Introduction
Papillary thyroid carcinoma (PTC) is one of the most
common types of endocrine malignancy and its prevalence is
increasing. The incidence of PTC has continually increased between
1973–1977 and 1998–2002 (1). With
increased awareness of thyroid nodular disease and advancements in
ultrasonography, impalpable small papillary thyroid microcarcinomas
(PTMCs) have been frequently detected. PTMC is defined as an
incidental PTC with a maximum diameter ≤1 cm by the World Health
Organization (WHO) (2). Within the
past two decades, the incidence of PTMC among all thyroid cancers
has nearly tripled in France (3).
Patients with PTMC typically have a good prognosis, with a
relatively low risk of developing distant metastases, resulting in
reported mortality rates for PTMC as low as 0.5% (4). Although the prevalence of PTMC has
increased in recent years, thyroid cancer-related mortality has not
changed (5). The results from a
meta-analysis of over 4,000 patients with PTMC demonstrated 28%
lymph node metastasis, 0.6% distant metastasis, 3.3% disease
recurrence and 0.3% tumor mortality incidences (6). These results suggest that the majority
of PTMCs are indolent tumors that may not progress. Identifying the
minority of patients with aggressive PTMCs is important in order to
offer the most appropriate treatment.
Research into B-Raf proto-oncogene serine/threonine
kinase (BRAF)V600E mutations in PTC have increased in
recent years. The activating somatic point mutation
BRAFV600E is the most frequent genetic alteration found
in PTC and is a prognostic biomarker for the aggressive behavior of
PTCs (7). BRAFV600E
mutation represents >90% of all the mutations found in the BRAF
gene (8,9). BRAFV600E mutation occurs in
between 29 and 83% of PTC cases (average, 45%), depending on the
population and geographical region being examined (8,10,11). The BRAFV600E mutation
occurs frequently in stage III and IV tumors (12).
Previous studies have reported an association
between BRAFV600E mutation and the severity of the
clinicopathological features exhibited by patients with PTC,
including those with multifocal disease and PTMC. However, to the
best of our knowledge, an association between BRAFV600E
mutation and solitary PTMC (sPTMC) has not yet been reported. In
the present study, the association between BRAFV600E
mutation and the clinicopathological features of patients with
sPTMC was analyzed in order to elucidate the molecular mechanisms
underlying the aggressive behavior of this tumor.
Materials and methods
Patients
The present study was retrospective and all patient
data were kept anonymous. The data of 108 patients with sPTMC who
had undergone thyroid surgery between December 2010 and December
2012 at the Cancer Institute and Hospital of the Chinese Academy of
Medical Sciences (Beijing, China) was reviewed. The diagnosis of
sPTMC was pathologically confirmed in all patients based on the
specimen size (maximum diameter ≤10 mm) and the presence of a
solitary PTMC tumor. The majority of patients underwent a lobectomy
and isthmus gland resection under general anesthesia. Central
compartment neck dissection was performed when an enlarged lymph
node or invasion of the thyroid capsule was detected during
surgery. All specimens were fixed in formalin and embedded in
paraffin. In the present study, archived histological specimens
from all patients were retrieved and analyzed. Specimen size and
the presence of a solitary tumor was confirmed through hematoxylin
and eosin staining. Other tumor features, such as histopathological
subtype were classified based on the World Health Organization
classification of tumors (2). Tumor
staging was performed according to the seventh edition of the
tumor-node-metastasis (TNM) classification by the American Joint
Committee on Cancer (13).
Patient medical histories were analyzed, including
their age at diagnosis, gender, lobe position of the tumor, primary
tumor diameter, histological subtype of PTC, lymph node status and
TNM stage. The tumors of all patients in the cohort were tested for
the BRAFV600E mutation.
DNA extraction and
BRAFV600E mutation analysis
Areas of the specimens that were enriched in tumor
cell populations was marked through hematoxylin and eosin staining
prior to analysis. Tissue was scraped from this preselected area
and transferred to an Eppendorf tube for DNA isolation using the
QIAamp® DNA Mini kit (Qiagen GmbH, Hilden, Germany) in
accordance with the manufacturer's protocol. The quality and
concentration of DNA samples was examined using a NanoDrop 1000
spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Exon 15 of the BRAF gene was amplified using PCR as
previously described (14).
Sequencing of the PCR products was performed using the BigDye
Terminator v3.1 Cycle Sequencing kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with the same primers. Sequencing reaction
products were put through capillary electrophoresis using a 3500xL
Genetic Analyzer (Applied Biosystems; Thermo Fisher Scientific,
Inc.).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 16.0; SPSS Inc., Chicago, IL, USA). Pearson's
Chi-squared or Fisher's exact tests were used to compare the
association between BRAFV600E mutations and
clinicopathological features. P<0.05 was considered to indicate
a statistically significant difference.
Results
Clinicopathological characteristics of
patients with sPTMC
Clinicopathological characteristics of the patients
are illustrated in Table I. There
were 27 (25%) male and 81 (75%) female patients in the present
study. The mean age of the patients was 42 years old (range, 22–66
years). There were 65 patients <45 years old (60.2%) and 43
patients ≥45 years old (39.8%). The average tumor size was 7.2 mm
(range, 2–10 mm). The number of patients with a tumor located in
the left lobe, right lobe or isthmus was 59 (54.6%), 45 (41.7%) and
4 (3.7%), respectively. Extrathyroid extension was present in 47
patients (43.5%). In total, 97 (89.8%) and 11 (10.2%) patients had
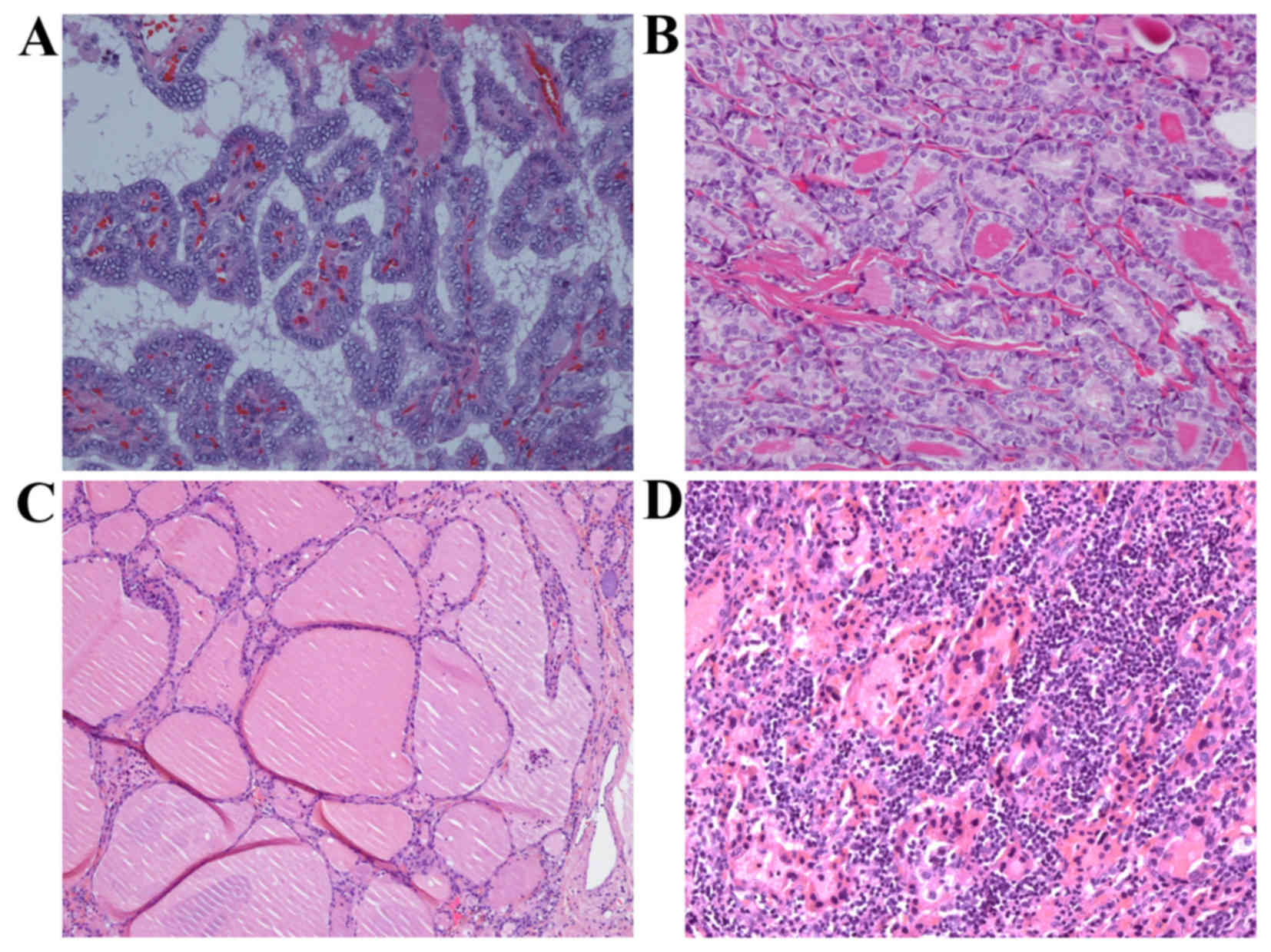
classic and follicular tumor subtypes, respectively (Fig. 1A and B). The lymph node status of 94
patients who underwent neck dissection was identified as the
following: N0 (n=52; 48.1%); N1a (n=32; 29.6%); and N1b (n=10;
9.3%). Nx lymph node status was identified in the remaining 14
patients (13.0%). Patient thyroid backgrounds were nodular goiter
(n=38; 35.2%), Hashimoto's thyroiditis (HT; n=5; 4.6%), lymphocytic
thyroiditis (LT; n=26; 24.1%), granulomatous thyroiditis (n=1;
0.9%) and normal (n=38; 35.2%) (Fig. 1B
and C). Sixty-five patients (60.2%) had T1 stage tumors, none
had T2 and 43 (39.8%) had T3 stage tumors. Patients' TNM stage was
separated into group I (n=83; 76.9%), group III (n=19; 17.6%) and
group IVa (n=6; 5.5%).
 | Table I.Clinicopathological characteristics of
patients with solitary papillary thyroid microcarcinomas. |
Table I.
Clinicopathological characteristics of
patients with solitary papillary thyroid microcarcinomas.
| Clinicopathological
characteristic | No. of patients
(%) |
|---|
| Age (years old) |
|
|
<45 | 65 (60.2) |
| ≥45 | 43 (39.8) |
| Gender |
|
| Male | 27 (25) |
|
Female | 81 (75) |
| Anatomic
location |
|
| Left | 59 (54.6) |
|
Right | 45 (41.7) |
|
Isthmus | 4 (3.7) |
| Tumor size (mm) |
|
| ≤5 | 15 (13.9) |
|
>5 | 93 (86.1) |
| Extrathyroid
extension |
|
|
Absent | 61 (56.5) |
|
Present | 47 (43.5) |
| Perineural
invasion |
|
|
Absent | 93 (86.1) |
|
Present | 15 (13.9) |
| Histological
subtype |
|
|
Classic | 97 (89.8) |
|
Follicular | 11 (10.2) |
| Tumor stage |
|
| T1 | 65 (60.2) |
| T3 | 43 (39.8) |
| Node stage |
|
| Nx | 14 (13.0) |
| N0 | 52 (48.1) |
| N1a | 32 (29.6) |
| N1b | 10 (9.3) |
| TNM stage |
|
| I | 83 (76.9) |
| III | 19 (17.6) |
| IVa | 6 (5.5) |
| Thyroid
background |
|
| Nodular
goiter | 38 (35.2) |
|
Hashimoto's thyroiditis | 5 (4.6) |
|
Lymphocytic thyroiditis | 26 (24.1) |
|
Granulomatous thyroiditis | 1 (0.9) |
|
Normal | 38 (35.2) |
Association between the
clinicopathological characteristics of patients with sPTMC and
BRAFV600E mutation
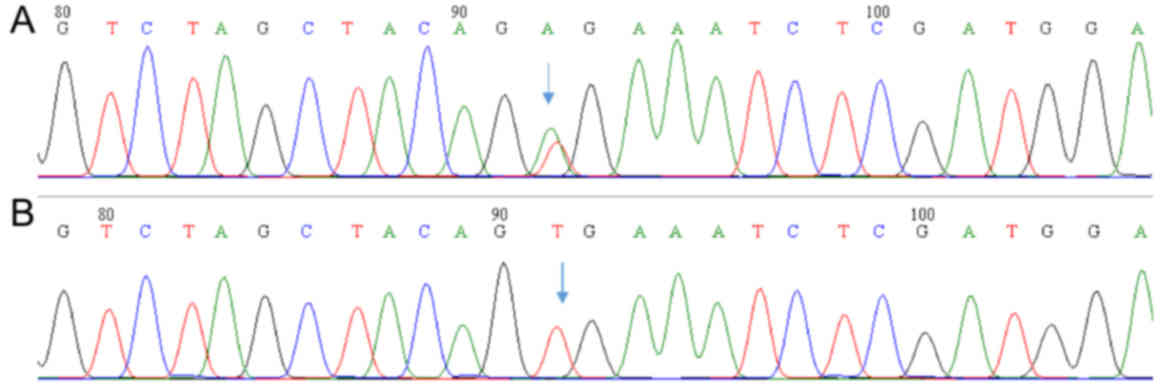
The BRAFV600E mutation was observed in 59
out of 108 patients (54.6%) through sequencing (Fig. 2). Table
II demonstrates the association between clinicopathological
characteristics and BRAFV600E mutation in sPTMC. The
following four clinicopathological characteristics were
significantly associated with BRAF mutation: Extrathyroid extension
(P=0.019, Pearson's Chi-squared test), tumor stage (P=0.032,
Pearson's Chi-squared test), advanced TNM stage groups (III/IVa;
P=0.007, Fisher's exact test) and thyroid background (P=0.010,
Fisher's exact test) There were more cases of the
BRAFV600E mutation in sPTMC where extrathyroid extension
was present compared with sPTMC where it was absent [32/47, (68.1%)
vs. 27/61, (44.3%)]. Of the patients, there were 65 with T1a
[BRAFV600E, n=30 (46.2%)] and 43 with T3
[BRAFV600E, n=29 (67.4%)]. In addition, out of the
patients 83 had TNM stage I [BRAFV600E, n=39 (47%)], 19
had TNM stage III [BRAFV600E, n=16 (84.2%)] and 6 had
TNM stage IVa [BRAFV600E, n=4 (66.7%)] sPTMCs.
BRAFV600E mutation frequency was higher in advanced
sPTMCs compared with early stage sPTMCs. The frequency of the
BRAFV600E mutation in patients with a normal thyroid
background (n=28; 73.7%) was higher compared with those with a
background of HT (n=1; 20%) or LT (n=10; 38.5%). Histological
examination identified 97 classic and 11 follicular sPTMCs among
the patients. The frequencies of the BRAFV600E mutation
were 55.7 and 45.5% (54/97 vs. 5/11) in patients with classic and
follicular sPTMCs, respectively. The mutation was more frequently
identified in classic subtype tumors compared with follicular
variants. However, statistical analysis showed no significant
difference in BRAFV600E mutation frequency between these
groups. The follicular sPTMC variant was uncommon in the study
group (11/108; 10.2%). There were no other subtypes identified in
the current study.
 | Table II.Association between
clinicopathological characteristics and BRAFV600E
mutation in solitary papillary thyroid microcarcinoma. |
Table II.
Association between
clinicopathological characteristics and BRAFV600E
mutation in solitary papillary thyroid microcarcinoma.
|
| BRAF gene
status |
|
|---|
|
|
|
|
|---|
| Clinicopathological
characteristic | Wild-type [no. of
patients/total (%)] | V600E mutation [no.
of patients/total (%)] | P-value |
|---|
| Age (years
old) |
|
| 0.081a |
|
<45 | 34/65 (52.3) | 31/65 (47.7) |
|
|
≥45 | 15/43 (34.9) | 28/43 (65.1) |
|
| Gender |
|
| 0.658a |
|
Male | 11/27 (40.7) | 16/27 (59.3) |
|
|
Female | 38/81 (46.9) | 43/81 (53.1) |
|
| Anatomic
location |
|
| 0.543b |
|
Left | 26/59 (44.1) | 33/59 (55.9) |
|
|
Right | 20/45 (44.4) | 25/45 (55.6) |
|
|
Isthmus | 3/4 (75) | 1/4 (25) |
|
| Tumor size
(mm) |
|
| 0.269a |
| ≤5 | 9/15 (60) | 6/15 (40) |
|
|
>5 | 40/93
(43) | 53/93 (57) |
|
| Fibrous capsular
invasion |
|
| 0.159a |
|
Absent | 13/22 (59.1) | 9/22 (40.9) |
|
|
Present | 36/86 (41.9) | 50/86 (58.1) |
|
| Extrathyroid
extension |
|
| 0.019a |
|
Absent | 34/61 (55.7) | 27/61 (44.3) |
|
|
Present | 15/47 (31.9) | 32/47 (68.1) |
|
| Perineural
invasion |
|
| 0.582a |
|
Absent | 41/93 (44.1) | 52/93 (55.9) |
|
|
Present | 8/15 (53.3) | 7/15 (46.7) |
|
| Histological
subtype |
|
| 0.541a |
|
Classic | 43/97 (44.3) | 54/97 (55.7) |
|
|
Follicular | 6/11 (54.5) | 5/11 (45.5) |
|
| Tumor stage |
|
| 0.032a |
|
T1a | 35/65 (53.8) | 30/65 (46.2) |
|
| T3 | 14/43 (32.6) | 29/43 (67.4) |
|
| Node stage |
|
| 0.495b |
| N0 | 21/52 (40.4) | 31/52 (59.6) |
|
|
N1a | 17/32 (53.1) | 15/32 (46.9) |
|
|
N1b | 4/10 (40) | 6/10 (60) |
|
| TNM stage |
|
| 0.007b |
| I | 44/83 (53) | 39/83 (47) |
|
|
III | 3/19 (15.8) | 16/19 (84.2) |
|
|
IVa | 2/6 (33.3) | 4/6 (66.7) |
|
| Thyroid
background |
|
| 0.010b |
| Nodular
goiter | 19/38 (50) | 19/38 (50) |
|
| HT | 4/5 (80) | 1/5 (20) |
|
| LT | 16/26 (61.5) | 10/26 (38.5) |
|
| GT | 0/1 | 1/1 (100) |
|
|
Normal | 10/38 (26.3) | 28/38 (73.7) |
|
Discussion
In the present study, BRAFV600E mutation
was present in 54.6% (59/108) of patients with sPTMC. This
frequency is higher when compared with another large Chinese study,
which reported a frequency of 40.1% that included multifocal cases
(15). Possible reasons for this
difference might be the detection method for BRAFV600E
mutation or the cases chosen for the studies. Guerra et al
(16) observed that the prevalence of
BRAFV600E mutation was higher when using pyrosequencing
than compared with BigDye Terminator sequencing. Another reason for
the differences in the results may be another effect of sPTMC that
was present in the multifocal cases. These results indicate that
BRAFV600E mutation is associated with prognostic factors
of sPTMC, including superficial (subscapular) tumor location and a
more advanced tumor stage. Similarly, other reports identified the
same association in PTC and PTMC (17–20).
BRAFV600E mutations have been identified in several
histological variants of PTC. The prevalence of
BRAFV600E mutations was higher in conventional PTC
(51–67.7%) compared with the follicular variant of PTC (17–24%)
(16,21–23). In
the current study, the prevalence of the BRAFV600E
mutation was not significantly associated with sPTMC subtype;
however, the prevalence was higher in the classic compared with the
follicular subtype (55.7 vs. 45.5%).
Several previous analyses have demonstrated that the
BRAFV600E mutation is a risk factor for disease
persistence or recurrence in PTC (21,22,24).
However, this could not be verified in the current study, as no
follow-up data was included in the analysis due to the positive
outcome of the patients.
The present study identified that the prevalence of
BRAFV600E mutation is lower in those with a background
of LT or HT compared with other conditions, such as nodular goiter
or a normal thyroid background. Lim et al (17) reported a similar result, observing
that PTC with a background of LT was significantly lower in those
with the BRAFV600E mutation compared with those with
wild-type BRAF (28.2 vs. 46.6%; P<0.001). In addition. Lang
et al (25) reported a
negative association between BRAFV600E mutation and HT.
The BRAFV600E mutation group in this study had
significantly lower rates of co-occurring HT compared with the
wild-type BRAF group (34.6 vs. 52.5%; P<0.001), which supports
the results found in the current study. These results suggest that
PTC with co-occurring HT or LT develop through a different
biological mechanism compared with PTCs in patients with other
thyroid backgrounds; however, this mechanism remains to be
elucidated (26). In future, the
respective roles and the association between BRAFV600E
mutation and thyroid background in the pathogenesis of PTC should
be evaluated.
In general, patients with PTC have a good prognosis.
Thus, the need for radioiodine ablation, the degree of thyroid
stimulating hormone suppression and the intervals between follow-up
visits are still in dispute, particularly in low-risk patients,
such as those with sPTMC. The results of the current study indicate
that the BRAFV600E mutation is associated with factors
that predict poor prognosis in patients with sPTMC. This suggests
that BRAFV600E mutation is a useful diagnostic and
prognostic marker for PTC. However, further investigations into the
underlying mechanisms and pathological diagnosis of PTMC are still
warranted. In future, molecular subtyping will be useful in
refining the risk stratification and personalizing the
postoperative management of patients with PTC (27).
Acknowledgements
The present study was supported by the Beijing Hope
Run Special Fund (grant no. LC2012B34).
References
|
1
|
Curado M, Edwards B, Shin H, Storm H,
Ferlay J, Heanue M and Boyle P: Cancer incidence in five
continents. IX. IARC Scientific Publications; Lyon: 2007
|
|
2
|
Lloyd R and De Lellis R P H: World health
organization classification of tumours: Pathology and genetics of
tumours of the endocrine organs. IARC Press International Agency
for Research on Cancer; Lyon, France: 2004
|
|
3
|
Leenhardt L, Grosclaude P and
Cherié-Challine L: Thyroid Cancer Committee: Increased incidence of
thyroid carcinoma in france: A true epidemic or thyroid nodule
management effects? Report from the French thyroid cancer
committee. Thyroid. 14:1056–1060. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roti E, Uberti EC degli, Bondanelli M and
Braverman LE: Thyroid papillary microcarcinoma: A descriptive and
meta-analysis study. Eur J Endocrinol. 159:659–673. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Besic N, Pilko G, Petric R, Hocevar M and
Zgajnar J: Papillary thyroid microcarcinoma: Prognostic factors and
treatment. J Surg Oncol. 97:221–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mazzaferri EL: Management of low-risk
differentiated thyroid cancer. Endocr Pract. 13:498–512. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xing M, Westra WH, Tufano RP, Cohen Y,
Rosenbaum E, Rhoden KJ, Carson KA, Vasko V, Larin A, Tallini G, et
al: BRAF mutation predicts a poorer clinical prognosis for
papillary thyroid cancer. J Clin Endocrinol Metab. 90:6373–6379.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xing M: BRAF mutation in thyroid cancer.
Endocr Relat Cancer. 12:245–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frasca F, Nucera C, Pellegriti G, Gangemi
P, Attard M, Stella M, Loda M, Vella V, Giordano C, Trimarchi F, et
al: BRAF(V600E) mutation and the biology of papillary thyroid
cancer. Endocr Relat Cancer. 15:191–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Riesco-Eizaguirre G and Santisteban P: New
insights in thyroid follicular cell biology and its impact in
thyroid cancer therapy. Endocr Relat Cancer. 14:957–977. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xing M: BRAF mutation in papillary thyroid
cancer: Pathogenic role, molecular bases, and clinical
implications. Endocr Rev. 28:742–762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu D, Liu Z, Condouris S and Xing M: BRAF
V600E maintains proliferation, transformation, and tumorigenicity
of BRAF-mutant papillary thyroid cancer cells. J Clin Endocrinol
Metab. 92:2264–2271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
AJCC, . American joint committee of
cancer, AJCC cancer staging manual. seventh. Springer; Chicago:
2010
|
|
14
|
Qiu T, Lu H, Guo L, Huang W, Ling Y, Shan
L, Li W, Ying J and Lv N: Detection of BRAF mutation in Chinese
tumor patients using a highly sensitive antibody
immunohistochemistry assay. Sci Rep. 5:92112015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng X, Wei S, Han Y, Li Y, Yu Y, Yun X,
Ren X and Gao M: Papillary microcarcinoma of the thyroid: Clinical
characteristics and BRAF(V600E) mutational status of 977 cases. Ann
Surg Oncol. 20:2266–2273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guerra A, Fugazzola L, Marotta V, Cirillo
M, Rossi S, Cirello V, Forno I, Moccia T, Budillon A and Vitale M:
A high percentage of BRAFV600E alleles in papillary thyroid
carcinoma predicts a poorer outcome. J Clin Endocrinol Metab.
97:2333–2340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lim JY, Hong SW, Lee YS, Kim BW, Park CS,
Chang HS and Cho JY: Clinicopathologic implications of the
BRAF(V600E) mutation in papillary thyroid cancer: A subgroup
analysis of 3130 cases in a single center. Thyroid. 23:1423–1430.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SJ, Lee KE, Myong JP, Park JH, Jeon
YK, Min HS, Park SY, Jung KC, Koo do H and Youn YK: BRAF V600E
mutation is associated with tumor aggressiveness in papillary
thyroid cancer. World J Surg. 36:310–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee X, Gao M, Ji Y, Yu Y, Feng Y, Li Y,
Zhang Y, Cheng W and Zhao W: Analysis of differential BRAF(V600E)
mutational status in high aggressive papillary thyroid
microcarcinoma. Ann Surg Oncol. 16:240–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi SY, Park H, Kang MK, Lee DK, Lee KD,
Lee HS, Kim SW, Lee EN and Hong JC: The relationship between the
BRAF(V600E) mutation in papillary thyroid microcarcinoma and
clinicopathologic factors. World J Surg Oncol. 11:2912013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JH, Lee ES and Kim YS:
Clinicopathologic significance of BRAF V600E mutation in papillary
carcinomas of the thyroid: A meta-analysis. Cancer. 110:38–46.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smith RA, Salajegheh A, Weinstein S,
Nassiri M and Lam AK: Correlation between BRAF mutation and the
clinicopathological parameters in papillary thyroid carcinoma with
particular reference to follicular variant. Hum Pathol. 42:500–506.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu H, Qiu T, Ying J, Guo C and Lyn N:
Correlation between BRAF V600E mutation and clinicopathologic
features of papillary thyroid carcinoma. Zhonghua Bing Li Xue Za
Zhi. 43:794–798. 2014.(In Chinese). PubMed/NCBI
|
|
24
|
Tufano RP, Teixeira GV, Bishop J, Carson
KA and Xing M: BRAF mutation in papillary thyroid cancer and its
value in tailoring initial treatment: A systematic review and
meta-analysis. Medicine (Baltimore). 91:274–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lang BH, Chai YJ, Cowling BJ, Min HS, Lee
KE and Youn YK: Is BRAFV600E mutation a marker for central nodal
metastasis in small papillary thyroid carcinoma? Endocr Relat
Cancer. 21:285–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SK, Song KH, Lim SD, Lim YC, Yoo YB,
Kim JS and Hwang TS: Clinical and pathological features and the
BRAF(V600E) mutation in patients with papillary thyroid carcinoma
with and without concurrent Hashimoto thyroiditis. Thyroid.
19:137–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niemeier LA, Akatsu H Kuffner, Song C,
Carty SE, Hodak SP, Yip L, Ferris RL, Tseng GC, Seethala RR, Lebeau
SO, et al: A combined molecular-pathologic score improves risk
stratification of thyroid papillary microcarcinoma. Cancer.
118:2069–2077. 2012. View Article : Google Scholar : PubMed/NCBI
|