of functional Th17 in the microenvironment of
DTC injury tissues, which is in agreement with the literature. A
number of studies have demonstrated that IL-17 induces vascular
endothelial growth factor expression, which subsequently induces
TGF-β (37–39). Numerous tumor cells express high
levels of TGF-β, which promotes tumor growth and metastasis
(39). The effect of TGF-β in the
differentiation of CD4+ T cells may be influenced by the cytokines
present in the tumor microenvironment. For example, IL-23 is able
to induce IL-17 production by memory and activated T cells and is
essential for the development, survival and growth of these cells
(40,41). In the present study, the differential
expression of IL-17 and IL-23 between malignant and benign lesions
indicates that the presence or absence of these cytokines may
influence disease progression. Therefore, the Th-17/IL-23 axis may
facilitate the malignant progression of cancer and the association
between IL-17 and IL-23 in the microenvironment indicates that the
two molecules may interact. The exact mechanism underlying this
interaction remains unclear; however; we hypothesize that these
molecules are important in the progression of cancer. Th17 cells
have been identified in a number of human tumor types, including
lymphoma (40), myeloma (41), breast (34) and ovarian cancer (42). In colorectal carcinomas, prognosis is
influenced by Th17 expression; patients with high Th17 expression
exhibit a poor prognosis (43). Zhang
et al (44) demonstrated that
the accumulation of IL-17-producing cells in the tumor
microenvironment leads to tumor progression in patients with
hepatocellular carcinoma by promoting angiogenesis. In the present
study, of the 19 patients who experienced recurrence or succumbed
to the disease, 14 (73.4%) exhibited high IL-17 protein expression,
which indicates that the IL-17 cytokine is involved in the
development and progression of tumors, and thus may present a
prognostic factor for poor survival. These results indicate an
association between infiltration of Th17 cells and poor prognosis,
which confirms that Th17 may inhibit antitumor responses. Based on
these results, we postulate that the growth and progression of
thyroid cancer may be positively influenced by two major
inflammatory components, IL-17 and IL-23.
Acknowledgements
The present study was supported by the National
Council of Scientific and Technological Development Process of
Brazil (grant no. HCRP 3156/2011). The authors would like to thank
Ms. Ana Maria Rocha (Department of Pathology, Faculty of Medicine
of Ribeirão Preto, University of São Paulo, São Paulo, Brazil) for
her technical assistance.
References
|
1
|
Talmadge JE, Donkor M and Scholar E:
Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer
Metastasis Rev. 26:373–400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qi W, Huang X and Wang J: Correlation
between Th17 cells and tumor microenvironment. Cell Immunol.
285:18–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Talmadge JE: Immune cell infiltration of
primary and metastatic lesions: Mechanisms and clinical impact.
Semin Cancer Biol. 21:131–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Josefowicz SZ, Lu LF and Rudensky AY:
Regulatory T cells: Mechanisms of differentiation and function.
Annu Rev Immunol. 30:531–564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bailey SR, Nelson MH, Himes RA, Li Z,
Mehrotra S and Paulos CM: Th17 cells in cancer: The ultimate
identity crisis. Front Immunol. 5:2762014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Z and O'Shea JJ: Th17 cells: A new
fate for differentiating helper T cells. Immunol Res. 41:87–102.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peck A and Mellins ED: Plasticity of
T-cell phenotype and function: The T helper type 17 example.
Immunology. 129:147–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barbi J, Pardoll D and Pan F: Treg
functional stability and its responsiveness to the
microenvironment. Immunol Rev. 259:115–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stritesky GL, Yeh N and Kaplan MH: IL-23
promotes maintenance but not commitment to the Th17 lineage. J
Immunol. 181:5948–5955. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kryczek I, Banerjee M, Cheng P, Vatan L,
Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, et
al: Phenotype, distribution, generation and functional and clinical
relevance of Th17 cells in the human tumor environments. Blood.
114:1141–1149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Azzazene D, Al Thawadi H, Al Farsi H,
Besbes S, Geyl C, Mirshahi S, Pardo J, Faussat AM, Jeannette S,
Therwath A, et al: Plasma endothelial protein C receptor influences
innate immune response in ovarian cancer by decreasing the
population of natural killer and TH17 helper cells. Int J Oncol.
43:1011–1018. 2013.PubMed/NCBI
|
|
13
|
Muranski P, Boni A, Antony PA, Cassard L,
Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K,
et al: Tumor-specific Th17-polarized cells eradicate large
established melanoma. Blood. 112:362–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sfanos KS, Bruno TC, Maris CH, Xu L,
Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB and Drake CG:
Phenotypic analysis of prostate-infiltrating lymphocytes reveals
TH17 and Treg skewing. Clin Cancer Res. 14:3254–3261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Demaria S, Pikarsky E, Karin M, Coussens
LM, Chen YC, El-Omar EM, Trinchieri G, Dubinett SM, Mao JT, Szabo
E, et al: Cancer and inflammation: Promise for biologic therapy. J
Immunother. 33:335–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guarino V, Castellone MD, Avilla E and
Melillo RM: Thyroid cancer and inflammation. Mol Cell Endocrinol.
321:94–102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okayasu I: The relationship of lymphocytic
thyroiditis to the development of thyroid carcinoma. Endocr Pathol.
8:225–230. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kebebew E, Treseler PA, Ituarte PH and
Clark OH: Coexisting chronic lymphocytic thyroiditis and papillary
thyroid cancer revisited. World J Surg. 25:632–637. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ugolini C, Basolo F, Proietti A, Vitti P,
Elisei R, Miccoli P and Toniolo A: Lymphocyte and immature
dendritic cell infiltrates in differentiated, poorly
differentiated, and undifferentiated thyroid carcinoma. Thyroid.
17:389–393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y and Wang W: Increasing incidence of
thyroid cancer in Shanghai, China, 1983–2007. Asia Pac J Public
Health. 27:223–229. 2015. View Article : Google Scholar
|
|
21
|
Nikiforov YE: Molecular diagnostics of
thyroid tumors. Arch Pathol Lab Med. 135:569–577. 2011.PubMed/NCBI
|
|
22
|
Zanetti BR, Carvalho-Galano DF, Feitosa
NL, Hassumi-Fukasawa MK, Miranda-Camargo FA, Maciel LM,
Ribeiro-Silva A and Soares EG: Differential expression of
immune-modulatory molecule HLA-E in non-neoplastic and neoplastic
lesions of the thyroid. Int J Immunopathol Pharmacol. 26:889–896.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Figueiredo Feitosa NL, Crispim JC,
Zanetti BR, Magalhães PK, Soares CP, Soares EG, Neder L, Donadi EA
and Maciel LM: HLA-G is differentially expressed in thyroid
tissues. Thyroid. 24:585–592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
American Thyroid Association (ATA)
Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid
Cancer, ; Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL,
Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, et
al: Revised American Thyroid Association management guidelines for
patients with thyroid nodules and differentiated thyroid cancer.
Thyroid. 19:1167–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Landskron G, De la Fuente M, Thuwajit P,
Thuwajit C and Hermoso MA: Chronic inflammation and cytokines in
the tumor microenvironment. J Immunol Res. 2014:1491852014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muzza M, Degl'Innocenti D, Colombo C,
Perrino M, Ravasi E, Rossi S, Cirello V, Beck-Peccoz P, Borrello MG
and Fugazzola L: The tight relationship between papillary thyroid
cancer, autoimmunity and inflammation: Clinical and molecular
studies. Clin Endocrinol (Oxf). 72:702–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
French JD, Weber ZJ, Fretwell DL, Said S,
Klopper JP and Haugen BR: Tumor-associated lymphocytes and
increased FoxP3+ regulatory T cell frequency correlate with more
aggressive papillary thyroid cancer. J Clin Endocrinol Metab.
95:2325–2333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ouyang W, Kolls JK and Zheng Y: The
biological functions of T helper 17 cell effector cytokines in
inflammation. Immunity. 28:454–467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang B, Kang H, Fung A, Zhao H, Wang T and
Ma D: The role of interleukin 17 in tumour proliferation,
angiogenesis, and metastasis. Mediators Inflamm. 2014:6237592014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mizutani K, Sud S, McGregor NA,
Martinovski G, Rice BT, Craig MJ, Varsos ZS, Roca H and Pienta KJ:
The chemokine CCL2 increases prostate tumor growth and bone
metastasis through macrophage and osteoclast recruitment.
Neoplasia. 11:1235–1242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ngiow SF, Teng MW and Smyth MJ: A balance
of interleukin-12 and −23 in cancer. Trends Immunol. 34:548–555.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF and
Peng G: Tumor microenvironments direct the recruitment and
expansion of human Th17 cells. J Immunol. 184:1630–1641. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kastelein RA, Hunter CA and Cua DJ:
Discovery and biology of IL-23 and IL-27: Related but functionally
distinct regulators of inflammation. Annu Rev Immunol. 25:221–242.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stritesky GL, Yeh N and Kaplan MH: IL-23
promotes maintenance but not commitment to the Th17 lineage. J
Immunol. 181:5948–5955. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeon SH, Chae BC, Kim HA, Seo GY, Seo DW,
Chun GT, Kim NS, Yie SW, Byeon WH, Eom SH, et al: Mechanisms
underlying TGF-beta1-induced expression of VEGF and Flk-1 in mouse
macrophages and their implications for angiogenesis. J Leukoc Biol.
81:557–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Langowski JL, Zhang X, Wu L, Mattson JD,
Chen T, Smith K, Basham B, McClanahan T, Kastelein RA and Oft M:
IL-23 promotes tumour incidence and growth. Nature. 442:461–465.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chizzolini C, Chicheportiche R, Alvarez M,
de Rham C, Roux-Lombard P, Ferrari-Lacraz S and Dayer JM:
Prostaglandin E2 synergistically with interleukin-23 favors human
Th17 expansion. Blood. 112:3696–3703. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Galand C, Donnou S, Crozet L, Brunet S,
Touitou V, Ouakrim H, Fridman WH, Sautès-Fridman C and Fisson S:
Th17 cells are involved in the local control of tumor progression
in primary intraocular lymphoma. PLoS One. 6:e246222011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Noonan K, Marchionni L, Anderson J,
Pardoll D, Roodman GD and Borrello I: A novel role of
IL-17-producing lymphocytes in mediating lytic bone disease in
multiple myeloma. Blood. 116:3554–3563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Charles KA, Kulbe H, Soper R,
Escorcio-Correia M, Lawrence T, Schultheis A, Chakravarty P,
Thompson RG, Kollias G, Smyth JF, et al: The tumor-promoting
actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in
mice and humans. J Clin Invest. 119:3011–3023. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tosolini M, Kirilovsky A, Mlecnik B,
Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH,
Pagès F and Galon J: Clinical impact of different classes of
infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in
patients with colorectal cancer. Cancer Res. 71:1263–1271. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang JP, Yan J, Xu J, Pang XH, Chen MS,
Li L, Wu C, Li SP and Zheng L: Increased intratumoral
IL-17-producing cells correlate with poor survival in
hepatocellular carcinoma patients. J Hepatol. 50:980–989. 2009.
View Article : Google Scholar : PubMed/NCBI
|