Introduction
Tumor-to-meningioma metastasis (TMM) is a fairly
uncommon phenomenon, with just over 100 cases published to date
since the first case reported by Fried in 1930 (1,2). Although
meningiomas have been reported as the most common intracranial
neoplasms to harbor metastasis, any benign or malignant tumor can
be a recipient (3–6). Despite certain radiological features
being suggestive of the diagnosis of TMM, such as atypical signal
changes in MRI suggesting the presence of another tumor within a
meningioma, they are neither sensitive nor specific, making the
pre-surgical diagnosis extremely difficult (7,8). The
prevalence of TMM increases with age and apparently there is no
gender difference. Only 7 cases of prostate cancer with TMM have
previously been described in the literature (6,9–13). The present study aimed to report a
case of prostate cancer TMM, and to discuss the relevant clinical
and neuroimaging aspects of this condition.
Case report
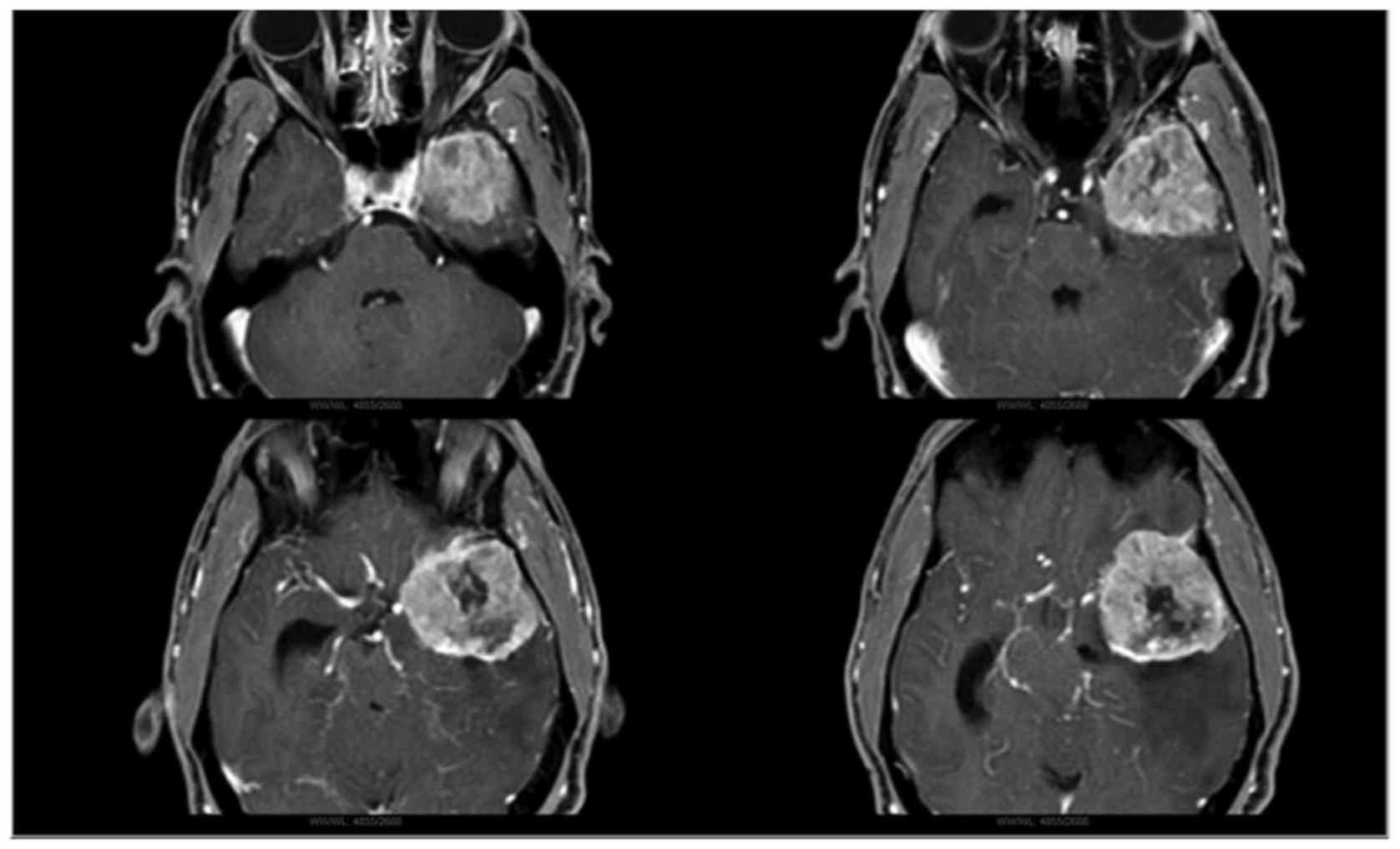
A 68-year-old male with a diagnosis of left sphenoid
wing meningioma, observed on brain magnetic resonance imagin (1.5T
device; Philips Medical Systems, Inc., Bothell, WA, USA), underwent
a Simpson II resection meningioma at Hospital das Clínicas,
University of Sao Paulo (Sao Paulo, Brazil) (Fig. 1). The patient experienced a good
post-operative clinical course and there were no signs of tumor
recurrence on imaging. However, 3 years after the resection, the
patient was admitted to the Emergency Department of the same
hospital complaining of headaches, poor visual acuity in the left
eye and ipsilateral eyelid droop. These symptoms were present for 1
month. A neurological exam at admission revealed normal levels of
consciousness and cognition, with no sensory or motor deficits.
Upon cranial nerve evaluation, there was impairment of the visual
acuity on the left side, associated with ipsilateral oculomotor
nerve palsy. The visual fields were preserved and no other cranial
nerve was altered.
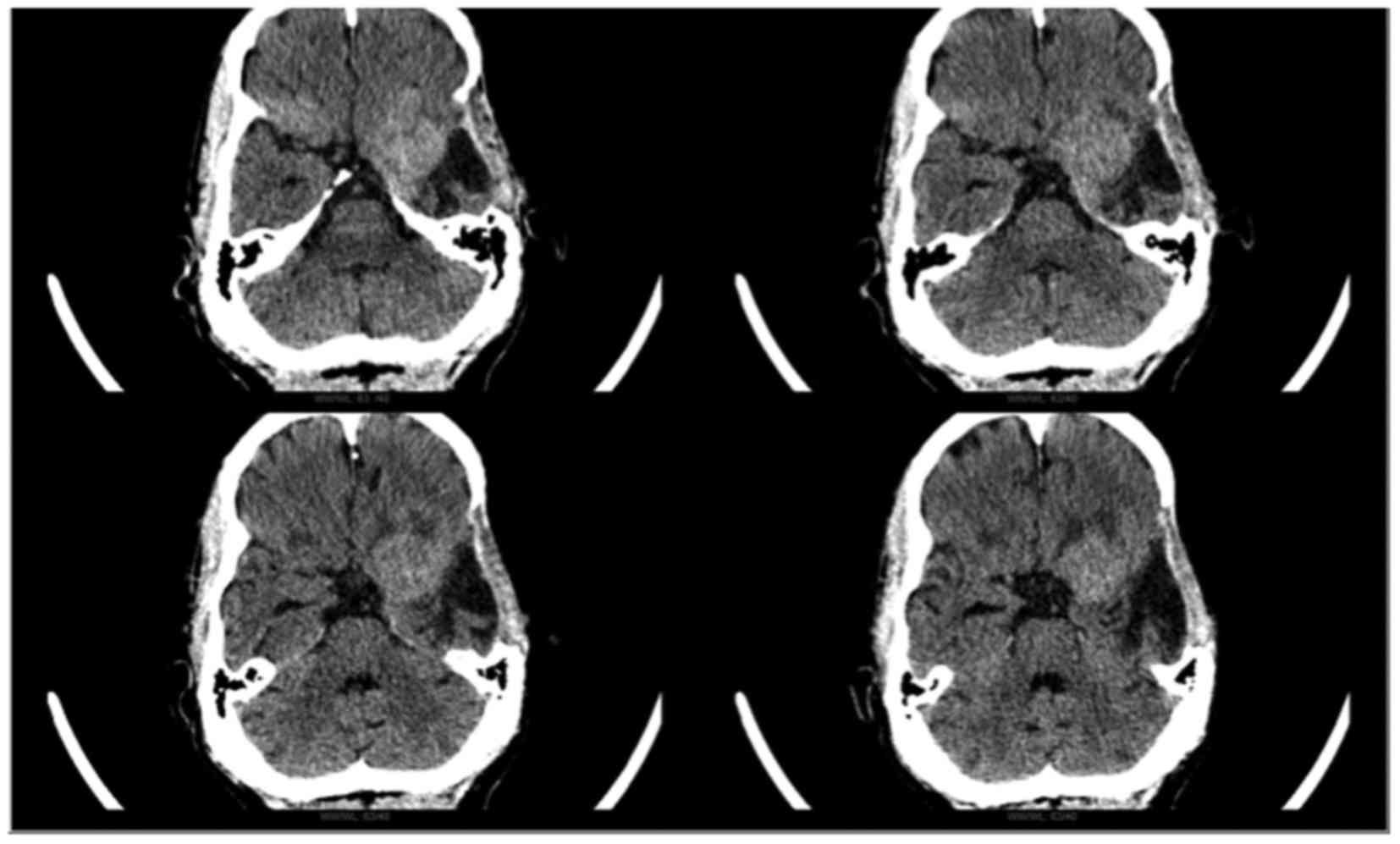
Head computed tomography (General Electric,
Milwaukee, WI, USA) revealed a hyperdense area suggestive of a
recurrent left sphenoid wing meningioma (Fig. 2). Gross total resection was performed
through the previous craniectomy access. A large gliotic area with
a cyst and enlarged subarachnoid space facilitated access to the
lesion. The tumor presented a fibrous aspect and bled profusely.
The tumor adhered to the middle fossa and left sphenoid wing, and
was resected with no complications. The intraoperative tumor aspect
was suggestive of a meningioma: A well-circumscribed yellowish
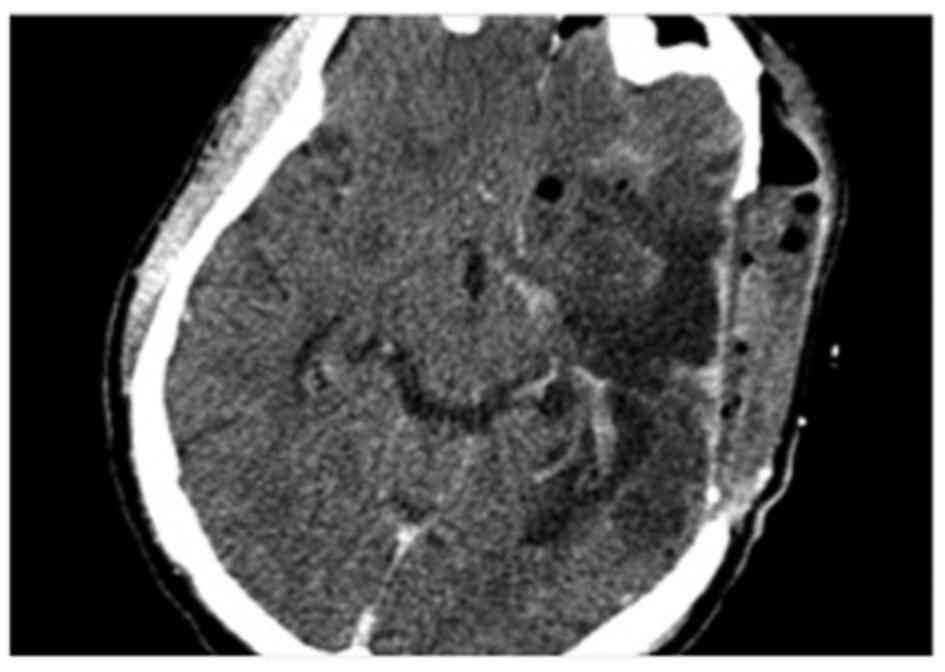
extra-axial lesion attached to dura-matter. Post-operative axial
head computed tomography (CT) scan was performed and showed
complete tumor removal (Fig. 3). Due
to septic shock from pneumonia on postoperative course and the
patient succumbing to the disease two months after surgery,
systemic cancer therapy was not performed.
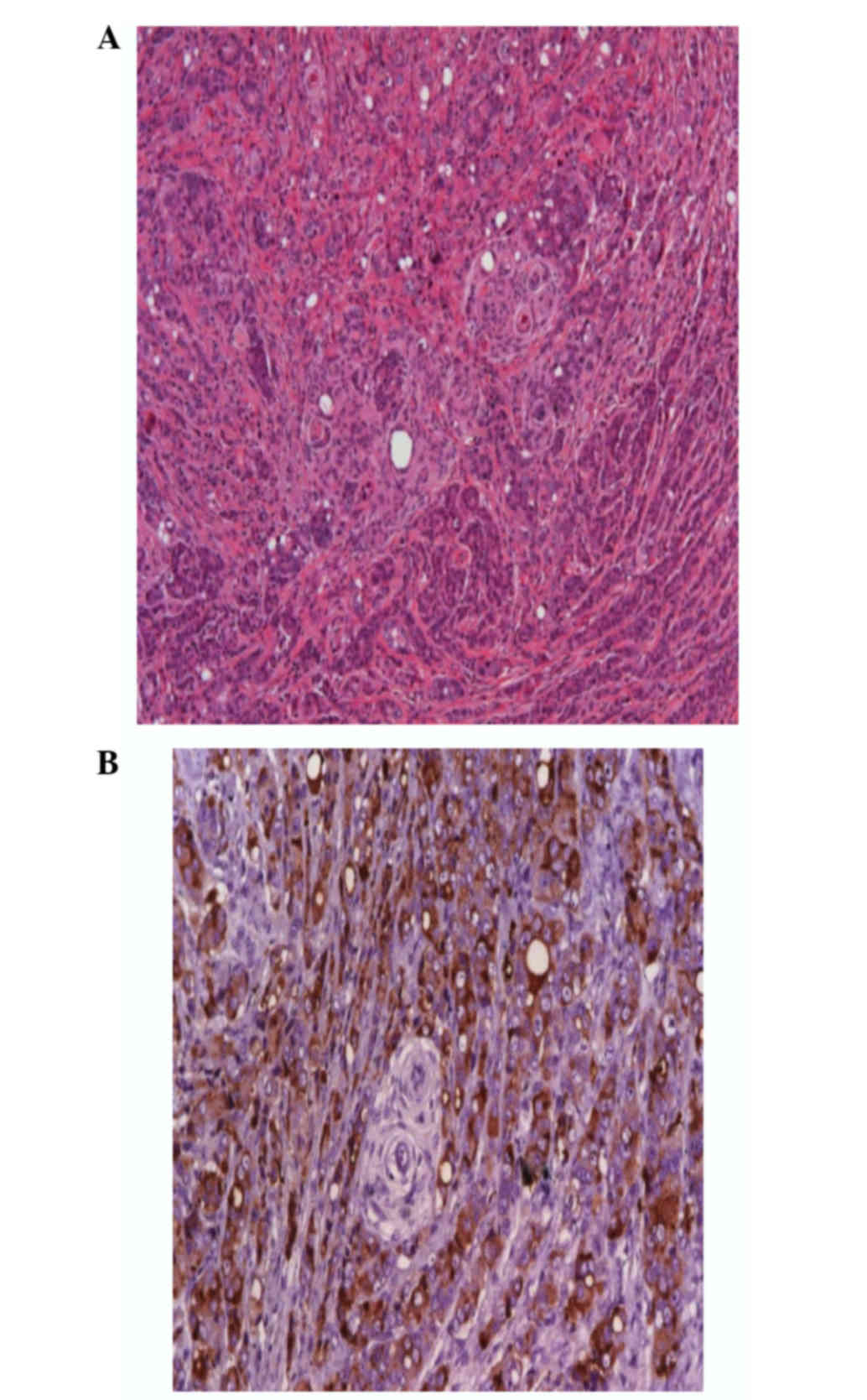
In the histological examination, the tumor was
identified as a metastatic adenocarcinoma inside a transitional
meningioma (Fig. 4). The surgical
specimen consisted of brownish tumor fragments totaling 4.2×3.8×0.8
cm with fibroelastic consistency. All the fragments underwent
fixation with formalin and histological examination. The
immunohistochemical examination favored a prostatic primary site,
with the following results: Negative reactivity for caudal-type
homeobox-2, cytokeratin 20, cytokeratin 7, thyroid transcription
factor-1 and vimentin, and positivity for epithelial membrane
antigen, prostatic acid phosphatase and prostate-specific antigen
(PSA). Subsequent investigation revealed a PSA level of 1,295 ng/ml
(reference value, <4.0 ng/ml) and signs of advanced metastatic
prostate disease on cancer staging.
Discussion
Prostate cancer TMM is a rare condition. To the best
of our knowledge, the present case is only the eighth case reported
in the literature. Table I summarizes
all the cases to date. The most commonly encountered malignant
donor tumor is lung adenocarcinoma in males and breast
adenocarcinoma in females, the latter mostly arising in women in
their fifth and sixth decades of life (7,8). In a
recent review of the literature (2),
114 cases of TMM were reported, with 54 breast cancer cases and 23
lung cancer cases, representing 47.4 and 20.2% of the total,
respectively.
 | Table I.Previously reported cases of prostate
cancer TMM, regarding clinical information, neuroimaging features
and histology. |
Table I.
Previously reported cases of prostate
cancer TMM, regarding clinical information, neuroimaging features
and histology.
| A, Autopsy
reports |
|---|
|
|---|
| Author, year | Age |
| Autopsy findings | (Ref.) |
|---|
| Döring, 1975 | 73 |
| Autopsy report:
Sudden enlargement of the meningioma leading to pressure on the
brain-stem, consequently causing a rapidly fatal disease course.
The patient succumbed at 10 h post-admission to hospital. | (10) |
|---|
| Chambers et
al, 1980 | 67 |
| Five cases of
carcinoma metastatic to intracranial meningioma or neurilemoma are
presented. Only one patient had TMM originating from metastatic
prostate carcinoma. Imaging study revealed hipodensity within
meningioma. | (11) |
|---|
|
| B, Treatment
reports |
|
| Author, year | Age | Signs/symptoms | Neuroimaging | Cancer staging | Histology | (Ref.) |
|
| Bernstein et
al, 1983 | 55 | 2-week history of
progressive left hemiparesis, recent onset of associated morning
nausea. | CT revealed a
calcified globoid mass with increased density at the right
parasagittal region, which enhanced with contrast material. A rim
of edema and a minimal shift of the midline were noted. | Metastatic lesions to
the spinal column, cranium, sternum and ribs. | Two distinct tissue
types: Dense fibrous/endotheliomatous meningioma, and a moderately
well-differentiated adenocarcinoma. Necrotic and hemorrhagic
centers were noted, and the malignant tumor appeared to insinuate
between meningeal cells in certain areas. | (9) |
| Cluroe, 2006 | 78 | Progressive lower
limb weakness, which partially responded to steroid therapy. | CT scan revealed a
parafalcine tumor extending into the brain on each side of the
falx, consistent with meningioma. | Not complete: Patient
had been treated elsewhere for prostate cancer 8 years
previously. | Two distinct
histological patterns: One pattern consisted of groups and nests of
epithelioid cells, with an increased mitotic index of 20/10
high-power fields. The second pattern consisted of more
typicalmeningiomatous area. Positive expression for EMA in the
meningiomatous and epithelioid areas. The distinct pattern of CK
staining confirmed the suspicion of two lesions, namely, a
carcinoma within a meningioma. PSA stain showed strong positivity
of the epithelioid areas only. | (12) |
| Pugsley et al,
2009 | 70 | 2 to 3-week history
of left-sided facial droop, left-sided weakness and subsequently
slurred speech. Headaches for several weeks prior. | 8.3-cm lobulated
dural lesion in the right parietal area with significant mass
effect and moderate midline shift to the left. | Two-year history of
metastatic prostate cancer | Two distinct
neoplasms arising from the dura: Meningothelial meningioma and
metastatic prostate adenocarcinoma. The meningioma was composed of
cells disposed in meningothelial nests and whorls with rare
psammoma bodies. The tumor was infiltrated by irregular nests and
cords of epithelial cells that stained for PSA. | (13) |
| Moody et al,
2012 | 58 | Numbness and
progressive weakness of the left toes and foot. | Dural-based,
2.4×2.6-cm lesion located in the area of the primary motor cortex
in the right frontal region with a significant amount of vasogenic
edema. | Metastatic prostate
cancer (Gleason 8) | PSA and PSAP
immunoreactive adenocarcinoma component admixed within a classic
meningioma. | (6) |
|
| 57 | New onset dizziness,
loss of concentration during driving and conversation, and weakness
in the right arm. | MRI showing a large,
6-cm left frontal mass containing blood, adjacent to a prominent
area of calvarial hyperostosis, with mass effect and surrounding
vasogenic edema. | Metastatic prostate
cancer (Gleason 8) | Metastatic prostate
carcinoma surrounded by meningioma areas. The former staining
strongly positive for CK (Cam 5.2) and PSA, intermingled within the
meningioma. |
|
Although cerebral metastases are by far the most
common intracranial tumors, the occurrence of TMM is still
considered a rare condition (2).
According to a study by Barz, among 8,371 autopsies, 4 cases
(0.05%) of a carcinoma metastasizing into a meningioma were found
(14). Notably, the yielding of less
common primary sites of the genitourinary system to such metastasis
has been reported, including in the kidneys and prostate (2).
Epidemiological and pathophysiological mechanisms
have been proposed to explain the meningioma's higher propensity to
harbor extracranial metastatic lesions. Table II outlines the various reasons
proposed. Apparently, a combination of epidemiological aspects and
the biology of meningiomas explain, at least in part, this
association. Epidemiological studies have shown that metastatic
cancer and meningioma occur simultaneously with high frequencies
during aging (15). The benign and
slow growth rate associated with high collagen and lipid content,
characteristic elements of meningiomas, render a favorable,
non-competitive environment for metastasis to develop (16). Another important point concerns the
hypervascularity of meningiomas, which may enhance the chances of
the meningioma to receive hematogenous metastasis (4,17). In
cases of breast cancer TMM, two relevant factors have been
postulated to play a role in metastatic seeding: i) High expression
of cell adhesion molecules, such as E-cadherin (18,19), and
ii) hormonal factors, particularly estrogen and progesterone
receptor expression (20,21). Lastly, overexpression of oncogenes in
meningioma and metastatic carcinoma could contribute to the
simultaneous occurrence (22,23).
 | Table II.Epidemiological and
pathophysiological mechanisms suggested for the predilection of
meningioma to harbor metastasis. |
Table II.
Epidemiological and
pathophysiological mechanisms suggested for the predilection of
meningioma to harbor metastasis.
| Proposed
mechanism | (Ref.) |
|---|
| High incidence of
meningioma among intracranial neoplasms | (15) |
| Increase in
incidence with age, as occurs with metastasis | (15) |
| Benign and slow
growth rate | (16) |
|
Hypervascularity |
| High collagen
content |
|
| High lipid
content |
|
| High expression of
cell adhesion molecules, such as E-cadherin | (15) |
| Overexpression of
oncogenes (e.g., c-Myc) |
|
| Hormonal factors
(estrogen and progesteron receptors) |
|
It is crucial to differentiate TMM from the
occurrence of collision tumors, which are due to the growth of two
isolated tumors that successively collide (24). In order to discriminate between these
two conditions, in 1968, Campbell et al (25) proposed four criteria for the diagnosis
of tumor-to-tumor metastasis, while in 1984, Pamphlett (26) depicted two diagnostic criteria for
TMM: i) At least partial enclosure of the metastatic focus by a rim
of benign histologically distinct host tumor tissue; and ii) the
proven existence of metastasizing primary carcinoma compatible with
the metastasis. The present case fulfills these criteria.
Even as the second most common cancer worldwide,
prostate cancer rarely metastasizes to the brain, with only
0.2–0.6% of such cases (9,27–29). In
general, central nervous system involvement is restricted to the
spine, involving the perineural and capsular lymphatic system, as
well as Batson's venous plexus (29).
Furthermore, certain studies have reported dural metastasis from
prostate cancer simulating meningiomas (30–34).
However, the present case represents a distinct entity: The
metastatic prostate cancer is surrounded by the recipient, benign
tumor (meningioma), meeting the aforementioned criteria (26) for ‘true’ TMM.
The present case has another distinctive feature in
that the TMM was the first clinical manifestation of an occult
primary prostate cancer. The histology report triggered a systemic
investigation, leading to the diagnosis of disseminated cancer.
However, the majority of the TMM cases reported to date had a
previous diagnosis of metastatic disease, rendering a pre-surgical
hypothesis of TMM (6,7,35).
Conversely, Caroli et al (8)
reported 3 cases of TMM in patients with occult cancer. Determining
the occurrence of TMM in a patient without a previous history of
metastatic disease remains a major challenge.
Clinical and neuroimaging features may be pivotal in
the pre-operative suspicion of TMM. From the clinical point of
view, three factors commonly associated with TMM were identified in
the present study: i) A previously known history of metastatic
disease, ii) uncontrolled systemic disease and iii) a rapid
deterioration of neurological condition. However, none of these
factors are specific for TMM, making a pre-operative diagnosis
unlikely. Besides clinical information, neuroimaging may be
extremely useful for assessing these patients. Table III highlights the most notable
characteristics associated with TMM. CT findings are described as
either a hyperdense or hypodense (when associated with necrosis)
area, which does not provide much information. However, atypical
changes of signal on routine MRI can suggest the coexistence of two
different tumors (6,8). The use of specialized, physiology-based
neuroimaging modalities, such as perfusion MRI (pMRI), magnetic
resonance spectroscopy and positron-emission tomography-CT
(PET-CT), may result in a superior diagnostic yield for this
condition (7,8). pMRI can potentially reveal two different
curve characteristics of regional cerebral blood volume, suggesting
different intratumoral vascular properties (36). When differentiating malignant tumors
from benign tumors, N-acetylaspartate (NAA)/choline (Cho), NAA/Cho
+ creatine (Cr), lactate/Cr and alanine/Cr ratios are useful.
Increased lipid/Cr and alanine/Cr ratios may enable metastasis and
meningiomas to be distinguished from other tumors. The most
striking characteristics of metastasis are a decrease in NAA, a
prominent increase in Cho and lactate lipid peaks, and a high
lipid/Cr ratio (37). Fukushima et
al (38) used PET-CT with
fluorodeoxyglucose and the positron-emitting radioactive isotope
fluorine-18 in a patient with lung adenocarcinoma metastasizing to
vestibular schwannoma that was suspected on pre-operative imaging
studies. Despite this not being a meningioma case, the rationale of
using a neuroimaging technique to assess the metabolic pattern of a
tumor may be applied for tumor-to-tumor metastasis (TTM) cases. The
tumor-to-normal ratio, one of the parameters used, was markedly
high, which offered corroborating evidence of TMM.
 | Table III.Neuroimaging features frequently
associated with TMM. |
Table III.
Neuroimaging features frequently
associated with TMM.
| Diagnostic
technique | Feature |
|---|
| CT | Hyperdense
lesion |
|
| Hypodense lesion
(when associated with necrosis) |
| MRI |
|
| Routine
MRI | Atypical signal
changes suggesting the presence of another tumor within a
meningioma. |
|
pMRI | Marked increase in
rCBV within a tumor. May reveal different curve characteristics
suggesting distinct intratumoral vascular properties. |
|
MRS | Increase in
alanine/Cr ratio is highly suggestive of a meningioma. A decrease
in NAA, a prominent increase in Cho and lactate lipid peaks, and a
high lipid/Cr ratio favor metastasis. |
| PET-CT | FDG may play a role
in the pre-operative diagnosis of TMM. An increase in T:N ratio may
be observed. |
The pre-operative high suspicion of TMM can modify
the treatment of TMM when compared with routine meningioma
excisions. Taking into consideration the possibility of TMM,
surgeons may adopt different surgical approaches. Likewise,
pathologists may look differently and systematically at the entire
tumor, reducing the chance of misdiagnosis if the TMM hypothesis
was not taken into account (7).
The present study is a case report of a patient with
a more likely preoperative diagnosis of a benign tumor based on
imaging studies that had a metastatic prostate cancer with
unfavorable postoperative course. In such cases, physicians caring
for patients with brain tumors should be familiar with TMMs, since
the preoperative diagnosis remains challenging for the majority of
cases. Clinical and neuroimaging features are crucial for this
purpose, giving further consideration to this hypothesis. However,
clinical and radiological findings are neither sensitive nor
specific for TMM diagnosis, and being subjected to
histopathological study is the only definitive diagnostic method.
Finally, the effort of forming a preoperative suspicion can be
rewarding, allowing the more appropriate conduct of the
professionals involved with the patient care.
References
|
1
|
Fried BM: Metastatic inoculation of a
meningioma by cancer cells from a bronchiogenic carcinoma. Am J
Pathol. 6:47–52.1. 1930.PubMed/NCBI
|
|
2
|
Erdogan H, Aydin MV and Tasdemiroglu E:
Tumor-to-tumor metastasis of the central nervous system. Turk
Neurosurg. 24:151–162. 2014.PubMed/NCBI
|
|
3
|
Petraki C, Vaslamatzis M, Argyrakos T,
Petraki K, Strataki M, Alexopoulos C and Sotsiou F: Tumor to tumor
metastasis: Report of two cases and review of the literature. Int J
Surg Pathol. 11:127–135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lanotte M, Benech F, Panciani PP, Cassoni
P and Ducati A: Systemic cancer metastasis in a meningioma: Report
of two cases and review of the literature. Clin Neurol Neurosurg.
111:87–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmitt HP: Metastases of malignant
neoplasms to intracranial tumours: The ‘tumour-in-a-tumour’
phenomenon. Virchows Arch A Pathol Anat Histopathol. 405:155–160.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moody P, Murtagh K, Piduru S, Brem S,
Murtagh R and Rojani AM: Tumor-to-tumor metastasis: Pathology and
neuroimaging considerations. Int J Clin Exp Pathol. 5:367–373.
2012.PubMed/NCBI
|
|
7
|
Sayegh ET, Henderson GA, Burch EA, Reis
GF, Cha S, Oh T, Bloch O and Parsa AT: Intrameningioma metastasis
of breast carcinoma. Rare Tumors. 6:53132014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caroli E, Salvati M, Giangaspero F,
Ferrante L and Santoro A: Intrameningioma metastasis as first
clinical manifestation of occult primary breast carcinoma.
Neurosurg Rev. 29:49–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bernstein RA, Grumet KA and Wetzel N:
Metastasis of prostatic carcinoma to intracranial meningioma. Case
report. J Neurosurg. 58:774–777. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Döring L: Metastasis of carcinoma of
prostate to meningioma. Virchows Arch A Pathol Anat Histol.
366:87–91. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chambers PW, Davis RL, Blanding JD and
Buck FS: Metastases to primary intracranial meningiomas and
neurilemomas. Arch Pathol Lab Med. 104:350–354. 1980.PubMed/NCBI
|
|
12
|
Cluroe AD: Metastasis to meningioma: Clues
and investigation. Pathology. 38:76–78. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pugsley D, Bailly G, Gupta R, Wilke D and
Wood L: A case of metastatic adenocarcinoma of the prostate arising
in a meningioma. Can Urol Assoc J. 3:E4–E6. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barz H: The incidence of metastatic
carcinomas in meningiomas. A report of 4 cases. Zentralbl Allg
Pathol. 127:367–374. 1983.(In German). PubMed/NCBI
|
|
15
|
Schoenberg BS, Christine BW and Whisnant
JP: Nervous system neoplasms and primary malignancies of other
sites. The unique association between meningiomas and breast
cancer. Neurology. 25:705–712. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Richardson JF and Katayama I: Neoplasm to
neoplasm metastasis. An acidophil adenoma harbouring metastatic
carcinoma: A case report. Arch Pathol. 91:135–139. 1971.PubMed/NCBI
|
|
17
|
Best PV: Metastatic carcinoma in a
meningioma: Report of a case. J Neurosurg. 20:892–894. 1963.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watanabe T, Fujisawa H, Hasegawa M,
Arakawa Y, Yamashita J, Ueda F and Suzuki M: Metastasis of breast
cancer to intracranial meningioma: Case report. Am J Clin Oncol.
25:414–417. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimada S, Ishizawa K and Hirose T:
Expression of E-cadherin and catenins in meningioma: Ubiquitous
expression and its irrelevance to malignancy. Pathol Int. 55:1–7.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Doyle S, Messiou C, Rutherford JM and
Dineen RA: Cancer presenting during pregnancy: Radiological
perspectives. Clin Radiol. 64:857–871. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grann VR, Troxel AB, Zojwalla NJ, Jacobson
JS, Hershman D and Neugut AI: Hormone receptor status and survival
in a population-based cohort of patients with breast carcinoma.
Cancer. 103:2241–2251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kozbor D and Croce CM: Amplification of
the c-myc oncogene in one of five human breast carcinoma cell
lines. Cancer Res. 44:438–441. 1984.PubMed/NCBI
|
|
23
|
Elmaci L, Ekinci G, Kurtkaya O, Sav A and
Pamir MN: Tumor in tumor: Metastasis of breast carcinoma to
intracranial meningioma. Tumori. 87:423–427. 2001.PubMed/NCBI
|
|
24
|
Anlyan FH, Heinzen BR and Carras R:
Metastasis of tumor to second different tumor: Collision tumors.
JAMA. 212:21241970. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Campbell LV Jr, Gilbert E, Chamberlain CR
Jr and Watne AL: Metastases of cancer to cancer. Cancer.
22:635–643. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pamphlett R: Carcinoma metastasis to
meningioma. J Neurol Neurosurg Psychiatry. 47:561–563. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lynes WL, Bostwick DG, Freiha FS and
Stamey TA: Parenchymal brain metastases from adenocarcinoma of
prostate. Urology. 28:280–287. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tremont-Lukats IW: Brain metastasis from
prostate carcinoma: The M.D. Anderson cancer center experience.
Cancer. 98:363–368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Catane R, Kaufman J, West C, Merrin C,
Tsukada Y and Murphy GP: Brain metastasis from prostatic carcinoma.
Cancer. 38:2583–2587. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lyons MK, Drazkowski JF, Wong WW, Fitch TR
and Nelson KD: Metastatic prostate carcinoma mimicking meningioma:
Case report and review of the literature. Neurologist. 12:48–52.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lippman SM, Buzaid AC, Iacono RP,
Steinbronn DV, Stanisic TH, Rennels MA, Yang PJ, Garewal HS and
Ahmann FR: Cranial metastases from prostate cancer simulating
meningioma: Report of two cases and review of the literature.
Neurosurgery. 19:820–823. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tagle P, Villanueva P, Torrealba G and
Huete I: Intracranial metastasis or meningioma? An uncommon
clinical diagnostic dilemma. Surg Neurol. 58:241–245. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kirkwood JR, Margolis MT and Newton TH:
Prostatic metastasis to the base of the skull simulating meningioma
en plaque. Am J Roentgenol Radium Ther Nucl Med. 112:774–778. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fink LH: Metastasis of prostatic
adenocarcinoma simulating a falx meningioma. Surg Neurol.
12:253–258. 1979.PubMed/NCBI
|
|
35
|
Wong A, Koszyca B, Blumbergs PC, Sandhu N
and Halcrow S: Malignant melanoma metastatic to a meningioma.
Pathology. 31:162–165. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jun P, Garcia J, Tihan T, McDermott MW and
Cha S: Perfusion MR imaging of an intracranial collision tumor
confirmed by image-guided biopsy. AJNR Am J Neuroradiol. 27:94–97.
2006.PubMed/NCBI
|
|
37
|
Bulakbasi N, Kocaoglu M, Ors F, Tayfun C
and Uçöz T: Combination of single-voxel proton MR spectroscopy and
apparent diffusion coefficient calculation in the evaluation of
common brain tumors. AJNR Am J Neuroradiol. 24:225–233.
2003.PubMed/NCBI
|
|
38
|
Fukushima Y, Ota T, Mukasa A, Uozaki H,
Kawai K and Saito N: Tumor to tumor metastasis: Lung adenocarcinoma
metastasizing to vestibular schwannoma suspected on preoperative
[18F]-fluorodeoxyglucose positron emission tomography imaging.
World Neurosurg. 78:553.e9–553.e13. 2012. View Article : Google Scholar
|