Introduction
According to worldwide cancer statistics (Globocan
2012), lung cancer is the most commonly diagnosed cancer among men,
and in women it is the third most commonly diagnosed (1). Approximately 2 million new cases of lung
cancer and 1.5 million lung cancer related deaths are recorded each
year (2). Mortality due to lung
cancer is estimated to increase up to 10 million per year in
another 15 years (3). There are two
major types of lung cancer, viz., small cell lung cancer
(SCLC), which is less common accounting for ~20% of the cancer
cases, and non-small cell lung cancer (NSCLC), which is the most
common, accounting for almost 85% of lung cancer cases (4). Despite the high incidence and associated
mortality, the available treatment options and prognosis for NSCLC
are not satisfactory. In a recent meta-analysis, we showed that
exercise at medium to high level intensity is associated with lower
risk of lung cancer, both in men and women, indicating the
lifestyle-based benefits for reducing lung cancer risk (5). Platinum-doublet chemotherapy, which is
the standard first-line therapy, has been shown to yield objective
responses with a median overall survival (OS) of 8–10 months in
approximately 30–40% of patients with NSCLC and there are
significant safety issues (6).
Considering that the efficacy of platinum-based therapies is
dependent on the mutational/single nucleotide polymorphisms (SNP)
status of the components of DNA-damage repair systems available in
the target cancer cells, there can be significant individual
differences in treatment responses. Thus, we showed earlier that
the SNPs XRCC1Arg399Gln and XPG His46His are associated with
significantly better treatment response in NSCLC patients to
platinum-based chemotherapy (7). On
the other hand, tyrosine kinase inhibitors (e.g., gefitinib,
erlotinib, and afatinib) are found to be more effective with nearly
65% response rate and a median OS of 24–36 months in NCLSC patients
with EGFR mutations (8). In addition
to chemotherapy and chemoradiotherapy, immunological approaches
have been shown to be effective in treating various cancers,
including NSCLC. Significant advances have been made in the last 3
years in the development of immunotherapies for NSCLC.
Immunotherapies include tumor antigen vaccination, monoclonal
antibodies targeting checkpoint pathways and also activated immune
cells (8–10). Several recent studies have shown the
efficacy of immunotherapies against advanced stage NSCLC patients
(10–12). Despite the promise of several
immunotherapies, their efficacy has not been consistent as noted in
the phase III FORTIS trial (13).
Response to immunotherapies can also be dependent on many other
factors, such as chemokines that influence immune cell function.
Thus, we reported earlier that polymorphism (−2518A/G) in the gene
coding for monocyte chemoattractant protein-1 (MCP-1) is associated
with elevated risk for NSCLC. MCP-1 is known to be a tumor
suppressor via pathways involving T-lymphocytes or independent of
lymphocytes (14). Similarly, we also
noted that the frequencies of polymorphisms in the human leukocyte
antigen (HLA) system, which is involved in the regulation of immune
response, HLA-A*0201, A*2601, B*1518, B*3802, DRB1*0401, DRB1*0402,
and DRB1*1201 are higher in the lung cancer patients than healthy
controls (15), and affect the tumor
immunity.
An earlier meta-analysis of 12 randomized controlled
trials of immunotherapies indicated a beneficial effect on OS with
few adverse effects (6). However, in
this analysis, 3 monoclonal antibody (Mab)-based trials with
cetuximab (targets EGFR) and one trial with trastuzumab (targets
HER2) as NSCLC Mab therapy subgroup, whereas these actually are
related to the growth factors and not immune system. A recent
meta-analysis of several clinical trials of immunotherapies,
including therapeutic vaccines and immune checkpoint inhibitors
showed that immunotherapies are well tolerated in advanced NSLSC
patients and improve OS and that specific antitumor immune response
simulating agents are more efficacious than immunomodulatory type
agents (16). A comparison of tumor
vaccine-based therapies with cellular immunotherapies, in a
meta-analysis revealed that cellular immunotherapies are more
effective in improving OS and progression-free survival (PFS) in
NSCLC patients (12). However, in
many of these meta-analyses, the number of included studies and the
total number of patients was lower and also, as mentioned above,
inclusion of unrelated therapies could have complicated the
outcomes and conclusions. Also, discordant results were seen in
certain clinical trials using immunotherapies against NSCLC as the
promising phase II study results were not observed in phase III
study, leading to premature termination of the clinical trials,
questioning the efficacy of immunotherapy (17). We have now conducted a meta-analysis
of 13 clinical trials assessing the efficacy and safety of Mab
therapies directed against tumor antigens and tumor antigen-based
vaccination therapies, as compared to
chemotherapy/chemoradiotherapy in NSCLC patients.
In the present meta-analysis, we analyzed results
from clinical trial studies that simultaneously compared
immunotherapies with placebo or chemotherapy/radiotherapy to assess
the beneficial effects of immunotherapies collectively or
separately in improving OS and PFS of NSCLC patients. Besides
efficacy, we also examined if immunotherapies are better in terms
of safety, by looking at the treatment-related adverse effects.
Materials and methods
Criteria for considering studies for
this review
Randomized controlled phase II and III clinical
trials on patients with NSCLC at stage III and IV, with
histological confirmation are included in this analysis. In these
studies, immunotherapies were administered to patients along with
chemotherapy or as monotherapy and the treatment effects were
compared to a control group of patients, who received either
chemotherapy alone or placebo. For the present meta-analysis, we
have included only vaccine-based and Mab-based immunotherapy
studies and not the immune cell-based adoptive immunotherapy
clinical trials. Studies that did not include proper controls were
excluded from this meta-analysis. Most of the patients in the
included studies had Eastern Cooperative Oncology Group (ECOG)
performance status of 0, 1 or 2 (18). Patients were excluded if they were on
concurrent systemic steroids, with metastases in bone requiring
immediate therapy, uncontrolled pleural effusions, serious
non-malignant disease or previous malignancies or if they had
myocardial infarction or other cardiovascular disease in the
6-month period prior to study. Approximately half of the included
studies were open-label, whereas the remaining are double-blind
studies.
Search methods
Literature search was completed on 10 January 2017
and publications that included complete relevant information and
data were collected. Following databases were searched: PubMed,
Google Scholar, Scopus, Web of Science and Cochrane Central
Register. Search MeSH terms included lung cancer, NSCLC,
immunotherapy, vaccine therapy for NSCLC, vaccination for NSCLC,
OS, clinical trials, PFS and Mab therapy. All the relevant
publications only in English language were collected and were
initially screened at the title and abstract level. Only clinical
trials at phase II and III level were included in this analysis.
Full reports and supplemental information files were retrieved as
per the relevance of the selected study.
Data collection and analysis and
quality assessment
All the authors of this meta-analysis participated
in the screening of collected publications and data extraction.
Data including patient baseline characteristics such as age, sex,
description and dosages of the administered treatment, tumor
histology, and disease stage, and treatment primary endpoint
measurements (PFS events, OS) and treatment related adverse effects
were collected, by filling out a pre-defined data collection form,
from each included study. OS, which was defined as the time from
randomization to censor or death due to any cause, was mostly
reported as median months and for the purposes of meta-analysis,
these values were converted to mean ± SD. The period from
randomization of patients to the progression of disease (or death
if it occurred first), during the study duration, as ascertained
and documented by the study investigators, was defined as PFS.
Treatment related adverse effects were evaluated to assess the
safety of immunotherapy procedure, and the adverse events (scoring
grade ≥3) reported in more than two clinical trials, as the number
of patients were collected. These include both hematological and
non-hematological effects. Hematological events recorded were
anemia, neutropenia, thrombocytopenia and the non-hematological
events include fatigue, vomiting, abdominal pain, fever, nausea,
and anorexia/loss of appetite.
Statistical analysis
Statistical analyses were conducted using the
methods described by the Cochrane Collaboration guidelines for
meta-analysis, using Review Manager (RevMan) version 5.3 software
(the Cochrane Collaboration). As mentioned above, OS was mostly
reported as median months and lower and upper limits of the range
and for the purposes of meta-analysis, these values were converted
to mean ± SD. Number of PFS events, were not given directly, was
deduced from the PFS plots in the individual trials. The effect of
immunotherapy on PFS and each of the adverse events were analyzed
by Mantel-Haenszel statistics, odds ratios (OR) in the
random-effect model, at 95% confidence intervals (CI). The effect
of immunotherapy on OS was assessed by mean difference analyses in
inverse variance (IV) fixed mode at 95% confidence intervals.
Sensitivity analysis was performed by excluding one study at a time
and also by removing study with highest weightage, among the
included data to examine influence of bias on the deduced
statistical significance and interpretation. Risk ratios were
calculated for adverse events and also for PFS at 95% confidence
intervals.
Results
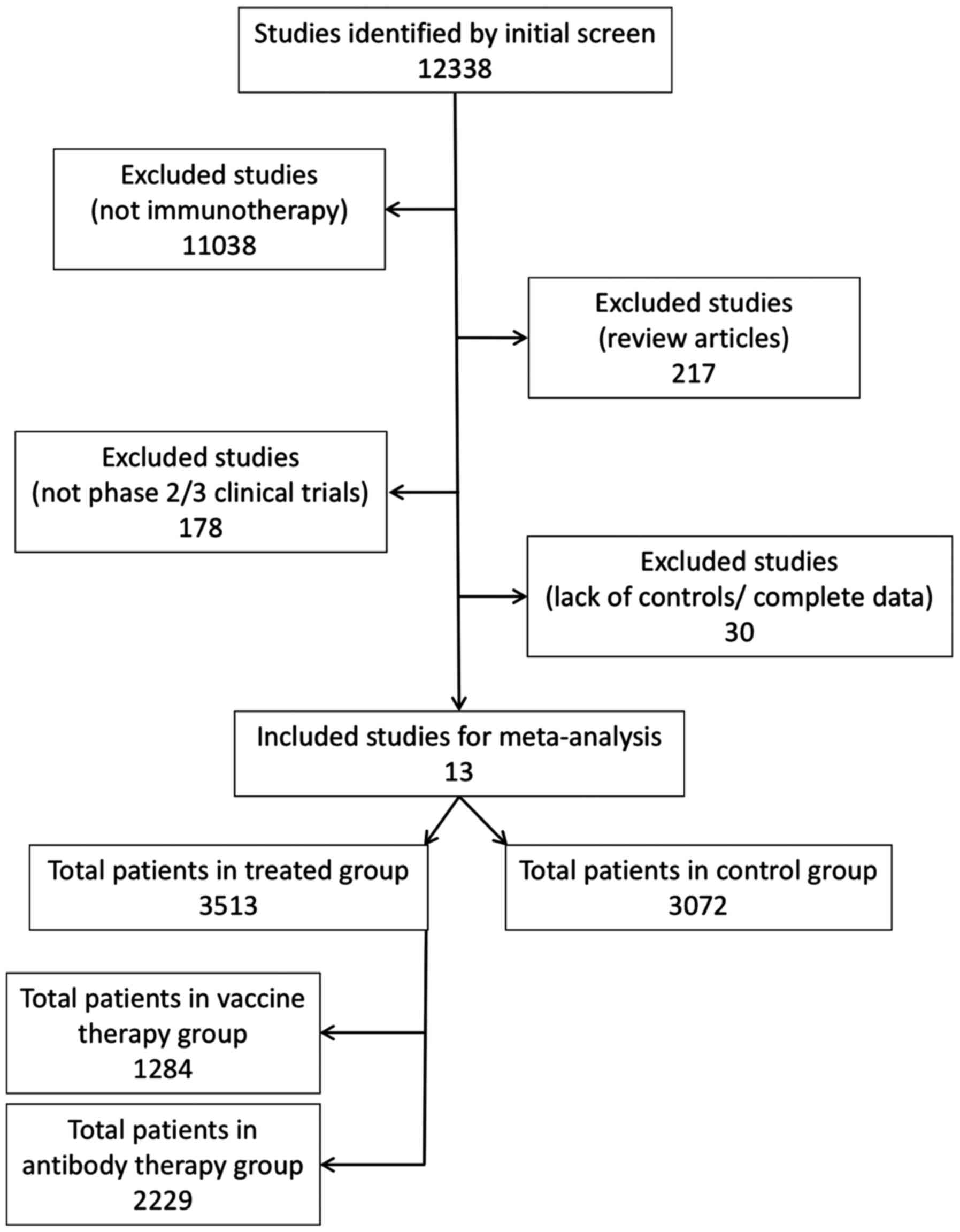
Search from all the databases yielded 12,338
studies, which contained the search MeSH terms either in the title
or in the abstract. Exclusion of studies was done through the
participation of all authors, who read the abstracts and a
collective decision was adopted. A total of 13 studies (11,19–29) were
identified on the basis of relevancy and inclusion/exclusion
criteria and completeness of the data in the study (Fig. 1). As immunotherapy of NSCLC is a
recently developed treatment approach, all the studies identified
are published after 2011. The total number of patients in all the
included studies was 6,585 and the number of patients who received
immunotherapy was 3,513, whereas the control group patients who
received chemotherapy or placebo were 3,072 (Fig. 1).
Patient characteristics
The demographic and baseline characteristics of
patients in the included studies are given in Table I. Average age of the patients in both
immunotherapy and control groups was ~62 years. Nearly 64.3% of the
patients who received immunotherapy were males while in control
chemotherapy group 62.9% were males. Tumors were of adenocarcinoma
type, by histological examination in nearly two-thirds of the
patients in both immunotherapy group (n=2,155) and in control group
(n=2,009) and next major histological type was squamous cell
carcinoma (Table I). ECOG performance
status, which is an indicator of the patient's daily living ability
and the progress of disease, is predominantly 0 or 1 for most of
the included patients, in both immunotherapy and
chemotherapy/placebo groups (18).
Majority of the patients are restricted for strenuous physical
activity, even though they could carry out light and sedentary
work.
 | Table I.Baseline characteristics of patients
included in the meta-analysis. |
Table I.
Baseline characteristics of patients
included in the meta-analysis.
| A,
Immunotherapy |
|---|
|
|
|
|
|
|
|
| ECOG status (no. of
patients) | Histology type
(n) |
|---|
| Author (ref.)
year | Study type | Type of
immunotherapy | No. of
patients | Age, years | Males, % | Immunotherapy
dosage | 0 | 1 | 2 | Ad | Sq |
|---|
| Quoix et al
(19) 2011 | Controlled
multicenter phase 2B | TG4010 genetic
vaccine (targets tumor MUC1) | 74 | 58.5 | 71.6 | 108 pfu;
cisp75 mg/m2 on day 1; gem 1.25 g/m2 on day
1, 8; every 3 weeks × 6 cycles | 20 | 53 | 1 | 47 | 19 |
| Lynch et al
(20) 2012 | Randomized,
double-blind phase 2B | Ipilimumab
(anti-cytotoxic T-cell lymphocyte-4 Mab) | 70 | 59 | 76 | 10 mg/kg
Ipilimumab; pacli 175 mg/m2; carbo AUC 6; i.v. dosing every 3 weeks
× 6 cycles | 19 | 51 |
| 35 | 21 |
| Borghaei et
al (21) 2015 | Randomized,
open-label phase 3 | Nivolumab human
IgG4 PD-1 immune checkpoint inhibitor Mab | 292 | 61 | 52 | 3 mg/kg, every 2
weeks; for 13.2 months minimum | 84 | 208 |
| 268 | 7 |
| Brahmer et
al (11) 2015 | Randomized,
open-label, multicenter, phase 3 | Nivolumab (IgG4
PD.1 Mab) | 135 | 62 | 82 | 3 mg/kg, every 2
weeks | 27 | 106 |
|
|
|
| Fehrenbacher et
al (22) 2016 | Multicenter,
open-label, randomized phase 2 | Atezolizumab
(anti-PD-L1 Mab) | 144 | 62 | 65 | Atezolizumab 1.2 g
fixed, i.v., on day 1, every 3 weeks | 46 | 96 |
| 95 | 49 |
| Herbst et al
(23) 2016 | Multicenter,
randomized, open-label, phase 2/3 | Pembrolizumab
(MK-3475; human IgG4 PD-1 Mab) | 344 | 63 | 62 | Pembrolizumab, 2
mg/kg, every 3 weeks | 112 | 229 | 3 | 240 | 76 |
| Herbst et al
(23) 2016 | Multicenter,
randomized, open-label, phase 2/3 | Pembrolizumab
(MK-3475; human IgG4 PD-1 Mab) | 346 | 63 | 62 | Pembrolizumab, 10
mg/kg, every 3 weeks | 120 | 225 | 1 | 244 | 80 |
| Quoix et al
(24) 2016 | Randomized,
double-blind multicenter, controlled phase 2b/3 | TG4010 genetic
vaccine | 111 | 63 | 65 | 108 pfu;
every week for 6 weeks then every 3 weeks; cisp 75mg/m2
on day 1; gem 1.25g/m2 on day 1, 8 | 33 | 77 |
| 95 | 13 |
| Reck et al
(25) 2016 | Open-label,
multicenter, phase 3 | Pembrolizumab
(MK-3475; human IgG4 PD-1 Mab) | 154 | 64.5 | 59.7 | Pembrolizumab, 200
mg every 3 weeks, 35 cycles | 54 | 99 |
| 125 | 29 |
| Rittmeyer et
al (26) 2017 | Open-label,
multicenter randomized controlled phase 3 | Atezolizumab
(Anti-PD-L1 Mab) | 425 | 63 | 61 | atezolizumab 1.2 g
fixed, i.v., on day 1, every 3 weeks | 155 | 270 |
| 313 | 112 |
| Butts et al
(27) 2014 | START randomized,
double-blind, multicenter phase 3 | Tecemotide vaccine
(MUC1-antigen-specific immunotherapy) | 829 | 61 | 68 | Subcutaneous
tecemotide (806 µg lipopeptide) + lipid-A+ liposome forming
lipids | 398 | 427 |
| 289 | 401 |
| Herbst et al
(28) 2011 | Double-blind,
multicenter placebo-controlled phase 3 | Bevacizumab
(recombinant, anti-vascular endothelial growth factor Mab) | 319 | 65 | 54 | Bevacizumab at 15
mg/kg, i.v., on day 1, every 3 weeks + erlotinib 150 mg/day | 129 | 166 | 23 | 242 | 23 |
| Giaccone et
al (29) 2015 | Phase 3 study | Belagenpumatucel-L
(whole tumor cell vaccine, of NSCLC) cells, transfected with a
human TGF-β2-antisense vector | 270 | 61.5 | 58 | 2.5×107
total cells were injected intradermally | 119 | 139 | 7 | 162 | 65 |
|
| B,
Chemotherapy/placebo |
|
|
|
|
|
|
|
|
| ECOG status (no. of
patients) | Histology type
(n) |
|
|
|
|
|
|
|
|
|
|
| Author (ref.)
year | Study type | Type of
chemotherapy | No. of
patients | Age, years | Males, % | Chemotherapy
dosage | 0 | 1 | 2 | Ad | Sq |
|
| Quoix et al
(19) 2011 | Controlled
multicenter phase 2B | Cisplatin +
gemcitabine | 74 | 58.5 | 73 | Cisp 75
mg/m2 on day 1; gem 1.25 g/m2 on day 1, 8;
every 3 weeks × 6 cycles | 20 | 54 | 0 | 55 | 11 |
| Lynch et al
(20) 2012 | Randomized
double-blind phase 2B | Paclitaxel +
carboplatin | 66 | 62 | 74 | Pacli 175
mg/m2; carbo AUC 6; i.v. dosing every 3 weeks × 6
cycles | 15 | 51 |
| 38 | 15 |
| Borghaei et
al (21) 2015 | Randomized
open-label, phase 3 | Docetaxel | 290 | 64 | 58 | Docetaxel 75
mg/m2 every 3 weeks; for 13.2 months minimum | 95 | 194 |
| 273 | 7 |
| Brahmer et
al (11) 2015 | Randomized
open-label, multicenter phase 3 | Docetaxel | 137 | 64 | 71 | Docetaxel 75
mg/m2 every 3 weeks | 37 | 100 |
|
|
|
| Fehrenbacher et
al (22) 2016 | Multicenter,
open-label, randomized phase 2 | Docetaxel | 143 | 62 | 53 | Docetaxel 75
mg/m2 on day 1, every 3 weeks | 45 | 97 |
| 95 | 48 |
| Herbst et al
(23) 2016 | Multicenter,
randomized, open-label, phase 2/3 | Docetaxel | 343 | 62 | 61 | Docetaxel 75
mg/m2 on day 1, every 3 weeks | 116 | 224 | 1 | 240 | 66 |
| Quoix et al
(24) 2016 | Randomized,
double-blind multicenter, controlled phase 2b/3 | Cisplatin +
gemcitabine | 111 | 59 | 63 | Cisp 75
mg/m2 on day 1; gem 1.25 g/m2 1, every 3
weeks + on day 1, 8; every 3 weeks × 6 cycles | 35 | 76 |
| 90 | 13 |
| Reck et al
(25) 2016 | Open-label,
multicenter, phase 3 |
Carboplatin/cisplatin/paclitaxel/gemcitabine | 151 | 66 | 62.9 | Varying
dosages | 53 | 98 |
| 124 | 27 |
| Rittmeyer et
al (26) 2017 | Open-label,
multicenter randomized controlled phase 3 | Docetaxel | 425 | 64 | 61 | Docetaxel 75
mg/m2, on day 1, every 3 weeks | 160 | 165 |
| 315 | 110 |
| Butts et al
(27) 2014 | START randomized,
double-blind multicenter, phase 3 | Placebo | 410 | 61.5 | 68 | Liposome forming
lipids | 167 | 239 |
| 163 | 171 |
| Herbst et al
(28) 2011 | Double-blind,
multicenter, placebo-controlled, phase 3 trial | Erlotinib (EGFR
inhibitor) | 317 | 64.8 | 54 | Erlotinib 150
mg/day | 121 | 176 | 20 | 90 | 17 |
| Giaccone et
al (29) 2015 | Phase 3 study | Placebo | 262 | 60.5 | 58 | 0.15%
intralipid | 130 | 119 | 6 | 141 | 81 |
In the present meta-analysis study, only
antibody-based and vaccine-based immunotherapies are included.
Cellular therapy studies were not included as many of these studies
are at early phase I stage. Out of the 13 studies, nine were
antibody-based immunotherapies, while the remaining four were
vaccine-based. Antibody therapies included ipilimu-mab, an
anti-cytotoxic T-cell lymphocyte-4 Mab (20); nivolumab, a human IgG4 PD-1
immune-checkpoint-inhibitor Mab (11,21);
atezolizumab, another anti-PD-L1 Mab (22,26);
pembrolizumab, also known as MK-3475, a human IgG4 PD-1 Mab
(23,25); and bevacizumab, a recombinant,
anti-vascular endothelial growth factor Mab (28). Vaccine-based immunotherapies included
TG4010 genetic vaccine, which is a suspension of a recombinant
modified vaccinia virus strain Ankara (MVA), coding for the MUC1
tumor-associated antigen and interleukin-2 (19,24);
tecemotide (L-BLP25), a tumor associated MUC1 antigen-specific
immunotherapy, that is capable of inducing a T-cell response to
MUC1 (27) and belagenpumatucel-L, an
allogeneic whole tumor cell vaccine consisting of four pCHEK/HBA2
(human transforming growth factor-β2-antisense vector)-transfected
NSCLC cell lines (29). Immunotherapy
dosages and chemotherapy dosages are shown in Table I. Dosage of antibodies varied
depending on the study and antibody used. Thus, ipilimumab was
given at 10 mg/kg as four doses plus paclitaxel and carboplatin
followed by two doses of placebo plus paclitaxel and carboplatin,
intravenously every 3 weeks for up to 18 weeks (20). Nivolumab was given at 3 mg/kg, every 2
weeks, for 11 to 13.2 months (11,21).
Atezolizumab was given at 1.2 g fixed dose, intravenously on day 1,
every 3 weeks, for at least 9 months (22,26).
Pembrolizumab was given at 2 mg or 10 mg/kg, every 3 weeks for 24
months (23) or at 200 mg every 3
weeks in 35 cycles (25). Bevacizumab
was given at 15 mg/kg, intravenously on day 1, every 3 weeks +
erlotinib 150 mg/day (28). TG4010
genetic vaccine was administered at 108 pfu along with
cisplatin 75 mg/m2 on day 1; gemcitabine 1.25
g/m2 on day 1 and 8, every 3 weeks in 6 cycles (19,24).
Tecemotide was administered subcutaneously, as 806 µg lipopeptide
with lipid-A and liposome forming lipids (27). Belagenpumatucel-L was given
intradermally at a dose of 2.5×107 total cells (29). Chemotherapy generally included
carboplatin, paclitaxel, docetaxel, cisplatin and gemcitabine. In
one study (28), EGFR inhibitor,
erlotinib was used as chemotherapy agent in control group patients.
Where chemotherapy was not the regimen for control group patients,
vehicle (used for immunotherapy) placebo (intralipid or liposome
forming lipids) was given.
Effect of immunotherapy on PFS and
OS
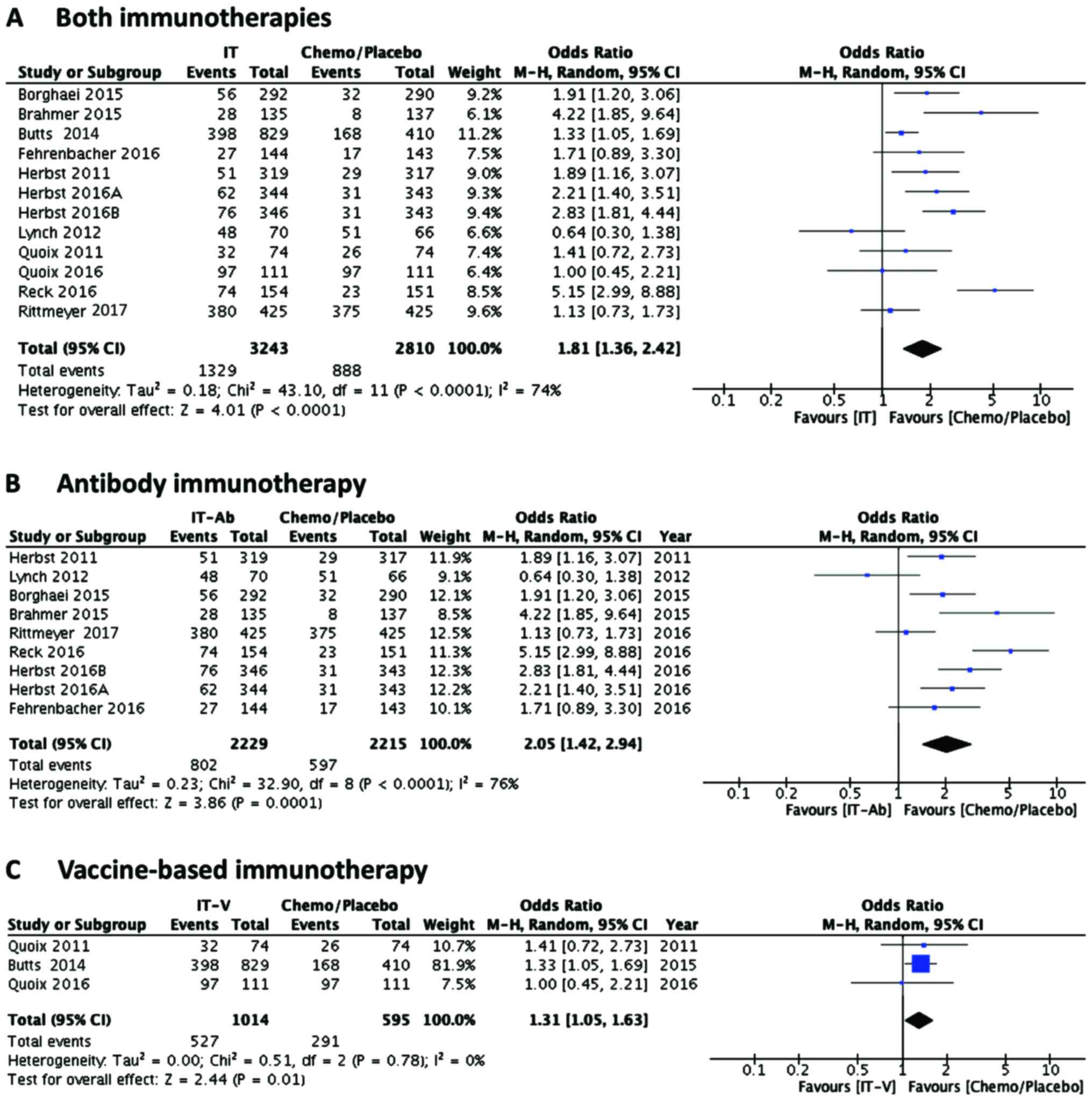
In pooled OR analysis (Mantel-Haenszel, random) of
both types of immunotherapies, PFS was found to be significantly
increased (Fig. 2A) in a higher
number of patients as compared to chemotherapy/placebo control
group (OR 1.81, 95% CI 1.36, 2.42; P<0.0001). Further separate
analysis of antibody therapy also revealed a significant beneficial
effect on PFS in antibody receiving patients (Fig. 2B) as compared to control group (OR
2.05, 95% CI 1.42, 2.94; P<0.0001). Even though the vaccine
therapy-receiving patients also showed better PFS compared to
control group (Fig. 2C), the
statistical significance (OR 1.31, 95% CI 1.05, 1.63; P<0.01)
was not as high as in the case of antibody therapy. These results
are in accordance with earlier results of a pooled analysis of the
beneficial effects of immunotherapies (12). Similar results were obtained by risk
ratio (RR) analysis (Table II).
Thus, RR for both types of immunotherapies was 1.297 (95% CI 1.211,
1.389; P=0) and for Mab therapy RR was 1.335 (95% CI 1.222, 1.458;
P=0). But RR for vaccine therapy was 1.063 and was not significant
(95% CI 0.96, 1.176; P=0.239).
 | Table II.Risk ratio analysis of PFS in
antibody-based and vaccine-based immunotherapy groups vs.
corresponding chemotherapy/placebo groups. |
Table II.
Risk ratio analysis of PFS in
antibody-based and vaccine-based immunotherapy groups vs.
corresponding chemotherapy/placebo groups.
|
| Immunotherapy |
Chemotherapy/placebo |
| 95% CI |
|
|---|
|
|
|
|
|
|
|
|---|
| PFS | Events | Non-events | Total | Events | Non-events | Total | Risk ratio | Lower | Upper | P-value |
|---|
| PFS (for all
immunotherapy) | 1,329 | 1,914 | 3,243 | 888 | 1,922 | 2,810 | 1.2968 | 1.2112 | 1.3885 | 0 |
| PFS (for
antibody-based therapy) | 802 | 1,427 | 2,229 | 597 | 1,618 | 2,215 | 1.3349 | 1.2223 | 1.4579 | 0 |
| PFS (for
vaccine-based therapy) | 527 | 487 | 1,014 | 291 | 304 | 595 | 1.0627 | 0.9604 | 1.1759 | 0.2392 |
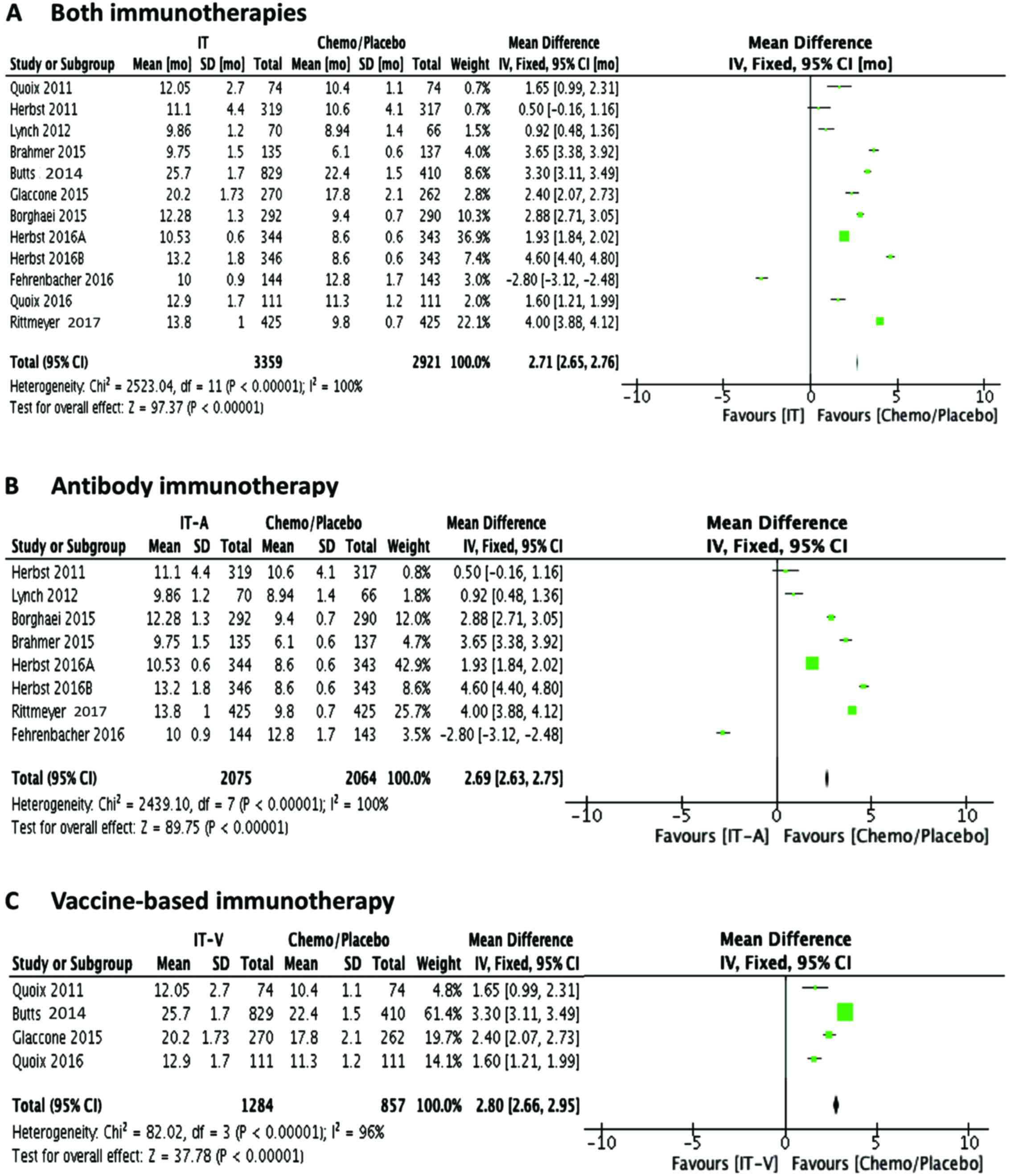
OS was significantly enhanced by both the
immunotherapies as compared to chemotherapy/placebo controls
(Fig. 3A), as revealed by mean
difference analysis in IV fixed mode (P<0.00001). Unlike in the
case of PFS, OS was greatly improved by both antibody-based therapy
(Fig. 3B; P<0.00001) and
vaccine-based therapy (Fig. 3C;
P<0.00001). These results are in agreement with earlier findings
showing the beneficial effects of immunotherapies on OS (12,16).
Effect of immunotherapies on treatment
related adverse effects
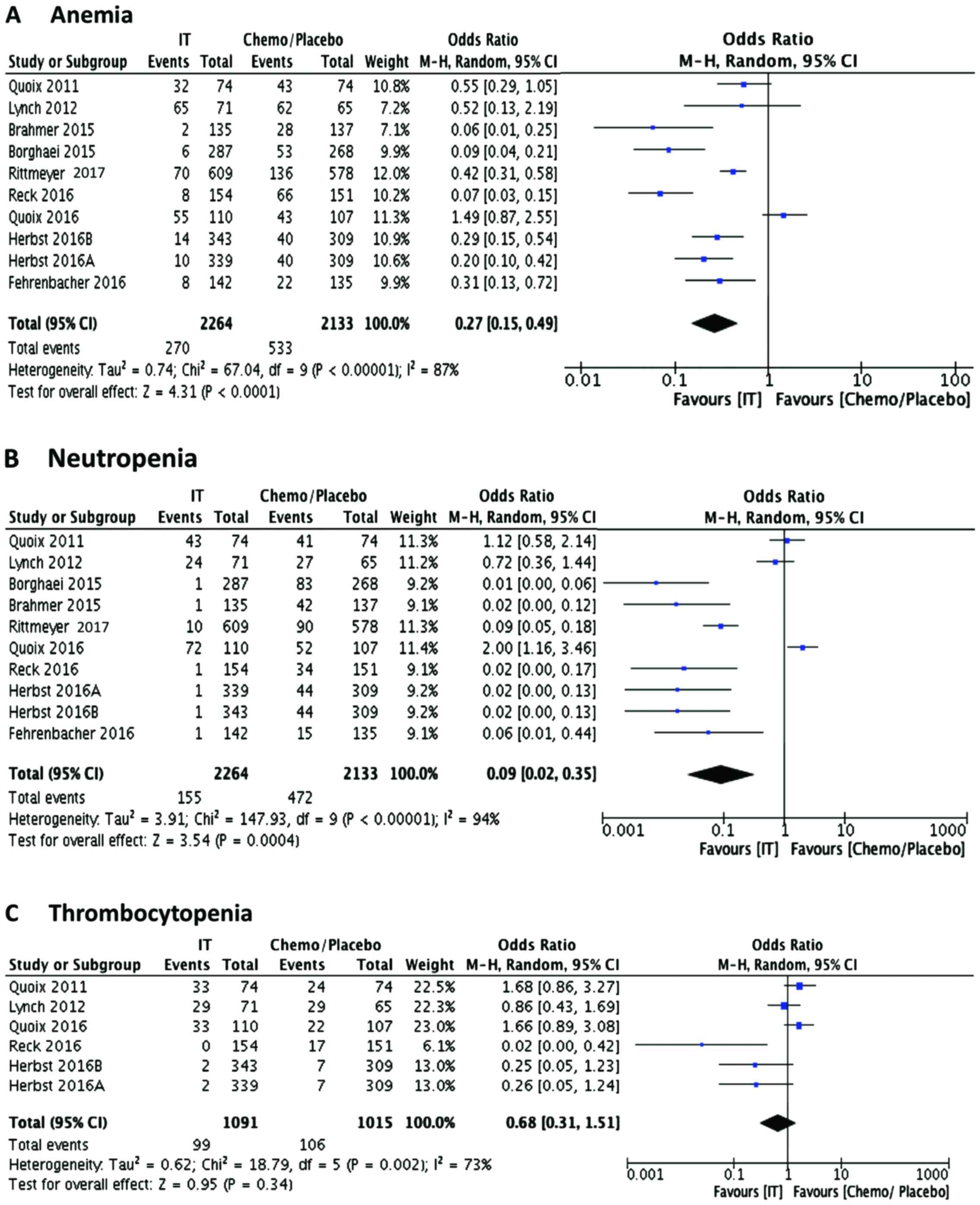
Earlier studies indicated that hematological adverse
effects were relatively lower in patients treated with immune check
point inhibitors whereas in vaccine treated patients these effects
were observed often (16). In the
present meta-analysis (OR, Mantel-Haenszel, random), we observed
that among the hematological adverse effects (Fig. 4), anemia (Fig. 4A; OR 0.27, 95% CI 0.15, 0.49;
P<0.0001) and neutropenia (Fig.
4B; OR 0.09, 95% CI 0.02, 0.35; P=0.0004) are significantly
lower in the immunotherapy-receiving patients (Table III). However, events of
thrombocytopenia (Fig. 4C; OR 0.68,
95% CI 0.31, 1.51; P=0.34) are not different compared to
chemotherapy/placebo control group. Thrombocytopenia events were
reported only in approximately half of the included studies, unlike
anemia and neutropenia. It has been shown earlier that
thrombocytopenia events are similar or even more common in patients
treated with immunomodulatory therapy (16). Among the non-hematological adverse
effects, neither abdominal pain nor loss of appetite/anorexia was
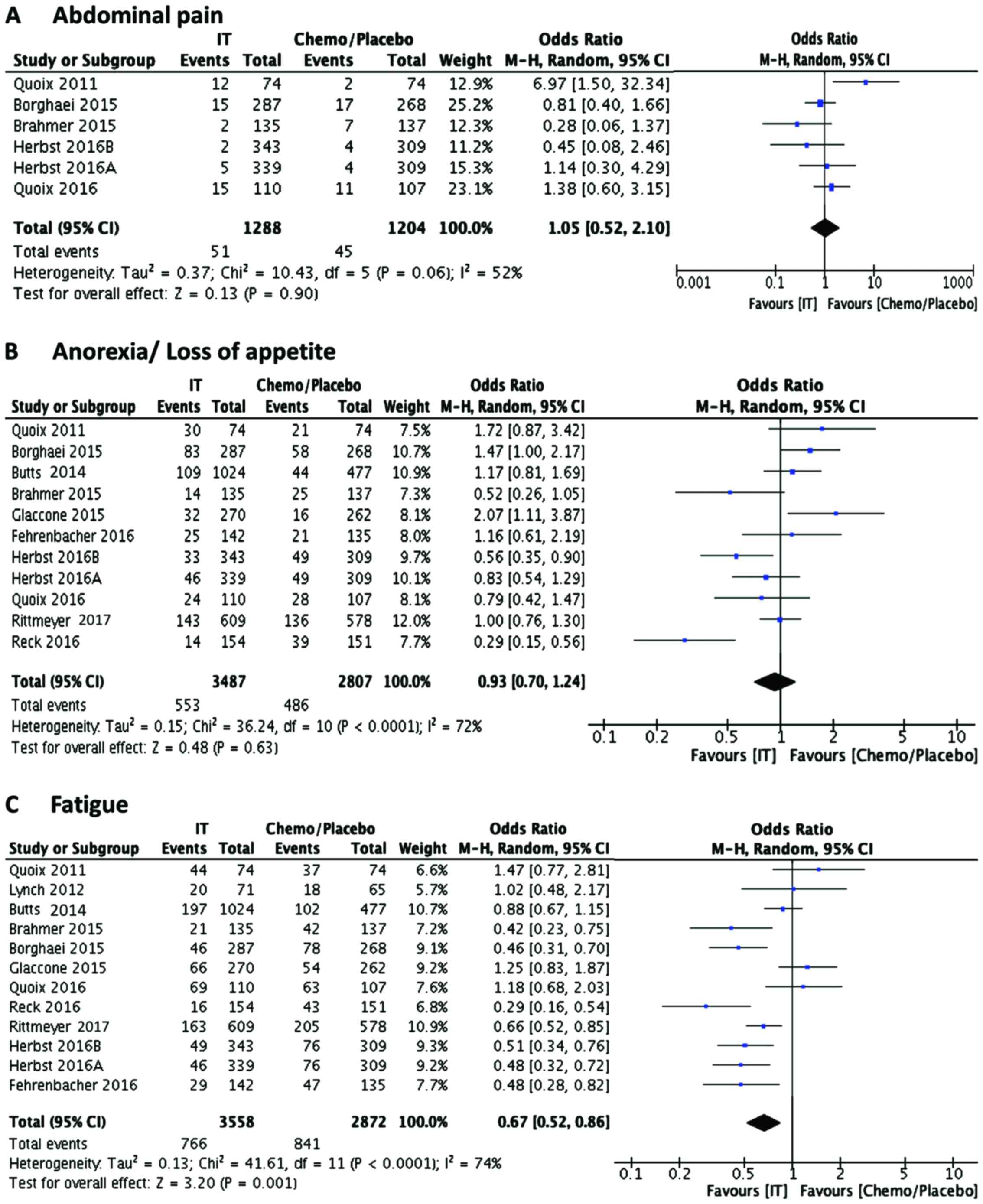
found to be different in the immunotherapy group (Fig. 5A and B). However, fatigue, which is a
commonly seen adverse effect appeared to be much less common in the
immunotherapy group, as compared to the control
chemotherapy/placebo group (Fig. 5C;
OR 0.67, 95% CI 0.52, 0.86; P<0.001). A closer look at the data
indicated that the number of patients with fatigue are much less in
the antibody treated group, compared to their controls, whereas the
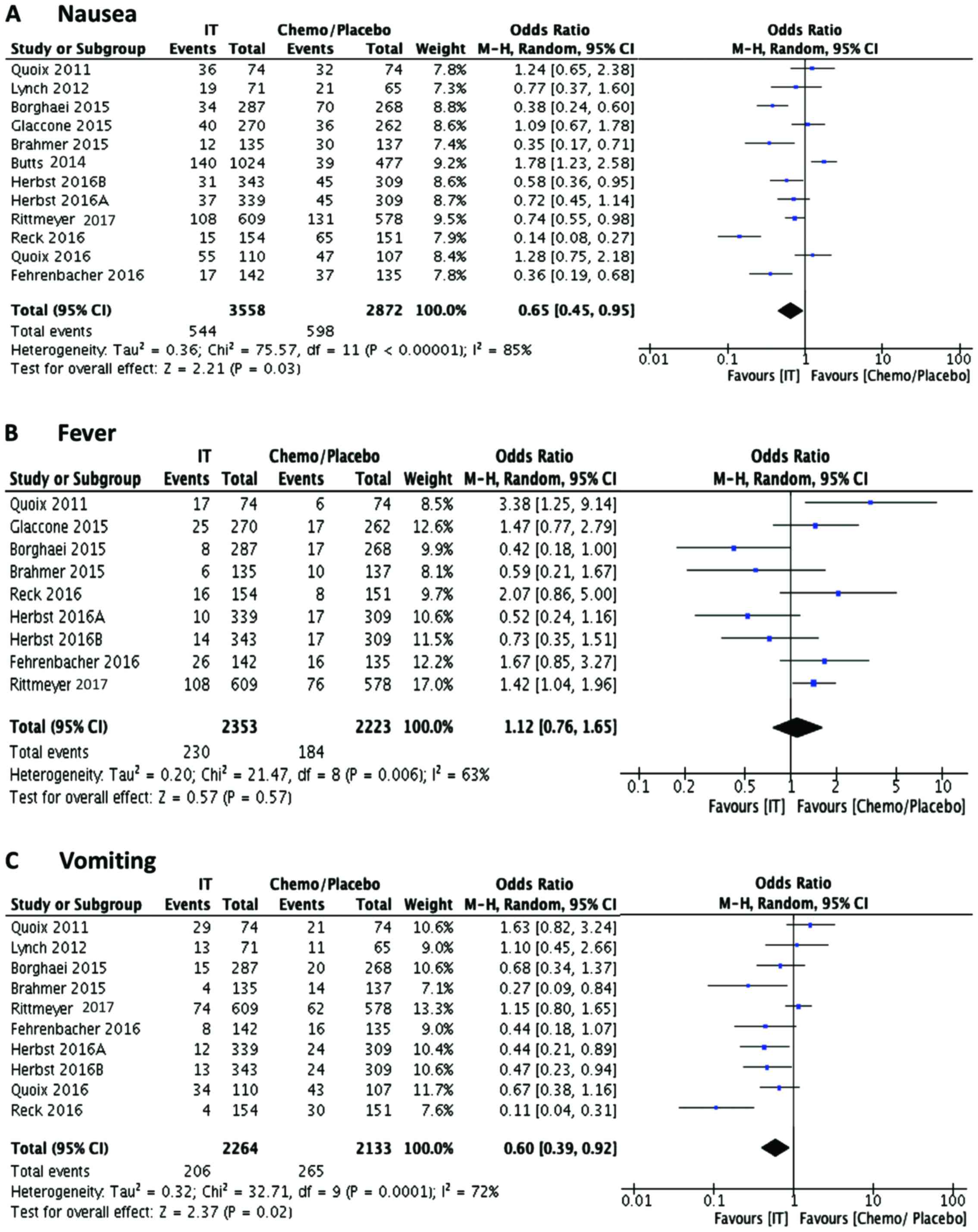
events seems to be higher in the vaccine treated group. Other
adverse effects including nausea, fever and vomiting are not much
different in the immunotherapy group (Fig. 6).
 | Table III.Risk ratio analysis of adverse
effects in immunotherapy vs. chemotherapy/placebo groups. |
Table III.
Risk ratio analysis of adverse
effects in immunotherapy vs. chemotherapy/placebo groups.
|
| Immunotherapy |
Chemotherapy/placebo |
| 95% CI |
|
|---|
|
|
|
|
|
|
|
|---|
| Event measured | Events | Non-events | Total | Events | Non-events | Total | Risk ratio | Lower | Upper | P-value |
|---|
| Anemia | 270 | 1,994 | 2,264 | 533 | 1,600 | 2,133 | 0.4773 | 0.4174 | 0.5457 | 0 |
| Neutropenia | 155 | 2,109 | 2,264 | 472 | 1,661 | 2,133 | 0.3094 | 0.2606 | 0.3673 | 0 |
|
Thrombocytopenia | 99 |
992 | 1,091 | 106 |
909 | 1,015 | 0.8689 | 0.6698 | 1.1272 | 0.2899 |
| Abdominal pain | 51 | 1,288 | 1,339 | 45 | 1,159 | 1,204 | 1.0191 | 0.6877 | 1.51 | 0.925 |
| Nausea | 544 | 3,014 | 3,558 | 598 | 2,274 | 2,872 | 0.7343 | 0.661 | 0.8158 | 0 |
| Fatigue | 766 | 2,792 | 3,558 | 841 | 2,031 | 2,872 | 0.7352 | 0.6755 | 0.8002 | 0 |
| Vomiting | 206 | 2,058 | 2,264 | 265 | 1,868 | 2,133 | 0.7324 | 0.6165 | 0.87 | 0.0004 |
| Anorexia | 553 | 2,934 | 3,487 | 486 | 2,321 | 2,807 | 0.916 | 0.8195 | 1.0238 | 0.1221 |
| Fever | 230 | 2,123 | 2,353 | 184 | 2,039 | 2,223 | 1.1809 | 0.9815 | 1.4209 | 0.0781 |
Sensitivity analysis
In order to assess the robustness and to eliminate
bias in the results, we re-analyzed the PFS and OS data by
excluding individual trials with highest or lowest weightage. Such
analysis did not qualitatively alter the obtained results and
conclusions (data not elaborated). The OS benefits were still noted
by immunotherapy over the chemotherapy/placebo controls. These
conclusions are in agreement with earlier study (16), indicating that the benefits of
immunotherapy are real and reproducible.
Discussion
Chemotherapy and chemoradiotherapy are the
first-line therapy for several cancers including NSCLC. Even though
objective responses have been noted with many of these therapies,
the efficacy is not observed in all the patients. Tyrosine kinase
receptor inhibitors showed better effects on OS and PFS but due to
resistance, their efficacy is lost and disease progression takes
place. A better understanding of immune mechanisms and host
antitumor responses led to the identification of potential
therapeutic opportunities employing the components of immune
system. These include different types of immunotherapies based on
the use of monoclonal antibodies against tumor specific antigens
and immune checkpoint pathways, immunomodulators, vaccination
against tumor antigens and activated immune cells that attack
tumors (8,30). In the present meta-analysis, we
examined the effectiveness of immunotherapies in improving PFS and
OS. A total of 13 multicenter international phase II and III
clinical trials addressing the efficacy of antibody therapies and
vaccine therapies for treating NSCLC patients are included in this
analysis. Among these studies, 9 were Mab-based therapies targeting
different antigens and 4 were vaccine-based, with different
immunogens. The immune check point pathway targets of the employed
Mabs were programmed death-1 (PD1), programmed death ligand-1
(PD-L1) and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4).
Vaccine therapies were targeting the MUC1 tumor-associated antigen
and using NSCLC cells transfected with human transforming growth
factor-β2-antisense vector.
In line with other studies, we observed that
immunotherapies offer better efficacy in terms of improved PFS and
OS. Surprisingly, we observed that Mab therapies are more effective
in improving PFS than the vaccine-based therapies (25,26,28). This
can be because of the less number of vaccine-based studies and less
number of patients in this analysis. An earlier meta-analysis
indicated that immunotherapies have relatively stronger effect in
improving PFS in low-stage NSCLC than in high-stage NSCLC patients
(12). It was also observed that
tumor histology is not related to disease progression. Combination
of chemotherapy with immunotherapy has been proposed to have
synergistic effect in inducing tumor cell death and by disrupting
the immune evasion pathways of the tumor cells (31). Blockade of PD-1/PD-L1 signaling with
checkpoint inhibiting antibodies has been shown to promote
antitumor T-cell functionality (32).
Efficacy of immunotherapies is likely related to the expression of
the corresponding targets in the selected patient population
(33,34), which should be considered as a patient
selection criterion for such clinical trials in future. However,
for PD-1/PD-L1 expression this dependence may not always be true
(8,21).
Incidence of moderate adverse effects such as
anorexia, abdominal pain, nausea, vomiting and fever appeared to be
similar in chemotherapy and immunotherapy groups. But incidence of
fatigue in immunotherapy receiving patients is less, suggesting an
important beneficial effect by this therapy in improving the
quality of life of patients. Also, the number of patients with
anemia and neutropenia are less in immunotherapy group indicating a
less secondary aggravated effect on hematological parameters. It
has been recognized that chemotherapy and chemoradiotherapy in the
treatment of NSCLC have adverse effects on bone marrow, leading to
anemia, neutropenia and also thrombocytopenia (35). It is possible that immunotherapy
assisted antitumor effects are helpful in strengthening the normal
cellular homeostatic mechanisms and in overcoming the toxic side
effects of chemotherapy. Better hematological parameters are likely
further improve the general health and the daily living ability of
the patient. Immunotherapy was earlier shown to be safe for agents
that trigger specific antitumor reaction (36,37). Thus,
overall immunotherapies are safe and well tolerated and when
combined with chemotherapy, they are somewhat protective against
the toxic effects of chemotherapeutics, particularly on blood
parameters. In as much as exercise also has beneficial effects in
reducing the risk of lung cancer (5),
it is of interest to assess whether a combination of immunotherapy
with medium level of supervised exercise has any added benefit to
the lung cancer patients, who are at 0–1 score for ECOG
performance.
A major limitation of the present meta-analysis is
the smaller number of included studies in vaccine-based
immunotherapy group. Even though we combined both Mab-based
therapies and vaccine-based therapies for comparison against
chemotherapy controls for getting a larger picture of the
effectiveness of immunotherapy, ideal comparison would be separate
comparisons for these two types of immunotherapies. This was done
for analyzing the effects on PFS and OS, where we noticed specific
effects on PFS. Another limitation is that we included one
Mab-based therapy study that targeted VEGF (28), instead of a direct tumor specific
antigen, we included this study, as VEGF is important for
neovascularization of the tumors and for tumor growth and is
secreted by tumor cells. Another important limitation is the
different treatment durations of the included studies, as this
could have influenced the observed PFS and OS. In as much as female
patients with NSCLC are known to have better survival and as it has
been seen in the present study that in all the included studies
there is a higher preponderance of males, it is important to
separate males from females when analyzing the beneficiary effects
of immunotherapy. This could not be done due to unavailability of
individual patient data for all the included studies. Future
analyses should take this into consideration to assess if males or
females show a different/better response to a given treatment.
In conclusion, immunotherapy of NSCLC is beneficial
and shows better efficacy than chemotherapy/placebo in improving
PFS and OS. Besides, immunotherapies, due to their less adverse
effects, seem to have beneficial impact on the quality of life of
patients and the daily living ability.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gordon SB, Bruce NG, Grigg J, Hibberd PL,
Kurmi OP, Lam KB, Mortimer K, Asante KP, Balakrishnan K, Balmes J,
et al: Respiratory risks from household air pollution in low and
middle income countries. Lancet Respir Med. 2:823–860. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haghgoo SM, Allameh A, Mortaz E, Garssen
J, Folkerts G, Barnes PJ and Adcock IM: Pharmacogenomics and
targeted therapy of cancer: Focusing on non-small cell lung cancer.
Eur J Pharmacol. 754:82–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Madureira P, de Mello RA, de Vasconcelos A
and Zhang Y: Immunotherapy for lung cancer: For whom the bell
tolls? Tumour Biol. 36:1411–1422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun JY, Shi L, Gao XD and Xu SF: Physical
activity and risk of lung cancer: A meta-analysis of prospective
cohort studies. Asian Pac J Cancer Prev. 13:3143–3147. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Zou ZH, Xia HL, He JX, Zhong NS
and Tao AL: Strengths and weaknesses of immunotherapy for advanced
non-small-cell lung cancer: A meta-analysis of 12 randomized
controlled trials. PLoS One. 7:e326952012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin ZY, Zhao XT, Zhang LN, Wang Y, Yue WT
and Xu SF: Effects of polymorphisms in the XRCC1, XRCC3, and XPG
genes on clinical outcomes of platinum-based chemotherapy for
treatment of non-small cell lung cancer. Genet Mol Res.
13:7617–7625. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gridelli C, Ascierto PA, Barberis MC,
Felip E, Garon EB, O'Brien M, Senan S, Casaluce F, Sgambato A,
Papadimitrakopoulou V and De Marinis F: Immunotherapy of non-small
cell lung cancer: Report from an international experts panel
meeting of the Italian Association of Thoracic Oncology. Expert
Opin Biol Ther. 16:1479–1489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forde PM, Kelly RJ and Brahmer JR: New
strategies in lung cancer: Translating immunotherapy into clinical
practice. Clin Cancer Res. 20:1067–1073. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kimura H, Matsui Y, Ishikawa A, Nakajima
T, Yoshino M and Sakairi Y: Randomized controlled phase III trial
of adjuvant chemo-immunotherapy with activated killer T cells and
dendritic cells in patients with resected primary lung cancer.
Cancer Immunol Immunother. 64:51–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dammeijer F, Lievense LA, Veerman GD,
Hoogsteden HC, Hegmans JP, Arends LR and Aerts JG: Efficacy of
tumor vaccines and cellular immunotherapies in non-small-cell lung
cancer: A systematic review and meta-analysis. J Clin Oncol.
34:3204–3212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramalingam S, Crawford J, Chang A,
Manegold C, Perez-Soler R, Douillard JY, Thatcher N, Barlesi F,
Owonikoko T, Wang Y, et al: Talactoferrin alfa versus placebo in
patients with refractory advanced non-small-cell lung cancer
(FORTIS-M trial). Ann Oncol. 24:2875–2880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang L, Shi GL, Song CX and Xu SF:
Relationship between genetic polymorphism of MCP-1 and
non-small-cell lung cancer in the Han nationality of North China.
Genet Mol Res. 9:765–771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang L, Wang LJ, Shi GL, Ni L, Song CX,
Zhang ZX and Xu SF: Analysis of HLA-A, HLA-B and HLA-DRB1 alleles
in Chinese patients with lung cancer. Genet Mol Res. 9:750–755.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou L, Wang XL, Deng QL, Du YQ and Zhao
NQ: The efficacy and safety of immunotherapy in patients with
advanced NSCLC: A systematic review and meta-analysis. Sci Rep.
6:320202016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cuppens K and Vansteenkiste J: Vaccination
therapy for non-small-cell lung cancer. Curr Opin Oncol.
26:165–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quoix E, Ramlau R, Westeel V, Papai Z,
Madroszyk A, Riviere A, Koralewski P, Breton JL, Stoelben E, Braun
D, et al: Therapeutic vaccination with TG4010 and first-line
chemotherapy in advanced non-small-cell lung cancer: A controlled
phase 2B trial. Lancet Oncol. 12:1125–1133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lynch TJ, Bondarenko I, Luft A,
Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H,
Cuillerot JM and Reck M: Ipilimumab in combination with paclitaxel
and carboplatin as first-line treatment in stage IIIB/IV
non-small-cell lung cancer: Results from a randomized,
double-blind, multicenter phase II study. J Clin Oncol.
30:2046–2054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Quoix E, Lena H, Losonczy G, Forget F,
Chouaid C, Papai Z, Gervais R, Ottensmeier C, Szczesna A,
Kazarnowicz A, et al: TG4010 immunotherapy and first-line
chemotherapy for advanced non-small-cell lung cancer (TIME):
Results from the phase 2b part of a randomised, double-blind,
placebo-controlled, phase 2b/3 trial. Lancet Oncol. 17:212–223.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: KEYNOTE-024 Investigators: Pembrolizumab versus
chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl
J Med. 375:1823–1833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: OAK Study Group: Atezolizumab versus docetaxel in
patients with previously treated non-small-cell lung cancer (OAK):
A phase 3, open-label, multicentre randomised controlled trial.
Lancet. 389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Butts C, Socinski MA, Mitchell PL,
Thatcher N, Havel L, Krzakowski M, Nawrocki S, Ciuleanu TE, Bosquée
L, Trigo JM, et al: START trial team: Tecemotide (L-BLP25) versus
placebo after chemoradiotherapy for stage III non-small-cell lung
cancer (START): A randomised, double-blind, phase 3 trial. Lancet
Oncol. 15:59–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Herbst RS, Ansari R, Bustin F, Flynn P,
Hart L, Otterson GA, Vlahovic G, Soh CH, O'Connor P and Hainsworth
J: Efficacy of bevacizumab plus erlotinib versus erlotinib alone in
advanced non-small-cell lung cancer after failure of standard
first-line chemotherapy (BeTa): A double-blind, placebo-controlled,
phase 3 trial. Lancet. 377:1846–1854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giaccone G, Bazhenova LA, Nemunaitis J,
Tan M, Juhász E, Ramlau R, Van den Heuvel MM, Lal R, Kloecker GH,
Eaton KD, et al: A phase III study of belagenpumatucel-L, an
allogeneic tumour cell vaccine, as maintenance therapy for
non-small cell lung cancer. Eur J Cancer. 51:2321–2329. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vesely MD, Kershaw MH, Schreiber RD and
Smyth MJ: Natural innate and adaptive immunity to cancer. Annu Rev
Immunol. 29:235–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Emens LA and Middleton G: The interplay of
immunotherapy and chemotherapy: Harnessing potential synergies.
Cancer Immunol Res. 3:436–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Postow MA, Callahan MK and Wolchok JD:
Immune checkpoint blockade in cancer therapy. J Clin Oncol.
33:1974–1982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Noguchi M, Sasada T and Itoh K:
Personalized peptide vaccination: A new approach for advanced
cancer as therapeutic cancer vaccine. Cancer Immunol Immunother.
62:919–929. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pol J, Bloy N, Buqué A, Eggermont A,
Cremer I, Sautès-Fridman C, Galon J, Tartour E, Zitvogel L, Kroemer
G, et al: Trial Watch: Peptide-based anticancer vaccines.
Oncoimmunology. 4:e9744112015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ettinger DS: Non-small cell lung cancer
treatment-related bone marrow toxicities. Semin Oncol. 32:S81–S85.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gelao L, Criscitiello C, Esposito A,
Goldhirsch A and Curigliano G: Immune checkpoint blockade in cancer
treatment: A double-edged sword cross-targeting the host as an
‘innocent bystander’. Toxins (Basel). 6:914–933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weber JS, Yang JC, Atkins MB and Disis ML:
Toxicities of immunotherapy for the practitioner. J Clin Oncol.
33:2092–2099. 2015. View Article : Google Scholar : PubMed/NCBI
|