Introduction
Lung cancer is among the most common causes of
cancer-associated mortality worldwide (1,2), and the
majority of cases are non-small cell lung cancer (NSCLC), which has
a poor 5-year survival rate (3).
Previous studies hypothesized that the cancer stem-like cell (CSC)
subpopulation resulted in cancer initiation, progression, drug
resistance, metastasis and recurrence. Since CSCs were first found
in leukemia (4), evidence for CSC
existence has been emerging in a variety of cancers (5–7), including
lung cancer (8–10). CSCs exhibited distinct
characteristics, including self-renewal, differentiation,
quiescence and heterogeneous tumorigenicity. These properties lead
to traditional therapy failure, making CSCs crucial targets for
successful cancer treatment (11).
Thioridazine (TDZ) was originally used as a
treatment for psychotic disease (12,13), and
has also been used to treat against drug-resistant microorganisms
(14,15). A recent study reported the potent
effect of TDZ on various types of cancer cells. TDZ was capable of
inhibiting mucosa-associated lymphoid tissue lymphoma translocation
protein 1 (MALT1) protease and provided potential applications in B
cell lymphoma (16). It is also
involved in the phosphoinositide 3-kinase (PI3K)/protein kinase B
(Akt)/mechanistic target of rapamycin pathway, contributing to
anti-angiogenesis effects and apoptosis in breast and ovarian
(17,18) cancers. In addition, TDZ induced cell
death in cancers, including cervical (19), prostate (20), gastric (21) and pancreatic cancers (16). Additionally, TDZ has been shown to
sensitize drug-resistance cancer cells by P-glycoprotein inhibition
(22) and be a top chemotherapeutic
for gefitinib-resistant NSCLC cells (23). Furthermore, TDZ has selectivity in
leukemia and breast CSCs as a dopamine receptor inhibitor (24,25).
Due to this background, the current study
hypothesized that TDZ would exert cytotoxic effects on lung CSCs.
In the present study, TDZ treatment was performed on lung cancer
stem-like A549 sphere cells, which were established and
characterized in our previous study (26). Cell viability and colony formation
ability of A549 spheres reduced following TDZ treatment.
Caspase-dependent apoptosis and G1 phase arrest were detected in
TDZ-treated A549 sphere cells. In vivo experiments
additionally showed that TDZ pretreatment inhibited initiation and
growth of mice xenografts derived from A549 sphere cells,
indicating it as a candidate for successful lung cancer
therapy.
Materials and methods
Cell culture and viability
analysis
Human lung cancer stem-like A459 sphere cells were
obtained and maintained as described in our previous study
(26), with a growth factor-defined
serum-free medium in ultra-low detachment 6-well plates. A549 cells
were incubated with serum-free Dulbecco's modified Eagle medium:
Nutrient mixture F-12 (GE Healthcare Life Sciences, Chalfont, UK)
medium in ultra-low attachment 6-well dishes (Corning, Tewksbury,
MA, USA). Growth factors including epidermal growth factor, basic
fibroblast growth factor and insulin-like growth factor 1 were
supplied at a concentration of 20 ng/ml (PeproTech, Rocky Hill, NJ,
USA) each day (A549 sphere cells). Three days subsequent to
seeding, the propagated spheroid bodies were collected and digested
by StemPro Accutase (Thermo Fisher Scientific Inc., Waltham, MA,
USA) to single cell suspension for subsequent experiments. Cell
viability was observed by microscopy or crystal violet staining and
quantitated by methyl thiazolyl tetrazolium (MTT) assay. Cells were
seeded in 24-well plates (2×105 cells/well) for direct
observation and in the 96-well plates (1×104 cells/well)
for indirect quantitation, respectively. Following adherence, TDZ
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) was added at
the indicated concentrations (0, 0.01, 0.1, 0.5, 1, 5, 10 and 15
µM). Two days later, cells in 24-well plates were photographed with
or without crystal violet staining. Cells in 96-well plates were
incubated with 20 ml MTT (Beyotime Institute of Biotechnology,
Haimen, China) for another 4 h at 37°C. Supernatants were discarded
and 100 µl dimethyl sulfoxide (DMSO; Guanghua Sci-Tech, Shanghai,
China) was added to each well and agitated. Cell viability was
assessed by absorbance of dual wavelength light (490 and 570 nm)
via a microplate reader (Tecan, Männedorf, Switzerland). All
experiments were repeated 3 times.
Colony formation assay
Cells were plated in 6-well plates (1×103
cells/well) for colony formation. TDZ was applied to treated cells
following adherence at indicated concentrations (0, 1, 5, 10 and 15
µM). After 12 days, colonies were fixed and subjected to crystal
violet staining for visualization. Images of plates containing
colonies were captured using a Canon EOS 650D digital camera
(Canon, Inc., Tokyo, Japan) and the number of colonies was counted.
Experiments were repeated 3 times.
Hoechst staining
Cells in 96-well plates (1×104
cells/well) received different treatments with TDZ (0, 1, 10 and 15
µM) for 48 h. Cells were then fixed with 4% paraformaldehyde
(Sigma-Aldrich; Merck Millipore) for 15 min and stained with 1
µg/ml Hoechst 33342 (Molecular Probes, Eugene, OR, USA) for 1 min.
Images of morphology were captured by fluorescence microscopy.
Experiments were repeated 3 times.
Flow cytometry
Cells were digested following a 1-day treatment with
TDZ (0, 1, 10 and 15 µM). For cell cycle analysis, cells were fixed
with 70% ethanol at 4°C for 1 h subsequent to being washed and
resuspended in phosphate-buffered saline. Cells were then
centrifuged at 1,000 × g for 3 min at room temperature,
prior to washing and incubation with 20 µg/ml RNase A (Generay,
Shanghai, China) for 30 min at 37°C in a water bath. Subsequently,
cells were stained for 30 min with 50 µg/ml PI (Sigma-Aldrich;
Merck Millipore). For Annexin V/PI staining, cells were prepared
using Annexin V-fluorescein isothiocyanate Apoptosis Detection kit
(eBioscience, San Diego, CA, USA), according to the manufacturer's
protocol. The fluorescence-activated cell sorting results were
collected using Accuri™ C6 (BD Biosciences, Franklin Lakes, NJ,
USA).
Western blotting
Western blotting was conducted according to the
standard procedures. Primary antibodies against survivin [cat no.
2808; rabbit monoclonal antibody (mAb); 1:1,000], cyclin-dependent
kinase 2 (CDK2; cat no. 2546; rabbit mAb; 1:1,000), Akt (cat no.
9272; Rabbit; 1:1,000), phosphorylated-Akt (Ser473) (D9E) (cat no.
4060; rabbit mAb; 1:2,000), caspase-8 precursor (caspase8; cat no.
9746; mouse mAb; 1:500), and poly ADP-ribose polymerase (PARP; cat
no. 9532; rabbit mAb; 1:1,000) were purchased from Cell Signaling
Technology (Beverly, MA, USA). GAPDH (cat no. CW0100M; mouse mAb;
1:3,000) was from CoWin Bioscience (Beijing, China). Secondary
antibodies including mouse anti goat IgG-HRP (cat no. sc-2354;
goat; 1:5,000) and rabbit anti goat IgG-HRP (cat no. sc-2922; goat;
1:5,000) were purchased from Santa Cruz Biotechnology (Dallas, TX,
USA).
Animal experiments
A total of 36 four-week old female BALB/c nude mice
were purchased from the Shanghai Laboratory Animal Centre and
raised in specific pathogen free conditions at the animal facility
of Shanghai Institute of Biochemistry and Cell Biology (both
Shanghai, China). All animal experiments were performed based on
the corresponding policy approved by the Institutional Animal Care
and Use Committee. To explore the in vivo anti-tumor effect
of TDZ, A549 sphere cells were pretreated with DMSO, 1 or 10 µM DZ
for 24 h. Cells were then mixed with Matrigel (2:1; BD Biosciences)
and injected subcutaneously into the right rear of nude mice
(2×105 cells/mouse) following digestion and counting.
Each group consisted of 6 mice. The tumor volumes were measured
using Vernier calipers every 3 days and calculated as follows:
Volume (mm3) = length × width × width / 2.
Statistical analysis
All the data were presented as the mean ± standard
deviation (SD) or mean + SD. R software was utilized for Student's
t-test or one-way analysis of variance to compare the difference
among groups. The non-parametric Friedman test was used to analyze
differences, followed by the Dunn's Multiple Comparison test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TDZ exhibited cytotoxicity in lung
CSCs
Lung cancer stem-like A549 sphere cells were
obtained as described in materials and methods. The cytotoxic
effect of TDZ on A549 sphere cells was analyzed. Morphological
alteration appeared in A549 sphere cells treated with TDZ (Fig. 1A). A concentration-dependent curve of
TDZ against cell viability through MTT assay indicated that a high
dose of TDZ significantly impacted cell growth (Fig. 1B), which was consistent with the
result of crystal violet staining (Fig.
1C). Similarly, the colony formation ability of A549 sphere
cells was markedly decreased by TDZ (Fig.
1D and E), inferring the dose-dependent inhibitory effect of
TDZ on lung CSCs.
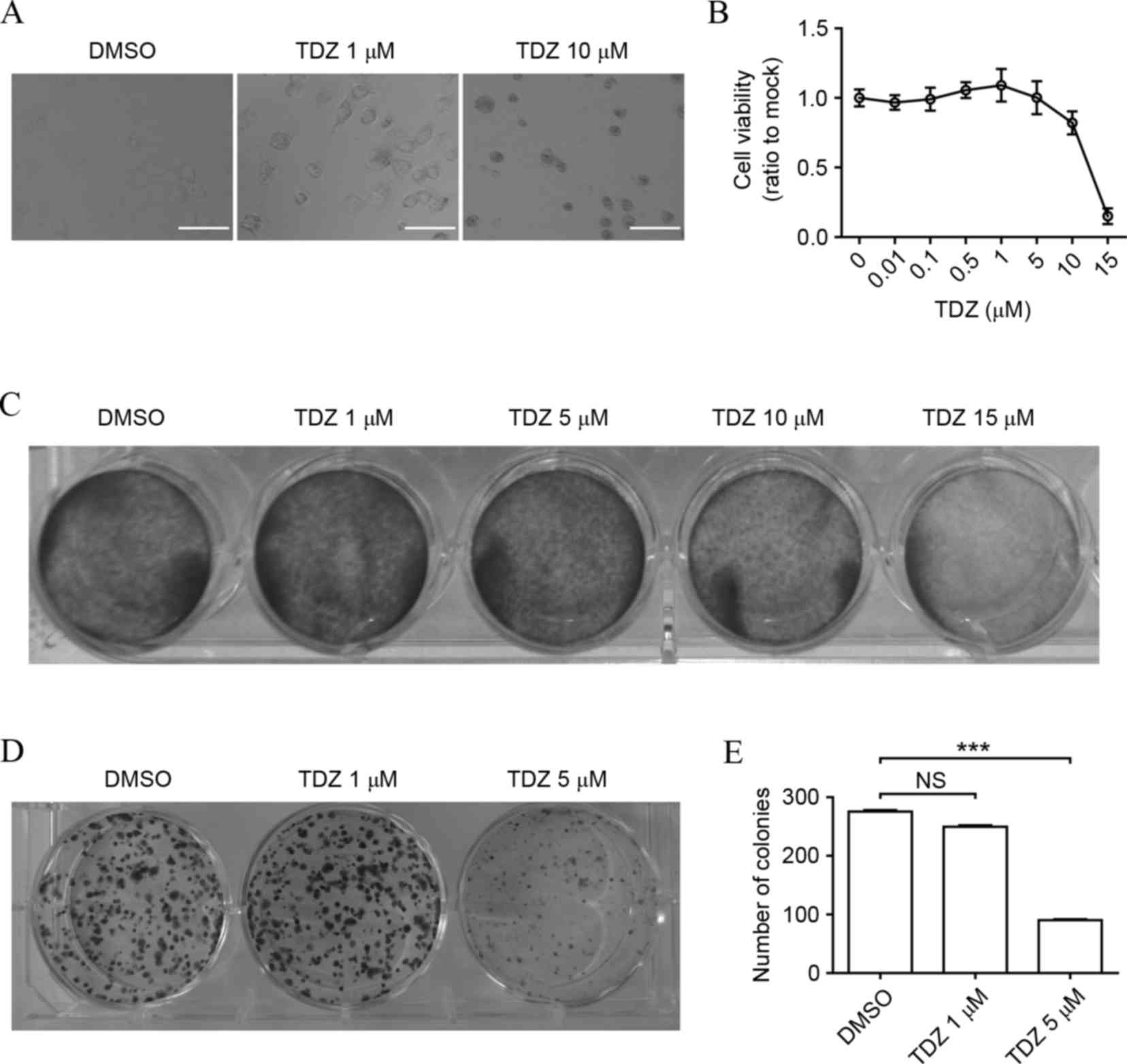 | Figure 1.TDZ exhibits cytotoxicity in lung
CSCs. (A) Morphology of A549 sphere cells altered following
treatment with 1 or 10 µM TDZ for 48 h. Scale bar, 200 µm. (B) Cell
viability of A549 sphere cells decreased in a dose-dependent manner
following TDZ treatment (0.01, 0.1, 0.5, 1, 5, 10 or 15 µM) for 48
h. Cell viability was determined by MTT assay (C) TDZ attenuated
A549 sphere cell viability. A549 sphere cells were treated with TDZ
(1, 5, 10 or 15 µM) for 48 h and then subjected to crystal violet
staining. (D) TDZ-treated A549 sphere cells formed fewer colonies,
as shown by crystal violet staining. (E) Statistical analysis of
colony formation assay. The y-axis indicated the total number of
colonies in 1 well. All experiments were repeated three times with
DMSO as a control. All data shown are expressed as the mean ±
standard deviation (n=3). ***P<0.001. TDZ, thioridazine; DMSO,
dimethyl sulfoxide; NS, no significance. |
TDZ induced apoptosis and cell cycle
arrest in A549 sphere cells
To investigate the mechanism of cell death and
proliferation inhibition, A549 sphere cells were stained by Hoechst
33342 following addition of TDZ. Significant nuclear fragmentation
was observed in cells under stress of high dose TDZ compared with
the DMSO-treated control (Fig. 2A).
The percentage of Annexin V-positive cells rose with the growing
concentration of TDZ (Fig. 2B).
Overall, these findings provided evidence that TDZ induced
apoptosis in A549 sphere cells. In addition, cell-cycle phase
distribution was analyzed. The proportion of cells in the G0/G1
phase enlarged as the dose of TDZ increased (Fig. 2C), indicating that TDZ was associated
with G1/S checkpoint activation and cell cycle arrest.
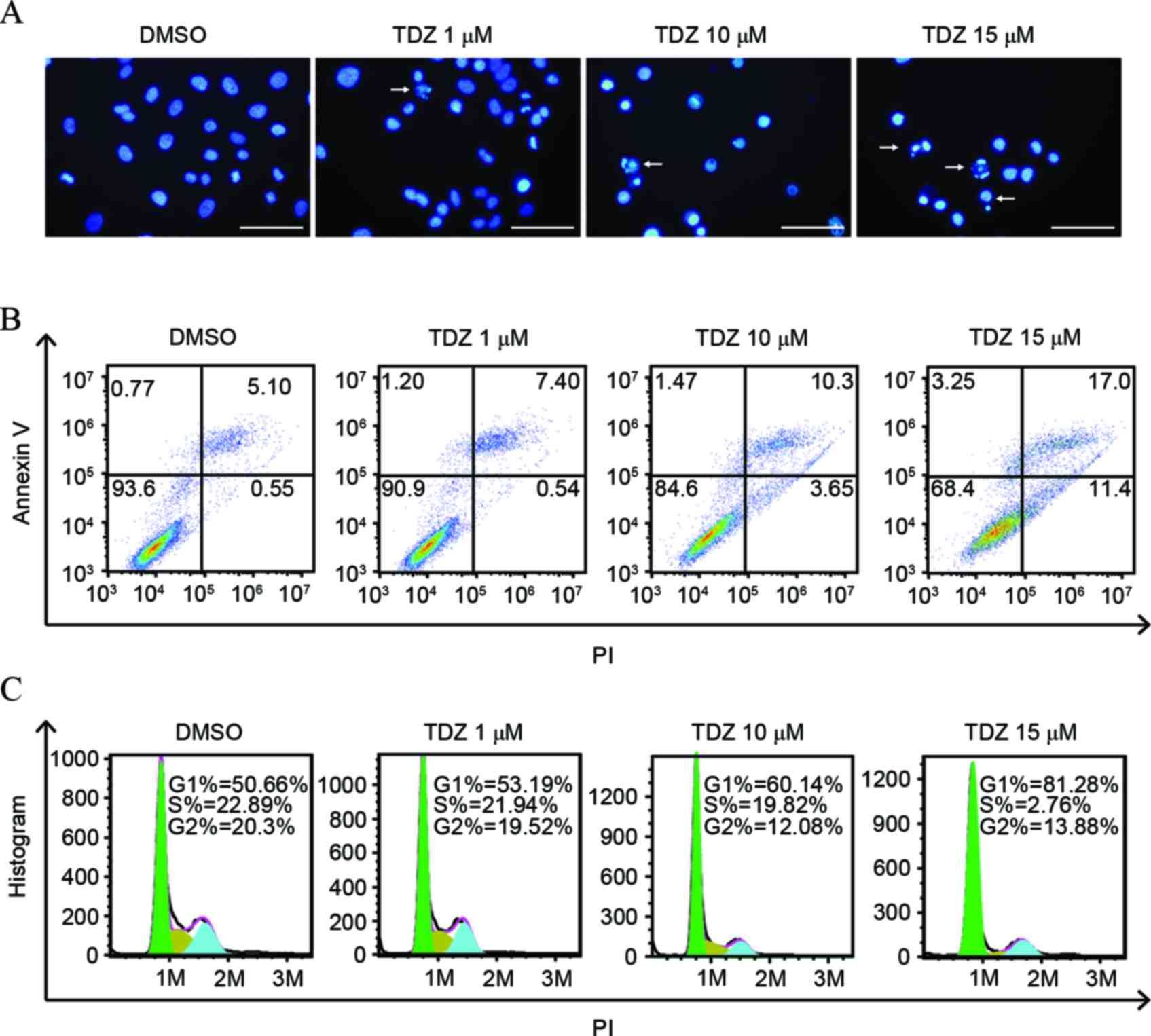 | Figure 2.TDZ induced apoptosis and cell cycle
arrest in A549 sphere cells. (A) Increased nucleic fragmentation
(arrow) was observed in A549 sphere cells following 2-day treatment
with TDZ (1, 10 or 15 µM), as detected by Hoechst staining. Scale
bar, 200 µm. (B) Apoptosis was detected in TDZ-treated (1, 10 or 15
µM) A549 sphere cells by Annexin V/PI double staining with
fluorescence-activated cell sorting analysis. (C) G1-phase cell
proportion increased with concentration of TDZ treatment (1, 10 or
15 µM) on A549 sphere cells. The areas of green, yellow and blue
equaled the percentage of cells in G1, S and G2 phase,
respectively. All experiments were repeated three times with DMSO
as a control. TDZ, thioridazine; PI, propidium iodide; DMSO,
dimethyl sulfoxide. |
TDZ induced caspase-dependent
apoptosis and Akt-associated cell cycle arrest
To additionally clarify the mechanism of apoptosis
and cell cycle arrest, expression levels of associated proteins
were examined by western blot. Decrease of procaspase8 and PARP
expression was detected in TDZ-treated A549-sphere cells along with
apoptosis repressors, such as survivin (Fig. 3A). Additionally, the protein levels of
cell cycle-associated pathways, including Akt, p-Akt and CDK2, were
downregulated (Fig. 3B). These
results indicated that TDZ induced cell death of A549 sphere cells
via caspase-dependent apoptosis and inhibited cell proliferation
through the Akt-CKD2 pathway.
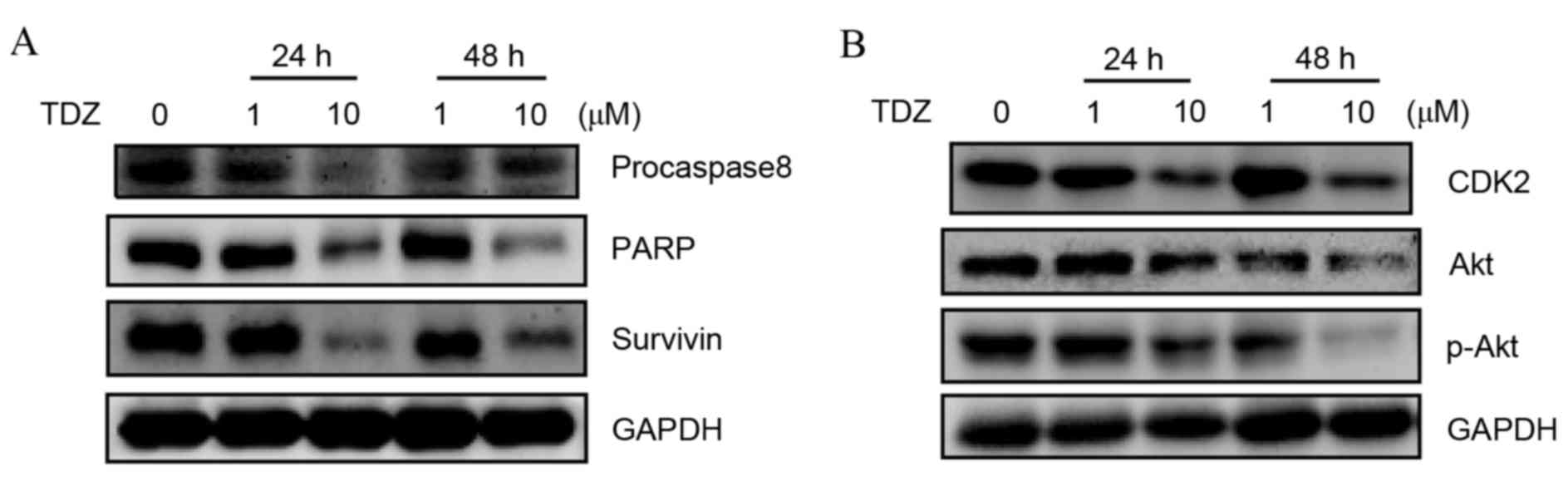 | Figure 3.TDZ induced caspase-dependent
apoptosis and Akt-CDK2 associated cell cycle progression. (A)
Decreased expression of apoptosis-associated proteins, including
procaspase8, PARP and survivin, in A549-sphere cells treated with
TDZ was detected using western blotting. (B) Decreased expression
of cell cycle associated proteins, including CDK2, Akt and p-Akt,
in A549-sphere cells treated with TDZ was detected using western
blotting. All experiments were repeated three times with GAPDH as a
loading control. TDZ, thioridazine; PARP, poly ADP-ribose
polymerase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; CDK2,
cyclin-dependent kinase 2; Akt, protein kinase B; p-Akt,
phosphorylated-protein kinase B. |
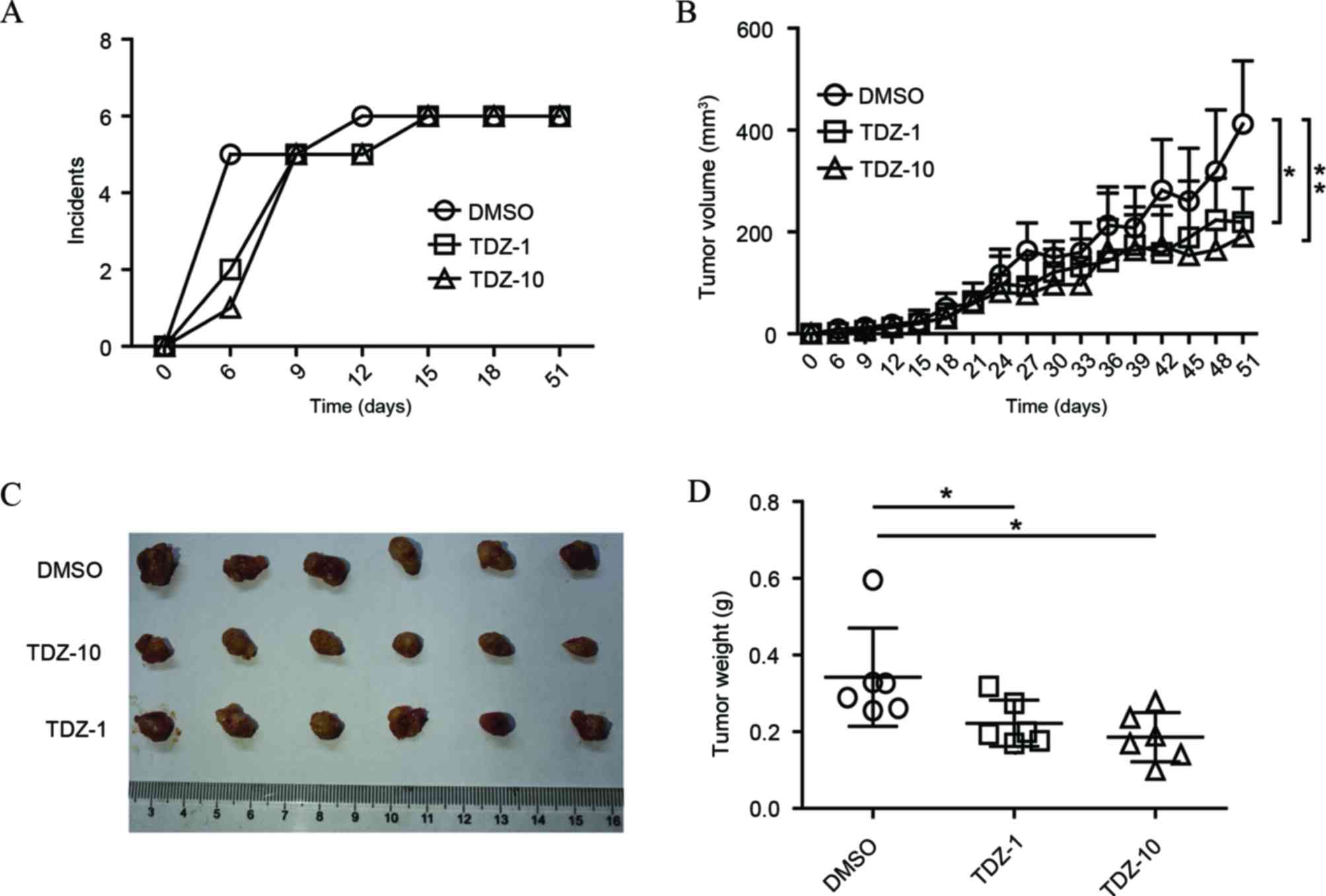
TDZ suppressed tumor initiation and
growth in vivo
The in vivo activity of TDZ on lung CSCs were
tested based on a mice xenograft model. In comparison to the
control, TDZ-pretreated A549 sphere cells showed increased latency
time on xenograft initiation with increasing TDZ concentration
(Fig. 4A). Although low dose TDZ
treatment (1 µM) presented little effect on A549 sphere cell
viability in vitro, the two doses of TDZ treatment (1 and 10
µM) showed significant inhibition of the growth of mice xenografts
derived by A549 sphere cells (Fig.
4B-D). Overall, these data disclosed the in vivo effect
of TDZ on repression of tumor initiation and progression.
Discussion
To the best of our knowledge, the present study
investigated for the first time the effect of TDZ on lung CSCs. As
more evidence for CSC existence is being found, novel
chemotherapeutics can be developed for CSC targeting. Akt is a key
regulator of cell proliferation, metabolism, survival and
apoptosis, and inhibitors for Akt are able to target CSCs in breast
cancer (27) and liver cancer
(28). TDZ, previously used for
patients with psychotic illnesses, is an antagonist of dopamine
receptor D2 family proteins, which has interactions with
Akt (29). It has been reported that
TDZ was able to inhibit the PI3K/Akt pathway (30) and induce cell death in various cancer
cells. TDZ treatment selectively affected leukemia CSCs rather than
normal stem cells (24), suggesting
TDZ as a potent therapeutic agent for CSCs. In the present study,
the Akt protein level decreased following TDZ-treatment, which was
consistent with the aforementioned studies and its downregulated
phosphorylation level may explain the apoptosis and G1/S arrest
presented following TDZ treatment (Figs.
2 and 3).
Isolation and identification of CSCs is required for
evaluating targeted drugs. Cell sorting and specific culture are
the main methods used to isolate CSCs. A549 sphere cells were
enriched and demonstrated to have lung CSC properties in our
previous study (26). In the current
study, TDZ exhibited anti-proliferation activity in A549 sphere
cells and altered their cell viability (Fig. 1). Notably, despite the fact that
low-dose TDZ treatment was not efficient in vitro, it
performed well in terms of tumor xenograft repression in
vivo (Fig. 4). The
anti-angiogenesis mechanism may contribute to the discrepancy, as
TDZ is capable of affecting vascular endothelial growth factor
receptor 2 (18). Furthermore, TDZ
has the capacity of relieving drug resistance of cancer cells,
indicating that combination of other therapeutics may achieve
improved results. In addition, TDZ promoted cancer cell death
induced by oncolytic adenovirus (31), inferring its potential application
with gene-virotherapy.
In conclusion, the present data illustrates that TDZ
shows robust inhibitory effect on lung CSCs in vitro and
in vivo, indicating its utility as a promising candidate
drug for lung CSC-targeted therapy.
Acknowledgements
The authors thank the staff at the Cell Biology
Platform for their assistance in flow cytometry assays. This study
was supported by National Natural Science Fund (grant no. 81272687)
and Zhejiang Provincial Natural Science Foundation of China (grant
no. LZ13H160004).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
She J, Yang P, Hong Q and Bai C: Lung
cancer in China: Challenges and interventions. Chest.
143:1117–1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
7
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roudi R, Madjd Z, Korourian A, Mehrazma M,
Molanae S, Sabet MN and Shariftabrizi A: Clinical significance of
putative cancer stem cell marker CD44 in different histological
subtypes of lung cancer. Cancer Biomark. 14:457–467. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu CC, Lin JH, Hsu TW, Su K, Li AF, Hsu
HS and Hung SC: IL-6 enriched lung cancer stem-like cell population
by inhibition of cell cycle regulators via DNMT1 upregulation. Int
J Cancer. 136:547–559. 2015.PubMed/NCBI
|
|
11
|
Zhou BB, Zhang HY, Damelin M, Geles KG,
Grindley JC and Dirks PB: Tumour-initiating cells: Challenges and
opportunities for anticancer drug discovery. Nat Rev Drug
Discovery. 8:806–823. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohman R and Axelsson R: Relationship
between prolactin response and antipsychotic effect of thioridazine
in psychiatric-patients. Eur J Clin Pharmacol. 14:111–116. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Realmuto GM, Erickson WD, Yellin AM,
Hopwood JH and Greenberg LM: Clinical comparison of thiothixene and
thioridazine in schizophrenic adolescents. Am J Psychiatry.
141:440–442. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Soolingen D, Hernandez-Pando R, Orozco
H, Aguilar D, Magis-Escurra C, Amaral L, van Ingen J and Boeree MJ:
The antipsychotic thioridazine shows promising therapeutic activity
in a mouse model of multidrug-resistant tuberculosis. PLoS One.
5:e126402010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thorsing M, Klitgaard JK, Atilano ML, Skov
MN, Kolmos HJ, Filipe SR and Kallipolitis BH: Thioridazine induces
major changes in global gene expression and cell wall composition
in methicillin-resistant Staphylococcus aureus USA300. PLoS One.
8:e645182013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagel D, Spranger S, Vincendeau M, Grau M,
Raffegerst S, Kloo B, Hlahla D, Neuenschwander M, von Kries J
Peter, Hadian K, et al: Pharmacologic inhibition of MALT1 protease
by phenothiazines as a therapeutic approach for the treatment of
aggressive ABC-DLBCL. Cancer Cell. 22:825–837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Byun HJ, Lee JH, Kim BR, Kang S, Dong SM,
Park MS, Lee SH, Park SH and Rho SB: Anti-angiogenic effects of
thioridazine involving the FAK-mTOR pathway. Microvascular Res.
84:227–234. 2012. View Article : Google Scholar
|
|
18
|
Park MS, Dong SM, Kim BR, Seo SH, Kang S,
Lee EJ, Lee SH and Rho SB: Thioridazine inhibits angiogenesis and
tumor growth by targeting the VEGFR-2/PI3K/mTOR pathway in ovarian
cancer xenografts. Oncotarget. 5:4929–4934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang S, Dong SM, Kim BR, Park MS, Trink B,
Byun HJ and Rho SB: Thioridazine induces apoptosis by targeting the
PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells.
Apoptosis. 17:989–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Csonka A, Spengler G, Martins A, Ocsovszki
I, Christensen JB, Hendricks O, Kristiansen JE, Amaral L and Molnar
J: Effect of thioridazine stereoisomers on the drug accumulation of
mouse lymphoma and human prostate cancer cell lines in vitro. In
vivo. 27:815–820. 2013.PubMed/NCBI
|
|
21
|
Mu J, Xu H, Yang Y, Huang W, Xiao J, Li M,
Tan Z, Ding Q, Zhang L, Lu J, et al: Thioridazine, an antipsychotic
drug, elicits potent antitumor effects in gastric cancer. Oncol
Rep. 31:2107–2114. 2014.PubMed/NCBI
|
|
22
|
Spengler G, Molnar J, Viveiros M and
Amaral L: Thioridazine induces apoptosis of multidrug-resistant
mouse lymphoma cells transfected with the human ABCB1 and inhibits
the expression of P-glycoprotein. Anticancer Res. 31:4201–4205.
2011.PubMed/NCBI
|
|
23
|
Sudo M, Mori S, Madan V, Yang H, Leong G
and Koeffler HP: Short-hairpin RNA library: Identification of
therapeutic partners for gefitinib-resistant non-small cell lung
cancer. Oncotarget. 6:814–824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sachlos E, Risueño Ruth M, Laronde S,
Shapovalova Z, Lee JH, Russell J, Malig M, McNicol JD, Fiebig-Comyn
A, Graham M, et al: Identification of drugs including a dopamine
receptor antagonist that selectively target cancer stem cells.
Cell. 149:1284–1297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ke XY, Lin Ng VW, Gao SJ, Tong YW, Hedrick
JL and Yang YY: Co-delivery of thioridazine and doxorubicin using
polymeric micelles for targeting both cancer cells and cancer stem
cells. Biomaterials. 35:1096–1108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Y, Xu HN, Huang WD, Ding M, Xiao J,
Yang D, Li H, Liu XY and Chu L: Targeting lung cancer stem-like
cells with TRAIL gene armed oncolytic adenovirus. J Cell Mol Med.
19:915–923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Korkaya H, Paulson A, Charafe-Jauffret E,
Ginestier C, Brown M, Dutcher J, Clouthier SG and Wicha MS:
Regulation of mammary stem/progenitor cells by
PTEN/Akt/beta-catenin signaling. PLoS Biol. 7:e10001212009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang XL, Jia Q, Lv L, Deng T and Gao J:
Tumorspheres derived from HCC Cells are enriched with cancer stem
cell-like cells and present high chemoresistance dependent on the
Akt pathway. Anticancer Agents Med Chem. 15:755–763. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arguello PA and Gogos JA: A signaling
pathway AKTing up in schizophrenia. J Clin Investig. 118:2018–2021.
2008.PubMed/NCBI
|
|
30
|
Rho SB, Kim BR and Kang S: A gene
signature-based approach identifies thioridazine as an inhibitor of
phosphatidylinositol-3′-kinase (PI3K)/AKT pathway in ovarian cancer
cells. Gynecol Oncol. 120:121–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dongmei D, Xianlong F, Yu Y, Hongyan L,
Qiang P and Haineng X: Inhibition of HeLa Cells by the combination
of Thioridazine and Oncolytic Adenovirus ZD55-TRAIL and the
exploration of its mechanism. Chinese J Cell Biol. 36:595–601.
2014.
|