Introduction
Zinc finger and BTB domain containing 7A (ZBTB7A),
also known as FBI, LRF or OCZF, is a member of the poxvirus and
zinc finger/broad-complex, tramtrack and bric-à-brac and Kruppel
family of transcriptional repressors, which serve essential
functions in cell transformation and malignancy (1). Previous studies have revealed that
ZBTB7A is implicated in the pathogenesis of several types of
cancer, including non-small cell lung and ovarian carcinomas,
gliomas, and T-cell and B-cell lymphomas (2–5). ZBTB7A is
considered to be an oncogene, but a previous study indicated that
it may also serve as a tumor suppressor in certain types of cancer,
including prostate cancer and melanoma (6,7). Although
ZBTB7A is overexpressed in human breast cancer (8), the involvement of ZBTB7A in breast
cancer remains unclear.
Transforming growth factor-β (TGF-β) is a ubiquitous
cytokine known to possess pleiotropic but context-dependent effects
on cell growth, differentiation and immune modulation (9–11). TGF-β
has three isoforms in mammals: TGF-β1, -β2 and -β3, of which TGF-β1
is the most active, accounting for 90% of body cell lines (12). Regarding the pathogenesis of breast
cancer, TGF-β1 exhibits a tumor-inhibiting effect in the early
stage, but as the tumor develops, this inhibition is often reversed
thereby promoting the development of tumors (13). It was revealed that TGF-β1 facilitates
the metastasis of tumor cells by affecting the tumor
microenvironment, enhancing invasion and suppressing immune cell
function (14). The
epithelial-mesenchymal transition (EMT) is the primary way by which
cells obtain tumor invasion and metastasis ability, and TGF-β1 is
an essential factor known to induce the EMT of cells (15–18). The
association between TGF-β1 and tumor development makes TGF-β1 a
potential target for cancer therapy aimed at inhibiting tumor
metastasis.
ZBTB7A and TGF-β1 are important factors in tumor
development. Furthermore, previous studies have suggested that
ZBTB7A is involved in the fine-tuning of TGF-β1 expression in
atherosclerosis (19), and that
TGF-β1 suppresses ZBTB7A expression in human bladder cancer cells
(20). Therefore, the present study
aimed to determine the association between ZBTB7A and TGF-β1 in
breast cancer cells and tissue.
Materials and methods
Cell culture
293T and human breast cancer MCF-7 cells (American
Type Culture Collection, Manassas, VA, USA) were maintained in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
heat-activated fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 2 mM glutamine, 100 U/ml penicillin and
100 µg/ml streptomycin at 37°C with 5% CO2.
Plasmid construction
The ZBTB7A-expressing plasmid was generated through
inserting the encoding region into a pcDNA3.1 expression vector
(Invitrogen; Thermo Fisher Scientific, Inc.) at the site of the
HindIII restriction enzyme. The ZBTB7A primers used were as
follows: Forward, 5′-CTTAAGCTTGCCACCATGGCCGGCGGCGTGG-3′ and
reverse, 5′-GTCAAGCTTTTAGGCGAGTCCGGCTGTGAAGTTAC-3′. TGF-β1 promoter
was subcloned into the pGL4.10 basic plasmid (Promega Corporation,
Madison, WI, USA) at the KpnI and HindIII restriction enzyme sites
to drive luciferase expression. The amplification primers used were
as follows: TGF-β1 PP1 (702 bp) forward,
5′-GTTGGTACCACAGTGGTCAAGAGCACA-3′ and reverse,
5′-GTTAAGCTTTGGGTCGGCAGGGGGTTTT-3′; TGF-β1 PP2 (512 bp) forward,
5′-GTTGGTACCGCTCAGTAAAGGAGAGCA-3′ and reverse,
5′-GTTAAGCTTTGGGTCGGCAGGGGGTTTT-3′.
Transient transfection and luciferase
activity assay
Transient gene delivery was performed to assess the
effect of ZBTB7A on TGF-β1 promoter activity in 293T and MCF-7
cells, as described previously (21,22).
Briefly, 1×105 cells were mixed with TGF-β1 promoter
constructs (0.75 µg), ZBTB7A expression vector (0.75 µg) and
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
Cells transfected with a pcDNA3.1 expression vector, rather than
ZBTB7A, were used as negative control. Cell extracts were prepared
48 h following transfection using a 1X lysis buffer (Promega
Corporation), and a 10 µl aliquot of the supernatant was mixed with
50 µl Luciferase Assay reagent (Promega Corporation) and analyzed
using a microplate luminometer. Luciferase activity was normalized
using a Renilla luciferase internal control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and semi-quantitative
RT-PCR
For RT-qPCR, total RNA was extracted from
2×105 293T or MCF-7 cells as indicated using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Isolated RNA
was reverse transcribed using the RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The following primers were used: ZBTB7A
forward, 5′-CATCTGCGAGAAGGTCATCC-3′ and reverse,
5′-TGTCCTGCCTGGTGAAGC-3′; TGF-β1 forward,
5′-ACAATTCCTGGCGATACCTC-3′ and reverse, 5′-TAAGGCGAAAGCCCTCAAT-3′;
GAPDH forward, 5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. Quantitative measurement of target
gene mRNA levels was performed using the ABI Prism 7900 Sequence
Detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The data were analyzed using the 2−ΔΔCq method
(23). The thermocycling conditions
maintained were as follows: 95°C for 10 min, followed by 40 cycles
of 95°C for 10 sec and 60°C for 60 sec. For each reaction, 100 ng
cDNA and SYBR-Green (Takara Bio, Inc., Otsu, Japan) were used. All
samples were run in triplicate and reported as target gene
expression levels relative to GAPDH. For semi-quantitative RT-PCR,
RNA was extracted and reverse transcribed as described above. The
following primers were used: ZBTB7A forward,
5′-TCCCGTTCCCCGACCACAGCA-3′ and reverse,
5′-ATCTGCCGGTCCAGGAGGTCG-3′; β-actin forward,
5′-ATCTGGCACCACACCT-3′ and reverse, 5′-CGTCATACTCCTGCTT-3′. The
expected PCR products of ZBTB7A and β-actin were 361 and 837 bp,
respectively. The PCR products were then visualized on 1.5%
Tris-borate-EDTA (TBE) agarose gels stained with ethidium
bromide.
Western blot analysis
A total of 2×105 MCF-7 cells were first
starved in serum-free DMEM for 24 h at 37°C and stimulated with 20
ng/ml TGF-β1 (Merck KGaA, Darmstadt, Germany) at 37°C for different
amounts of time (0–6 h). Subsequently, the cells were harvested by
centrifugation at 500 × g for 10 min at room temperature, and total
protein was extracted using lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Protein concentration was
evaluated using a BCA Protein Assay kit (cat. no. P0012; Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol, and 30 µg total protein per lane was separated using 10%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes.
The membranes were blocked with 5% non-fat dry milk for 1 h at room
temperature, followed by incubation with various primary antibodies
at 4°C overnight. The primary antibodies were against ZBTB7A (cat.
no. 175918; dilution, 1:1,000; Abcam, Cambridge, MA, USA), protein
kinase B (Akt) and anti-phosphorylated Akt (cat. nos. 4691 and
4060; dilution, 1:500; Cell Signaling Technology, Inc., Danvers,
MA, USA). Loading variations were normalized against β-actin, which
was identified using the anti-β-actin antibody (cat. no. 4970;
dilution, 1:1,000; Cell Signaling Technology, Inc.). Membranes were
subsequently washed three times with PBST (pH 7.4). The membranes
were then incubated for 1 h at room temperature with horseradish
peroxidase (HRP)-conjugated secondary antibodies (cat. no. AP-132P;
dilution, 1:5,000; Merck Millipore, Darmstadt, Germany).
Subsequently, the membranes were incubated with Enhanced
Chemiluminescence Western Blotting Detection Reagent (Beijing
ComWin Biotech Co., Ltd., Beijing, China) according to the
manufacturer's instructions, and the blots were visualized by
exposure to X-ray films in the dark. Densitometric analyses were
conducted using Scion Image software (version 4.1; Scion
Corporation, Frederick, MD, USA), normalizing against β-actin.
Immunofluorescent staining
MCF-7 cells were washed twice with PBS. Following
fixation with 4% paraformaldehyde for 20 min at room temperature,
cells were permeabilized with 0.2% Triton X-100 for 30 min at room
temperature. Immunofluorescent staining was performed as described
previously (24). DNA was stained
using DAPI (Sigma-Aldrich; Merck KGaA). The ZBTB7A (cat. no.
175918; Abcam) and TGF-β1 (cat. no. 130348; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) antibodies were used at a
dilution of 1:300 and incubated for 2 h at room temperature.
Following this, they were washed three times with PBS, and then
incubated for 1 h at room temperature with fluorescein
isothiocyanate-conjugated secondary antibodies (cat. no. BA1101;
dilution, 1:100; Boster Biological Technology Co., Ltd., Wuhan,
China). Cells were examined using a confocal laser scanning
microscope and images were produced using the QImaging system
(QCAPTURE PRO 7; Surrey, BC, Canada) using a 10X oil-immersion
objective (magnification, ×100).
Electrophoretic mobility shift assay
(EMSA)
Nuclear extract was extracted from MCF-7 cells using
a Nuclear Extraction kit (Applygen Technologies, Inc., Beijing,
China). Biotin-labeled TGF-β1 probes were synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China). The sequences were as follows:
Probe 1 wild-type, 5′-CTGTTTGCGGGGCGGAGC-3′ and mutant,
5′-CTGTTTGCAAAACGGAGC-3′; probe 2 wild-type,
5′-TTCCTGGGTGGGGCCGGGGGCGGCT-3′ and mutant,
5′-TTCCTGGGTAAAACCAAAAACGGCT-3′. EMSA was performed using a
LightShift Chemiluminescent EMSA kit (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Briefly,
biotin-labeled probes (100 or 200 fM) and 20 µg nuclear extract
prepared from the MCF-7 cells were incubated for 20 min at room
temperature. Free probes were separated from DNA-protein complexes
using electrophoresis on a native 6% polyacrylamide gel in 0.5X TBE
buffer. Following electrophoresis, the DNA was transferred to a
positively charged nylon membrane, cross-linked and detected using
the chemiluminescence reagent. IgG (cat. no. A7016; Beyotime
Institute of Biotechnology) was used as a negative control of
ZBTB7A.
LY294002 inhibition experiment
MCF-7 cells with or without pretreatment with 10 µM
or 25 µM LY294002 (Calbiochem, Merck Millipore, Darmstadt,
Germany), a PI3K inhibitor, for 0.5 h were cultured in the presence
of 20 ng/ml TGF-β1 for 6 h at 37°C. Cells were harvested, and
ZBTB7A mRNA expression was analyzed using semi-quantitative RT-PCR,
and ZBTB7A protein expression was determined using
immunofluorescence assays (magnification, ×100) and western
blotting.
Tissue microarray and human cancer
genomics analysis
A tissue microarray (BR2086; US Biomax, Inc.,
Rockville, MD, USA) consisting of 185 breast cancer samples was
used. These samples were histologically interpretable and were
analyzed for the correlation between ZBTB7A and TGF-β1.
Immunohistochemical staining was performed as detailed in our
previous study (25). The tissues
were blocked with 1% bovine serum albumin (Roche Diagnostics,
Basel, Switzerland) at room temperature for 1 h. Rabbit polyclonal
antibody directed against ZBTB7A (cat. no. 175918; dilution, 1:50;
Abcam) and mouse monoclonal antibody against TGF-β1 (cat. no.
130348; dilution, 1:25; Santa Cruz Biotechnology, Inc.) were used.
In addition, ZBTB7A mRNA data for breast cancer were downloaded
from the Bittner breast data set on Oncomine (www.oncomine.org; Thermo Fisher Scientific, Inc.).
This dataset was used for correlation analysis between ZBTB7A and
TGF-β1 expression levels. The correlation analysis was performed
using GraphPad Prism software (version 5.0; GraphPad Software,
Inc., La Jolla, CA, USA). Spearman's rank correlation coefficients
were generated to determine the degree of the correlation.
Statistical analysis
All experiments were performed ≥3 times, and the
results were expressed as the mean ± standard deviation unless
otherwise stated. GraphPad Prism software (version 5.0) was used
for statistical analysis. Comparisons between two groups were
performed using the two-tailed Student's t-test. Comparisons among
multiple groups were performed using one-way analysis of variance
with post hoc intergroup comparisons using the Tukey test.
Spearman's rank-correlation coefficients were used to assess the
correlation between ZBTB7A and TGF-β1 expression levels. P<0.05
was considered to indicate a statistically significant
difference.
Results
TGF-β1 induces the expression of
ZBTB7A in MCF-7 cells
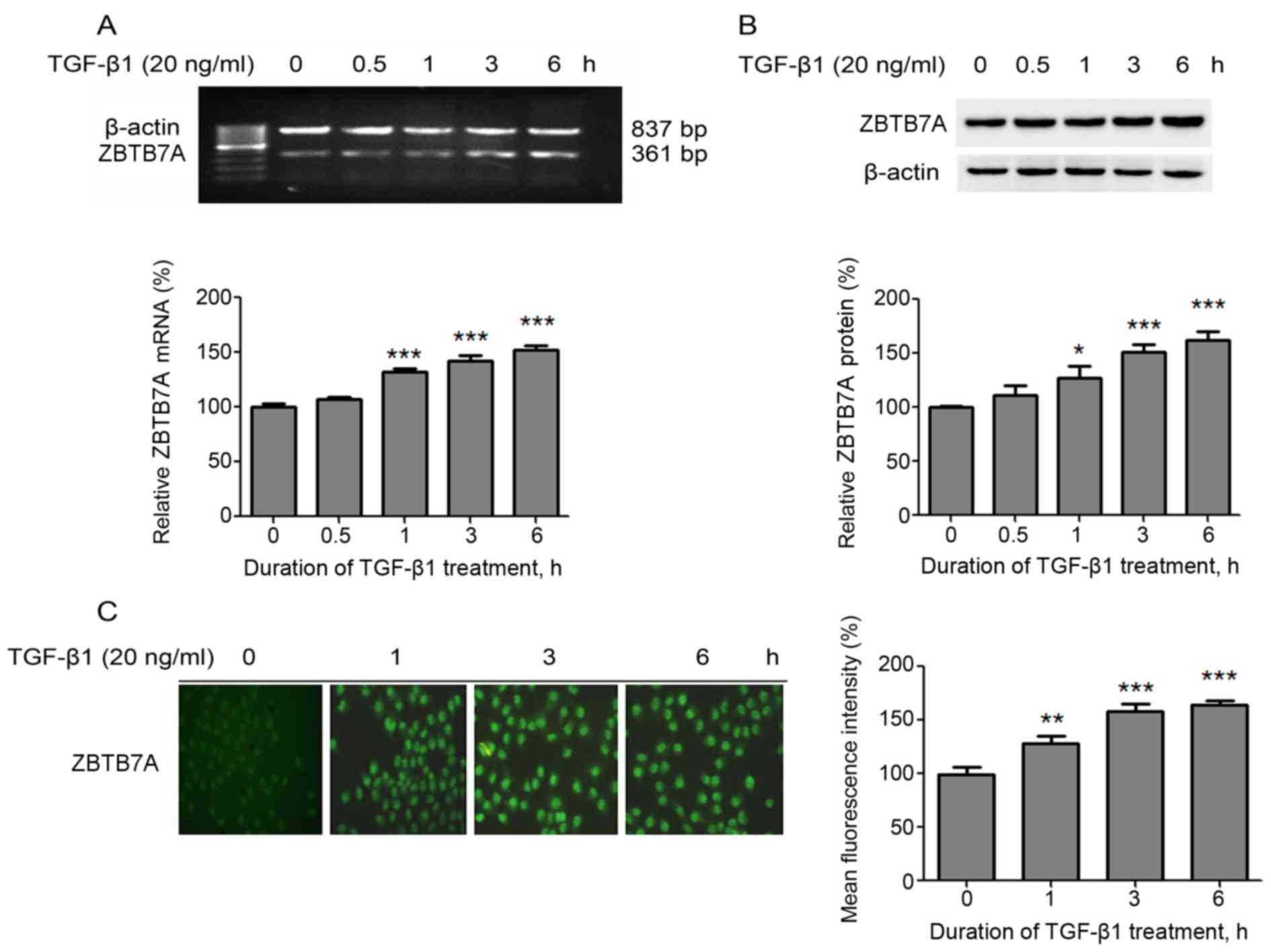
First, the effect of TGF-β1 on the expression of
ZBTB7A was evaluated in MCF7 cells. RT-qPCR analysis results
demonstrated that the ZBTB7A mRNA expression levels were
significantly upregulated following TGF-β1 treatment, in a
time-dependent manner, compared with untreated cells (Fig. 1A). Similarly, western blot analysis
and immunofluorescence assay results revealed that ZBTB7A protein
expression levels were significantly increased following TGF-β1
treatment, in a time-dependent manner, compared with untreated
cells (Fig. 1B and C). These data
supported the notion that TGF-β1 induces the expression of ZBTB7A
in MCF-7 cells.
TGF-β1 induces the expression of
ZBTB7A via the phosphoinositide 3-kinase (PI3K)-Akt signaling
pathway
The PI3K-Akt signaling pathway is an important
pathway in terms of tumor proliferation, invasion and metastasis,
and it is known to be a non-classical pathway which involves TGF-β
signaling. Therefore, the involvement of the PI3K-Akt signaling
pathway in TGF-β1-induced ZBTB7A expression was determined. TGF-β1
was demonstrated to increase the expression of phosphorylated Akt
without altering the expression of total Akt (Fig. 2A). Furthermore, when TGF-β1 treatment
was combined with the PI3K inhibitor LY294002, the mRNA and protein
expression levels of ZBTB7A decreased compared with TGF-β1
treatment alone (Fig. 2B-D). These
results indicated that the PI3K-Akt signaling pathway may, at least
in part, be responsible for TGF-β1-induced ZBTB7A expression in
breast cancer cells.
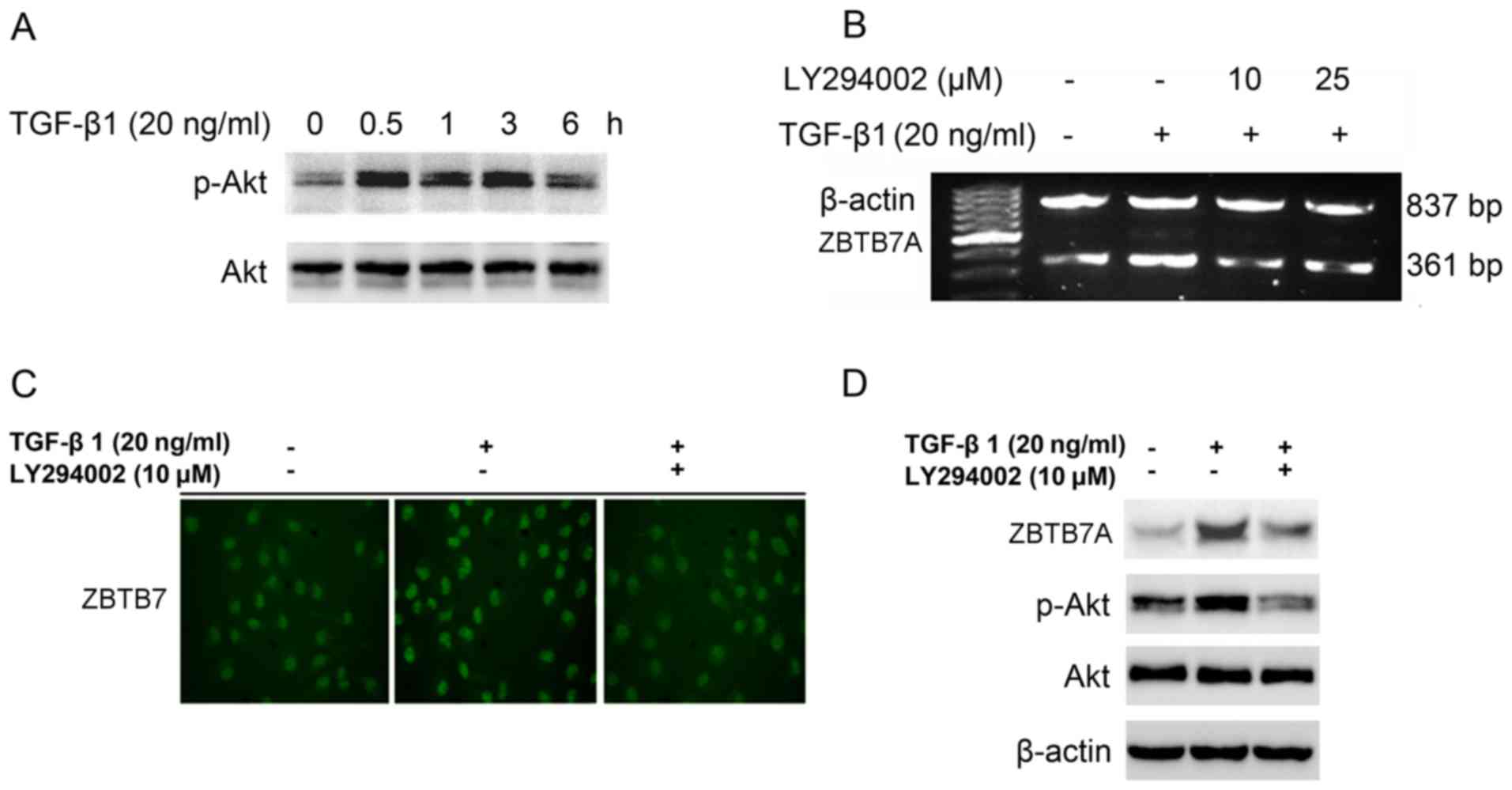 | Figure 2.TGF-β1 induces the expression of
ZBTB7A via the PI3K-Akt signaling pathway. (A) MCF-7 cells were
treated with 20 ng/ml TGF-β1 for 0–6 h, and then cells were
harvested and the indicated proteins were analyzed using western
blotting. MCF-7 cells with or without pretreatment with 10 µM or 25
µM LY294002, a PI3K inhibitor, for 0.5 h were cultured in the
presence of 20 ng/ml TGF-β1 for 6 h. Cells were harvested, and
ZBTB7A mRNA expression was analyzed using (B) semi-quantitative
RT-PCR, and ZBTB7A protein expression was determined using (C)
immunofluorescence assays (magnification, ×100) and (D) western
blotting. TGF-β1, transforming growth factor-β1; ZBTB7A, zinc
finger and BTB domain containing 7A; PI3K, phosphoinositide
3-kinase; Akt, protein kinase B; p-, phosphorylated. |
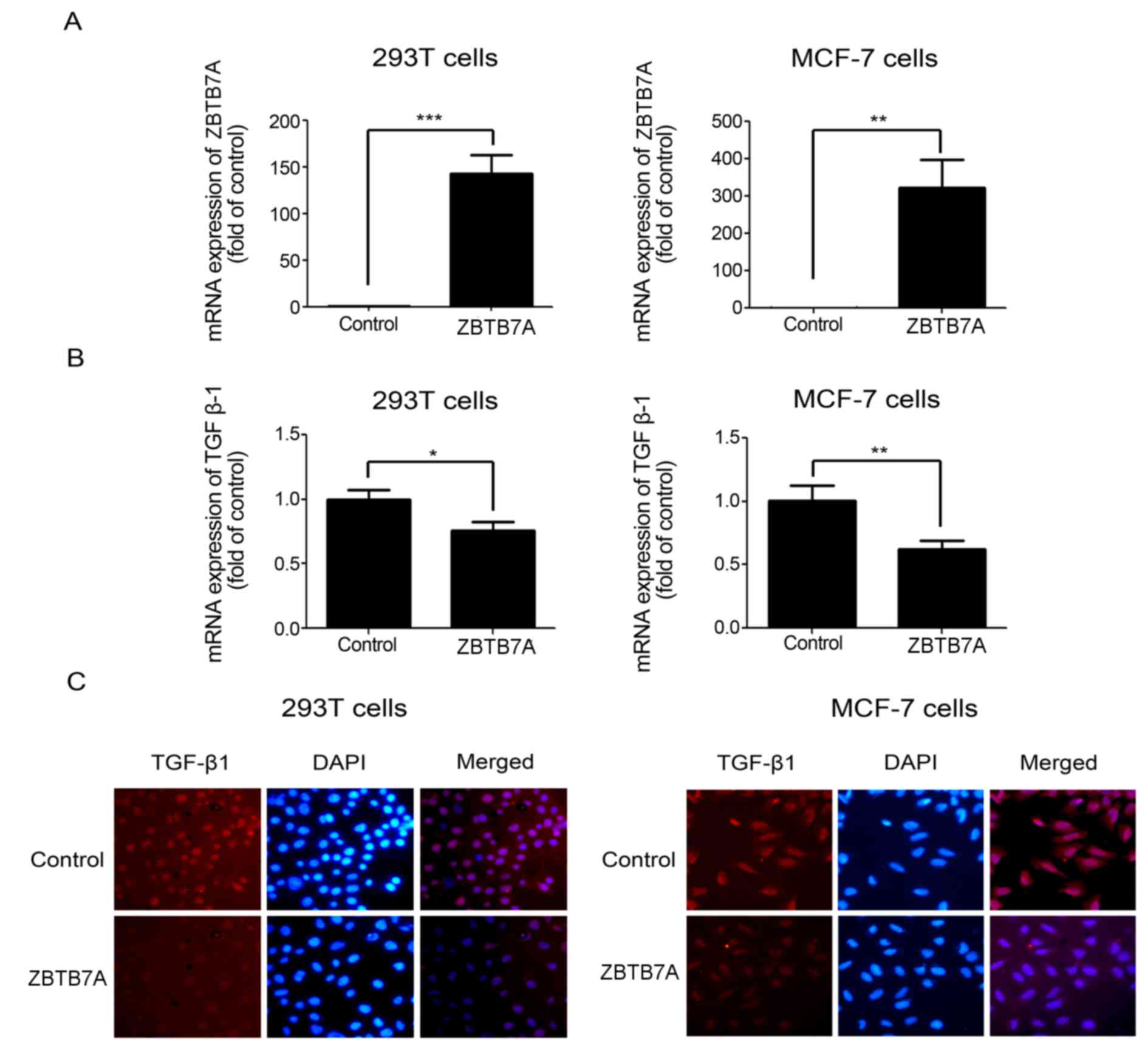
ZBTB7A inhibited the expression of
TGF-β1 in 293T and MCF-7 cells
To determine the effect of ZBTB7A on TGF-β1
expression, 293T and MCF7 cells were transfected with the ZBTB7A
expression plasmid (Fig. 3A). As
presented in Fig. 3B and C, the
ectopic expression of ZBTB7A in 293T and MCF7 cells led to
significant downregulation of TGF-β1 mRNA and protein expression
compared with the control cells. These results indicated that
ZBTB7A is a modulator of TGF-β1 expression and signaling in 293T
and MCF7 cells.
ZBTB7A suppresses the promoter
activity of TGF-β1 indirectly
The mechanism by which ZBTB7A regulates the
expression of TGF-β1 was further investigated. The promoter region
of TGF-β1, containing 702 bp upstream of the transcription start
site, was cloned and used to drive the expression of the luciferase
reporter in 293T and MCF-7 cells (PGL4.10-TGF-β1 PP1, −702-+1).
Deletion of the 5′ end of the TGF-β1 PP1 promoter generated the 512
bp fragment upstream of the transcription start site
(PGL4.10-TGF-β1 PP2, −512-+1). The luciferase assay demonstrated
that ZBTB7A significantly inhibited the promoter activities of
TGF-β1 PP1 and TGF-β1 PP2, suggesting that the −512-+1 fragment of
the TGF-β1 promoter is an important regulation site for ZBTB7A in
293T and MCF-7 cells (Fig. 4A).
ZBTB7A is a transcriptional factor, so it was hypothesized that
ZBTB7A may suppress TGF-β1 promoter activity through directly
binding to the promoter of TGF-β1 PP2. To confirm this hypothesis,
two probes within the −512-+1 fragment of TGF-β1 promoter sequence
were utilized for EMSA. Unexpectedly, no specific shifted band with
the anti- ZBTB7A antibody was observed in the EMSA assay (Fig. 4B). This suggested that ZBTB7A
suppressed the promoter activity of TGF-β1 in an indirect manner,
leading to the downregulation of TGF-β1 in MCF-7 cells.
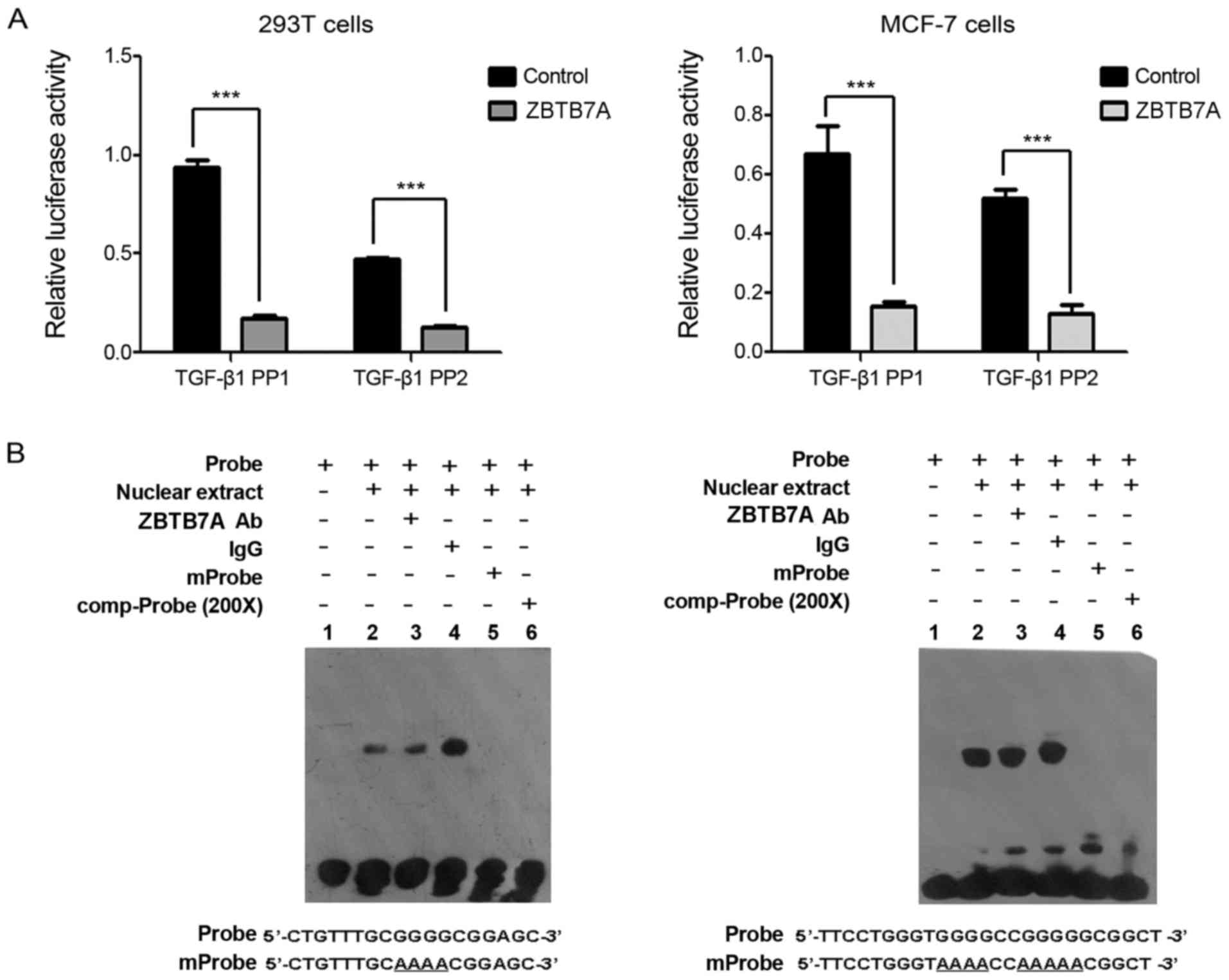 | Figure 4.ZBTB7A suppresses the promoter
activity of TGF-β1 indirectly. (A) ZBTB7A inhibited the promoter
activity of TGF-β1. MCF-7 and 293T cells were co-transfected with
plasmids of ZBTB7A, PGL4.10-TGF-β1 PP1 or PGL4.10-TGF-β1 PP2, and
Renilla. Subsequently, the cells were harvested and
subjected to a dual-luciferase reporter assay. (B) Direct binding
analysis between ZBTB7A and promoter sequences of TGF-β1 in MCF-7
cells. An electrophoretic mobility shift assay was performed. IgG
was used as a negative control. Data are presented as the mean ±
standard deviation following three independent experiments.
***P<0.001, with comparisons indicated by lines. ZBTB7A, zinc
finger and BTB domain containing 7A; TGF-β1, transforming growth
factor-β1; IgG, immunoglobulin G; Comp-probe, unlabeled competitive
probe; mProbe, unlabeled mutant probe; Ab, antibody. |
Correlation between ZBTB7A and TGF-β1
expression in breast cancer tissue
To further identify the association between ZBTB7A
and TGF-β1 expression in breast cancer, a tissue microarray
(BR2086), consisting of 185 breast cancer cases, was used. No
significant correlation (r=−0.077; P=0.296; Table I) was identified between the
expression of ZBTB7A and TGF-β1 upon analysis of the human breast
cancer tissue microarray, regardless of breast cancer types. To
determine the association between ZBTB7A and TGF-β1 expression, the
mining Bittner breast data set on Oncomine was also used. In
agreement with the results of the tissue microarray, no significant
correlation (r=−0.001; P=0.982) was identified between the mRNA
expression levels of ZBTB7A and TGF-β1 in breast cancer tissue (335
cases; data not shown). These data suggested that there was no
significant correlation between the expression of ZBTB7A and TGF-β1
in breast cancer tissue.
 | Table I.Correlation between ZBTB7A and TGF-β1
expression in breast cancer. |
Table I.
Correlation between ZBTB7A and TGF-β1
expression in breast cancer.
|
|
| ZBTB7A expression, n
(%) |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | n | − | + | Spearman's R | P-value |
|---|
| TGF-β1
expression |
|
|
| −0.077 | 0.296 |
| − | 104 | 5 (4.8) | 99 (95.2) |
|
|
| + | 81 | 7 (8.6) | 74 (91.4) |
|
|
Discussion
Embryonic fibroblasts (MEF) from ZBTB7A-null mice
are resistant to cellular or viral oncogene-induced carcinogenic
transformation. On the other hand, ectopic expression of ZBTB7A
makes MEF cells susceptible to oncogenic transformation (26). These results suggest an important
function for ZBTB7A in carcinogenesis. ZBTB7A is considered to be
an oncogenic transcription factor in multiple types of cancer,
including in colorectal and oral cancer (27,28).
However, previous studies have revealed that ZBTB7A may also act as
a tumor suppressor through the transcriptional repression of
glycolysis in colon cancer (29),
suppression of melanoma cell adhesion molecule in melanoma
(7), and inhibition of a SRY-box
9-dependent pathway for tumor invasion and the bypassing of
cellular senescence in prostate cancer (6). Therefore, ZBTB7A has dual but
context-dependent effects on tumor formation and development.
ZBTB7A has been demonstrated to be aberrantly expressed in breast
cancer tissue (8). It is thus
important to elucidate whether the elevated expression of ZBTB7A in
breast cancer functions as an oncogene, or as a compensatory tumor
suppressor gene.
Initial studies on TGF-β concerned its ability to
induce malignant behavior in normal fibroblasts (30), which led to the idea that TGF-β was an
essential promoting factor in tumorigenesis. However, TGF-β was
later demonstrated to possess profound growth suppressive effects
on numerous cell types, including epithelial cells (31). Thus, TGF-β possesses pleiotropic but
context-dependent effects on tumor transformation. In the early
stage of breast cancer, TGF-β suppresses tumor formation, but in
the late stage of breast cancer it promotes tumor development
(13). Notably, TGF-β signaling was
demonstrated to be activated in bone metastases from patients with
breast cancer (32). Abnormal
upregulation of TGF-β is positively correlated with the
progression, angiogenesis and metastasis of breast cancer,
resulting in adverse clinical outcomes in the late stage of the
disease (33). TGF-β1 is known as the
predominant isoform in breast tissue and cells, thus, TGF-β1 was
selected for the present study.
ZBTB7A and TGF-β1 possess dual, but
context-dependent effects on tumor formation and development.
Furthermore, ZBTB7A has been demonstrated to be a potential
transcriptional regulator of TGF-β1 expression in human
atherosclerotic arteries (19). In
addition, TGF-β1 suppresses ZBTB7A expression in human bladder
cancer cells (20). In the present
study, the association between ZBTB7A and TGF-β1 was investigated
in breast cancer. First, the effect of TGF-β1 on ZBTB7A expression
was evaluated, which revealed that TGF-β1 significantly induced the
expression of ZBTB7A through the PI3K-Akt signaling pathway in
MCF-7 cells. TGF-β1 has been reported to suppress ZBTB7A expression
in human bladder cancer cells, which is inconsistent with the
results of the present study in breast cancer cells. The
inconsistency may be explained by the context-dependent function of
TGF-β1 and ZBTB7A in different tumor stages and tumor types, as
described in previous studies (2–7,9–11). Next,
the effect of ZBTB7A on TGF-β1 expression was evaluated, and it was
revealed that the ectopic expression of ZBTB7A led to significant
downregulation of TGF-β1 mRNA and protein expression levels in
MCF-7 and 293T cells. The potential mechanisms of ZBTB7A-induced
TGF-β1 expression were further evaluated, and ZBTB7A was
demonstrated to inhibit the promoter activity of TGF-β1 PP1
(−702-+1 fragment) and TGF-β1 PP2 (−512-+1 fragment), suggesting
that ZBTB7A regulates the transcription of TGF-β1 through the
fragment of −512-+1 within TGF-β1 promoter sequences. However, the
EMSA assay confirmed that ZBTB7A did not bind to the TGF-β1
promoter directly, indicating that ZBTB7A may suppress the promoter
activity of TGF-β1 indirectly.
Taken together, the results of the present study
suggested a negative feedback loop between ZBTB7A and TGF-β1 in
breast cancer cells. To further confirm the results obtained from
the cell model, a tissue microarray with 185 breast cancer samples
was used, and the association between ZBTB7A and TGF-β1 expression
was assessed. No significant correlation between the expression of
ZBTB7A and TGF-β1 in breast cancer tissue was identified from the
clinical data. The results of the tissue microarray were further
confirmed using the Bittner breast data set in Oncomine, with 335
breast cancer cases. The results obtained from clinical analysis
supported the existence of the negative feedback loop between
ZBTB7A and TGF-β1 in breast cancer, resulting in the balance and
homeostasis of ZBTB7A and TGF-β1 expression.
In conclusion, to the best of our knowledge, this is
the first study to reveal a negative feedback loop mechanism
between ZBTB7A and TGF-β1 in breast cancer, which may aid in
understanding the pleiotropic functions of ZBTB7A in the TGF-β
signaling network and in breast cancer progression.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81502276, 81272355,
81170807 and 81372824), Major Projects of Science and Technology of
Health and Family Planning Commission of Hunan Province (grant no.
A2017013) and the Natural Science Foundation of Hunan Province
(grant no. 2016JJ4077).
References
|
1
|
Maeda T, Hobbs RM, Merghoub T, Guernah I,
Zelent A, Cordon-Cardo C, Teruya-Feldstein J and Pandolfi PP: Role
of the proto-oncogene Pokemon in cellular transformation and ARF
repression. Nature. 433:278–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Apostolopoulou K, Pateras IS, Evangelou K,
Tsantoulis PK, Liontos M, Kittas C, Tiniakos DG, Kotsinas A,
Cordon-Cardo C and Gorgoulis VG: Gene amplification is a relatively
frequent event leading to ZBTB7A (Pokemon) overexpression in
non-small cell lung cancer. J Pathol. 213:294–302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang L, Siu MK, Wong OG, Tam KF, Lam EW,
Ngan HY, Le XF, Wong ES, Chan HY and Cheung AN: Overexpression of
proto-oncogene FBI-1 activates membrane type 1-matrix
metalloproteinase in association with adverse outcome in ovarian
cancers. Mol Cancer. 9:3182010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maeda T, Merghoub T, Hobbs RM, Dong L,
Maeda M, Zakrzewski J, van den Brink MR, Zelent A, Shigematsu H,
Akashi K, et al: Regulation of B versus T lymphoid lineage fate
decision by the proto-oncogene LRF. Science. 316:860–866. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rovin RA and Winn R: Pokemon expression in
malignant glioma: An application of bioinformatics methods.
Neurosurg Focus. 19:E82005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang G, Lunardi A, Zhang J, Chen Z, Ala U,
Webster KA, Tay Y, Gonzalez-Billalabeitia E, Egia A, Shaffer DR, et
al: Zbtb7a suppresses prostate cancer through repression of a
Sox9-dependent pathway for cellular senescence bypass and tumor
invasion. Nat Genet. 45:739–746. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu XS, Genet MD, Haines JE, Mehanna EK,
Wu S, Chen HI, Chen Y, Qureshi AA, Han J, Chen X, et al: ZBTB7A
suppresses melanoma metastasis by transcriptionally repressing
MCAM. Mol Cancer Res. 13:1206–1017. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aggarwal A, Hunter WJ III, Aggarwal H,
Silva ED, Davey MS, Murphy RF and Agrawal DK: Expression of
leukemia/lymphoma-related factor (LRF/POKEMON) in human breast
carcinoma and other cancers. Exp Mol Pathol. 89:140–148. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lampropoulos P, Zizi-Sermpetzoglou A,
Rizos S, Kostakis A, Nikiteas N and Papavassiliou AG: Prognostic
significance of transforming growth factor beta (TGF-β) signaling
axis molecules and E-cadherin in colorectal cancer. Tumour Biol.
33:1005–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou L, Lopes JE, Chong MM, Ivanov II, Min
R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al:
TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by
antagonizing RORgammat function. Nature. 453:236–240. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo S, Kleemann GA, Ashraf JM, Shaw WM and
Murphy CT: TGF-β and insulin signaling regulate reproductive aging
via oocyte and germline quality maintenance. Cell. 143:299–312.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flanders KC and Wakefield LM: Transforming
growth factor-(beta)s and mammary gland involution; functional
roles and implications for cancer progression. J Mammary Gland Biol
Neoplasia. 14:131–144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moses H and Barcellos-Hoff MH: TGF-beta
biology in mammary development and breast cancer. Cold Spring Harb
Perspect Biol. 3:a0032772011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Derynck R and Akhurst RJ: Differentiation
plasticity regulated by TGF-beta family proteins in development and
disease. Nat Cell Biol. 9:1000–1004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johansson J, Tabor V, Wikell A, Jalkanen S
and Fuxe J: TGF-β1-induced epithelial-mesenchymal transition
promotes monocyte/macrophage properties in breast cancer cells.
Front Oncol. 5:32015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lv ZD, Kong B, Li JG, Qu HL, Wang XG, Cao
WH, Liu XY, Wang Y, Yang ZC, Xu HM and Wang HB: Transforming growth
factor-β 1 enhances the invasiveness of breast cancer cells by
inducing a Smad2-dependent epithelial-to-mesenchymal transition.
Oncol Rep. 29:219–225. 2013.PubMed/NCBI
|
|
17
|
Walsh LA and Damjanovski S: IGF-1
increases invasive potential of MCF 7 breast cancer cells and
induces activation of latent TGF-β1 resulting in epithelial to
mesenchymal transition. Cell Commun Signal. 9:102011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng B, Yang X, Liu J, He F, Zhu Z and
Zhang C: Focal adhesion kinase mediates TGF-beta1-induced renal
tubular epithelial-to-mesenchymal transition in vitro. Mol Cell
Biochem. 340:21–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dhaouadi N, Li JY, Feugier P, Gustin MP,
Dab H, Kacem K, Bricca G and Cerutti C: Computational
identification of potential transcriptional regulators of TGF-β1 in
human atherosclerotic arteries. Genomics. 103:357–370. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li W, Kidiyoor A, Hu Y, Guo C, Liu M, Yao
X, Zhang Y, Peng B and Zheng J: Evaluation of transforming growth
factor-β1 suppress Pokemon/epithelial-mesenchymal transition
expression in human bladder cancer cells. Tumour Biol.
36:1155–1162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zu X, Yu L, Sun Q, Liu F, Wang J, Xie Z,
Wang Y, Xu W and Jiang Y: SP1 enhances Zbtb7A gene expression via
direct binding to GC box in HePG2 cells. BMC Res Notes. 2:1752009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Z, Zhong L, Krishack PA, Robbins S,
Cao JX, Zhao Y, Chung S and Cao D: Structure and promoter
characterization of aldo-keto reductase family 1 B10 gene. Gene.
437:39–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao C, She T, Wang L, Su Y, Qu L, Gao Y,
Xu S, Cai S and Shou C: Daucosterol inhibits cancer cell
proliferation by inducing autophagy through reactive oxygen
species-dependent manner. Life Sci. 137:37–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhong J, Cao RX, Zu XY, Hong T, Yang J,
Liu L, Xiao XH, Ding WJ, Zhao Q, Liu JH and Wen GB: Identification
and characterization of novel spliced variants of PRMT2 in breast
carcinoma. FEBS J. 279:316–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maeda T, Hobbs RM and Pandolfi PP: The
transcription factor Pokemon: A new key player in cancer
pathogenesis. Cancer Res. 65:8575–8578. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao Y, Yao YH, Li L, An WF, Chen HZ, Sun
LP, Kang HX, Wang S and Hu XR: Pokemon enhances proliferation, cell
cycle progression and anti-apoptosis activity of colorectal cancer
independently of p14ARF-MDM2-p53 pathway. Med Oncol. 31:2882014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sartini D, Lo Muzio L, Morganti S, Pozzi
V, Di Ruscio G, Rocchetti R, Rubini C, Santarelli A and Emanuelli
M: Pokemon proto-oncogene in oral cancer: potential role in the
early phase of tumorigenesis. Oral Dis. 21:462–469. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu XS, Haines JE, Mehanna EK, Genet MD,
Ben-Sahra I, Asara JM, Manning BD and Yuan ZM: ZBTB7A acts as a
tumor suppressor through the transcriptional repression of
glycolysis. Genes Dev. 28:1917–1928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sanchez-Capelo A: Dual role for TGF-beta1
in apoptosis. Cytokine Growth Factor Rev. 16:15–34. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duangkumpha K, Techasen A, Loilome W,
Namwat N, Thanan R, Khuntikeo N and Yongvanit P: BMP-7 blocks the
effects of TGF-β-induced EMT in cholangiocarcinoma. Tumour Biol.
35:9667–9676. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang Y, He W, Tulley S, Gupta GP,
Serganova I, Chen CR, Manova-Todorova K, Blasberg R, Gerald WL and
Massagué J: Breast cancer bone metastasis mediated by the Smad
tumor suppressor pathway. Proc Natl Acad Sci USA. 102:pp.
13909–13914. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bierie B and Moses HL: Tumour
microenvironment: TGFbeta: The molecular Jekyll and Hyde of cancer.
Nat Rev Cancer. 6:506–520. 2006. View
Article : Google Scholar : PubMed/NCBI
|