Introduction
Recent improvements in chemotherapy, including the
use of biological agents, have been shown to prolong the survival
of patients with metastatic colorectal cancer (mCRC), with a
recorded median overall survival time of 30 months (1,2). For
patients with mCRC and liver metastasis, liver resection combined
with systemic therapies resulted in a 5-year survival rate of
25–40% (3). Combination regimens
using various biological agents with cytotoxic chemotherapy
achieved high response rates and a reduced tumor size (4). This enables liver resection for mCRC
patients with borderline resectable, as well as un-resectable,
liver metastasis.
Tumor response to chemotherapy is the initial step
in the selection of patients who are most likely to benefit from
surgery. Changes in the characteristics of liver metastases may be
assessed with respect to surgical and oncological viewpoints. In
addition to the technical considerations, the ability of the tumor
to be resected and its biology are the most important factors that
determine the outcome of the patients. Candidates for tumor
resection of liver metastases should have surgically resectable
masses and be oncologically stable. The patients should also be
unlikely to relapse within a short period of time following surgery
(5–11).
The Response Evaluation Criteria in Solid Tumors
(RECIST) model is widely used to evaluate tumor response, however,
since its introduction, there has been increasing concern regarding
the use of traditional tumor response criteria (12,13). This
is due to RECIST being limited in its application in assessing the
response of tumors to biological agents that exhibit a cytostatic
mechanism of action. For patients with metastatic gastrointestinal
stromal tumors, RECIST using anatomical information only (such as
tumor size), has been shown to significantly underestimate the
initial tumor response to imatinib (14). This is since patients that exhibit a
stable response to imatinib have a similar outcome to those who
achieve a complete response (CR) or partial response (PR) when
evaluated using RECIST (15). Several
studies have shown that morphological response is an improved
alternative to RECIST for predicting the outcome of patients with
colorectal liver metastases (16–18).
However, the potential clinical application of morphological
evaluation has not been attempted for the selection of patients
most likely to benefit from surgery. The present study examined
whether evaluations that included morphological criteria were
useful in selecting the best therapeutic strategy for patient
treatment.
Patients and methods
Patients
A total of 50 patients with mCRC and unresectable
liver metastasis were recruited for the present retrospective
study. The patients had histologically confirmed and measurable
mCRC. Each patient underwent oxaliplatin-based chemotherapy, with
or without bevacizumab, between May 2008 and November 2012 at the
Saitama Medical Center (Jichi Medical University, Saitama, Japan).
The present study was approved by the Research Ethics Committee of
Jichi Medical University.
Imaging analysis
Tumor morphology was assessed using enhanced
computed tomography (CT) and characterized according to the
criteria previously described (16):
Group 1, homogeneous low attenuation with a thin, sharply-defined
tumor-liver interface; group 3, heterogeneous attenuation with a
thick, poorly-defined tumor-liver interface; and group 2,
intermediate morphology that could be rated as either group 1 or 3.
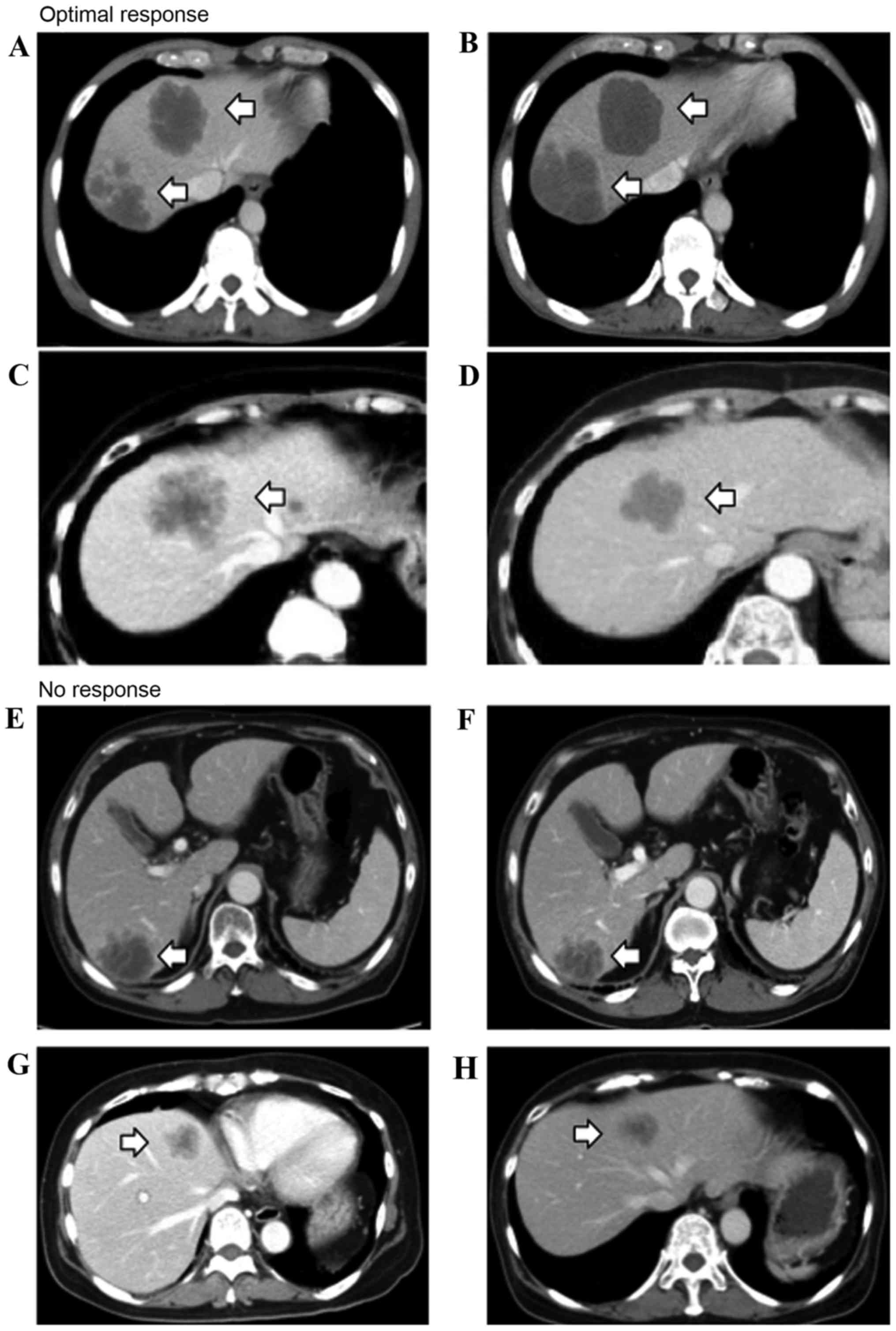
A change in morphology between group 3 or 2 to group 1 was defined
as an optimal response (OR; Fig. 1),
and a group 3 to group 2 change was defined as an incomplete
response (IR). The absence of marked changes in tumor morphology
was defined as no response (NR; Fig.
1). In patients with multiple tumors, morphological response
was assigned based on changes observed in the majority of the
tumors. Response to chemotherapy was also determined using
RECIST.
Statistical analysis
Fisher's exact test was used to examine the
association between two categorical variables. Continuous
comparison of the variables between two groups was performed.
Unpaired t-tests were used for those variables that followed a
normal distribution, and the non-parametric Mann-Whitney-Wilcoxon
test was used for those variables that did not follow a normal
distribution. P<0.05 was considered to indicate a statistically
significant difference. Values are shown as the mean ± standard
error. Progression-free survival (PFS) and overall survival (OS)
data were plotted as Kaplan-Meier curves, and the differences among
the groups were compared using a log-rank test.
Results
Characteristics of patients
The present study included 34 men and 16 women with
a median age of 65 years (range, 45–83 years). A total of 28
patients presented with primary colon tumors, while the other 22
patients were diagnosed with tumors of the rectum. Liver metastasis
was detected simultaneously in 34 patients and metachronously in 16
patients. Solitary liver metastases were observed in 19 patients
and multiple metastases were observed in 31 patients. A total of 30
patients presented with metastasis in extrahepatic regions,
including 10 in the lymph nodes, 9 in the lung, 5 in the
intra-peritoneum, 2 in the bone, 2 in the pelvic node at the
anastomotic site, one in the spleen and one in the ovarian. The
size of the largest metastasis ranged from 9 to 140 mm, with a
median size of 47 mm. All patients underwent oxaliplatin-based
chemotherapy with or without bevacizumab as the first line of
treatment. The treatment regimens were mFOLFOX6 in 15 patients,
which included 200 mg/m2 folinic acid, 400
mg/m2 5-FU and 85 mg/m2 oxaliplatin on day 1,
followed by 46 h of continuous infusion with 2,400 mg/m2
5-FU on days 1 and 2 and XELOX in the remaining 35 patients, which
consisted of 2,000 mg/m2 capecitabine on days 1–15) and
130 mg/m2 oxaliplatin on day 1. A total of 19 patients
were also treated with bevacizumab, anti-vascular endothelial
growth factor (VEGF) antibody: For one patient treated with
mFOLFOX6 regimen, 5 mg/kg bevacizumab was administrated on day 1
and q14d, and for 18 patients treated with XELOX regimen, 7.5 mg/kg
bevacizumab was administrated on day 1 and q21d.
Morphological response rate
The morphological response rate following treatment
with or without bevacizumab is shown in Table I. There were 14 responders (28.0%),
including 7 patients with an OR (14.0%) and 7 patients with an IR
(14.0%). A total of 36 patients (72.0%) showed NR. The rate of
patients classified as OR/IR who were treated with bevacizumab was
47.4% (9/19), whereas for patients who did not receive bevacizumab,
the rate was 19.4% (6/31).
 | Table I.Morphological response rate according
to treatment with or without bevacizumab. |
Table I.
Morphological response rate according
to treatment with or without bevacizumab.
| Treatment | Patients, n | Optimal response, n
(%) | Incomplete response,
n (%) | No response, n
(%) |
|---|
| Total | 50 | 7/50
(14.0) | 7/50 (14.0) | 36/50 (72.0) |
| Chemotherapy with
bevacizumab | 19 | 5/19
(26.3) | 4/19 (21.1) | 10/19 (52.6) |
| Chemotherapy without
bevacizumab | 31 | 2/31 (6.5) | 4/31 (12.9) | 25/31 (80.6) |
CT evaluations according to RECIST and
morphological criteria
Using RECIST, 10 patients had a CR/PR, 27 had stable
disease (SD) and 13 had progressive disease (PD). No significant
difference was observed in PFS time between those patients who were
classified as CR/PR and those patients who had SD/PD (10.9 months
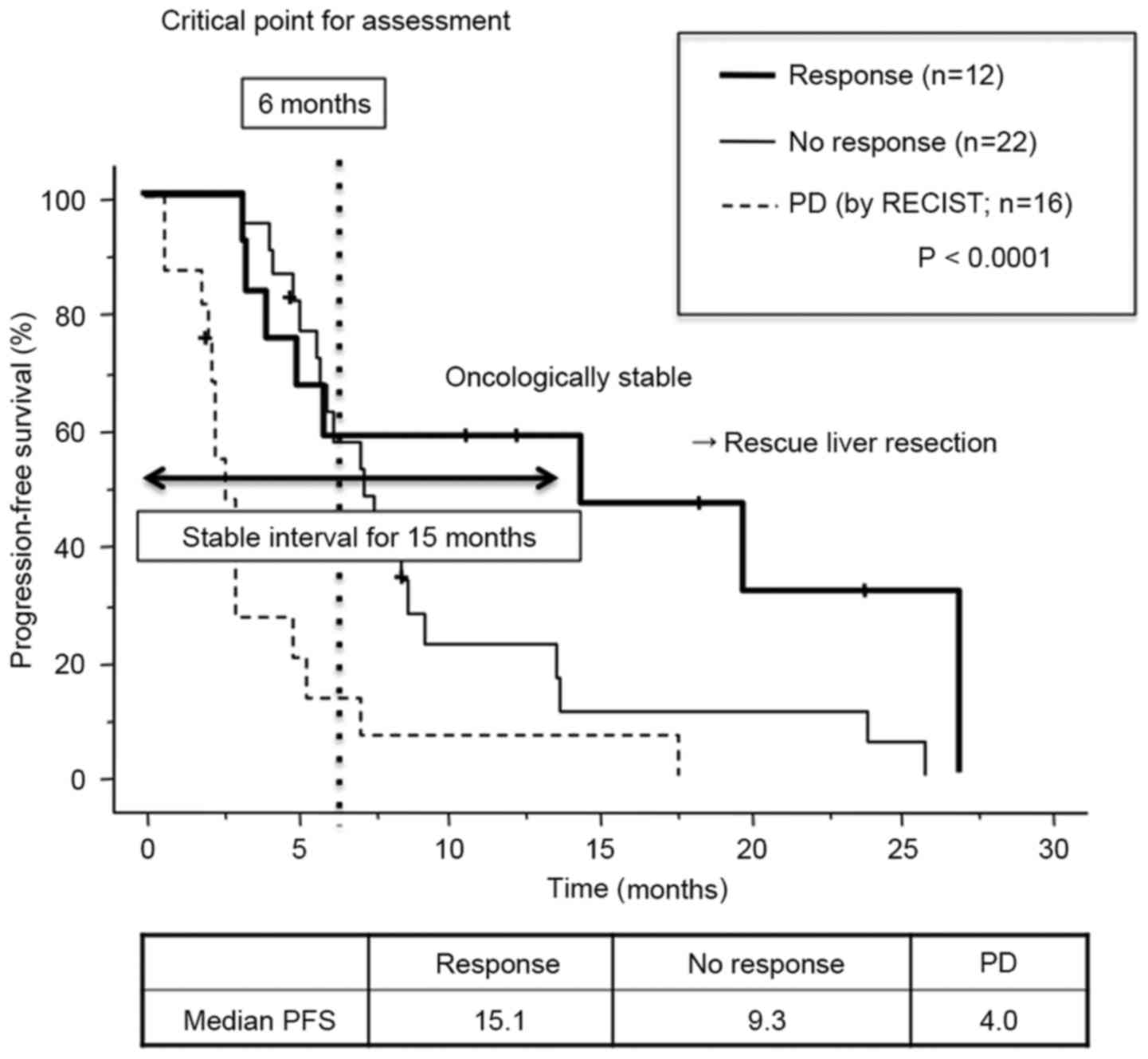
for CR/PR vs. 8.6 months for SD/PD; P=0.2604; Fig. 2A). According to the morphological
criteria, 14 patients were classified as having OR/IR, while 36
patients had NR. PFS time for patients showing OR/IR was
significantly improved when compared with patients showing NR (13.2
months for OR/IR vs. 8.7 months for NR; P=0.0426; Fig. 2B). For those patients who showed PD,
they also had a short PFS period (4.0 months; n=16) compared with
the SD and CR/PR groups (P<0.0001). Therefore, these patients
were classified as group 1 and used as a comparison to patients in
the other groups using RECIST and morphological criteria. The
RECIST criteria did not show any significant difference in PFS time
between patients with CR/PR and SD (11.5 months for CR/PR vs. 11.0
months for SD; Fig. 2C).
Morphological criteria, however, revealed an increase in the
difference in PFS time between those patients with OR/IR and those
with NR (15.1 months for OR/IR vs. 9.3 months for NR; P<0.0001;
Fig. 2D). Among those patients who
had SD, those treated with bevacizumab had improved median PFS
times compared with those who did not receive bevacizumab (13.1
months for bevacizumab and chemotherapy vs. 10.0 months for
chemotherapy alone; P=0.3415). This indicated that bevacizumab may
exhibit an antitumor effect that does not result in a reduction in
tumor size or morphological criteria. Comparison of the survival
curves between the OR/IR and NR groups showed that they remained
close to each other up to 6 months after chemotherapy (the second
period of CT evaluation), but the curves then diverged from each
other subsequent to this date. The median PFS was >15 months for
patients classified as having an OR or IR. These results indicated
that tumors in patients showing OR/IR AT 6 months' post-treatment
were oncologically stable, which would make the patients candidates
for surgical intervention, including rescue liver resection
(Fig. 3).
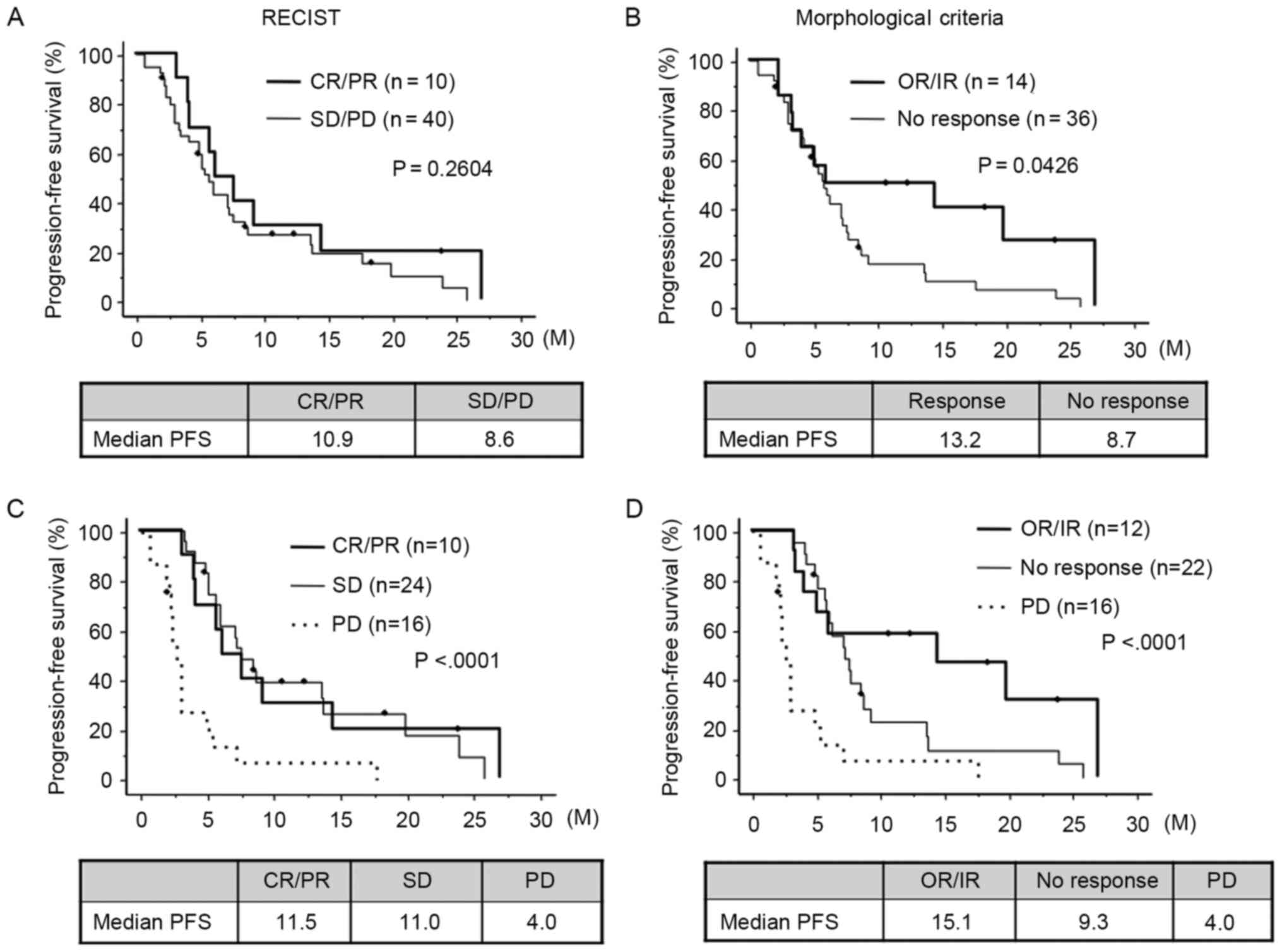 | Figure 2.PFS estimated by RECIST and
morphological criteria. (A) Comparison of PFS between patients
showing CR/PR and SD/PD estimated by RECIST. (B) Comparison of PFS
between patients showing OR/IR and NR estimated by morphological
criteria. (C) Comparison of PFS between CR/PR, SD and PD.
Morphological criteria enhanced the difference in PFS between
patients with OR/IR and those with NR. (D) Comparison of PFS
between OR/IR, no response and PD. Bevacizumab may exhibit
antitumor effects without reducing tumor size and morphological
criteria may detect a cytostatic effect of bevacizumab. PFS,
progression-free survival; CR, complete response; PR, partial
response; SD, stable disease; PD, progressive disease; RECIST,
Response Evaluation Criteria in Solid Tumors; NR, no response; IR,
incomplete response; OR, optimal response. |
Univariate analysis
Univariate analysis revealed that morphological
response was the only significant prognostic factor for PFS
following chemotherapy (Table II),
therefore multivariate analysis was not carried out.
 | Table II.Univariate analysis concerning the
prediction of progression free survival in the 50 patients. |
Table II.
Univariate analysis concerning the
prediction of progression free survival in the 50 patients.
| Factors | n | Coefficient (95%
CI) | P-value |
|---|
| Gender |
| 0.816
(0.419–1.588) | 0.549 |
|
Male | 29 |
|
|
|
Female | 21 |
|
|
| Primary tumor
site |
| 0.758
(0.412–1.393) | 0.372 |
|
Colon | 28 |
|
|
|
Rectum | 22 |
|
|
| Occurrence of
metastasis |
| 1.436
(0.769–2.685) | 0.256 |
|
Simultaneous | 34 |
|
|
|
Metachronous | 16 |
|
|
| Number of liver
metastasis |
| 1.159
(0.630–2.133) | 0.634 |
|
<5 | 27 |
|
|
| ≥5 | 23 |
|
|
| Extra hepatic
lesions |
| 0.600
(0.321–1.123) | 0.110 |
|
Positive | 30 |
|
|
|
Negative | 20 |
|
|
| Size of largest
metastasis, cm |
| 0.886
(0.479–1.639) | 0.701 |
|
<5 | 29 |
|
|
| ≥5 | 21 |
|
|
| Bevacizumab |
| 0.889
(0.462–1.710) | 0.725 |
|
Yes | 19 |
|
|
| No | 31 |
|
|
| RECIST |
| 0.565
(0.266–1.198) | 0.136 |
|
CR/PR | 10 |
|
|
|
SD/PD | 40 |
|
|
| Morphological
change |
| 2.131
(1.005–4.517) | 0.048a |
|
Response | 14 |
|
|
| No
response | 36 |
|
|
Representative optimal response
case
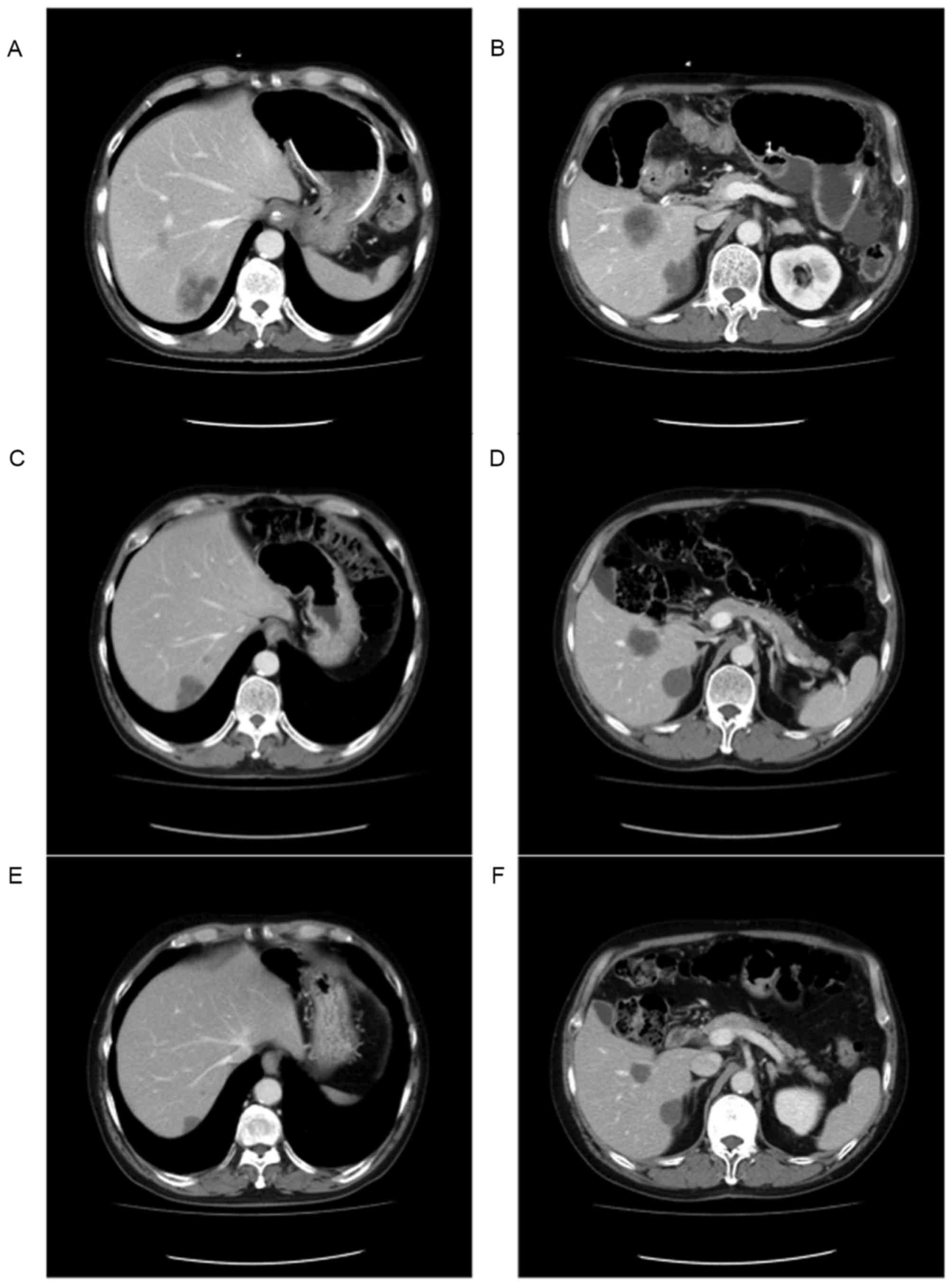
A 60-year-old man presented with CRC and
unresectable liver metastasis. The CT images shown in Fig. 4A and B indicate that the patient had
multiple liver metastases. The tumors appeared to exhibit
heterogeneous attenuation with a thick, poorly-defined tumor-liver
interface, which placed the patient in group 3, based on the
morphological criteria. The patient was subsequently treated with
mFOLFOX6 and bevacizumab, including 200 mg/m2 folinic
acid, 400 mg/m2 5-FU and 85 mg/m2 oxaliplatin
on day 1 and 5 mg/kg bevacizumab on day 1 and q14d, followed by 46
h of continuous infusion with 2,400 mg/m2 5-FU on days 1
and 2, following resection of the ascending colon. After 2 months,
the tumors in the liver had changed to become classified as
homogeneous low attenuation masses with a thin, sharply-defined
tumor-liver interface, which now placed the patient in group 1
(Fig. 4C and D). At the same time,
the liver tumors demonstrated an OR; however, during the study,
tumors that were OR were not classified as being oncologically
stable and resectable, and as such, the patient continued receiving
chemotherapy. At 16 months post-chemotherapy, the size of tumors
were reduced (Fig. 4E and F) and
showed no accumulation on positron emission tomography-CT (data not
shown), therefore, a resection of the liver tumors was performed.
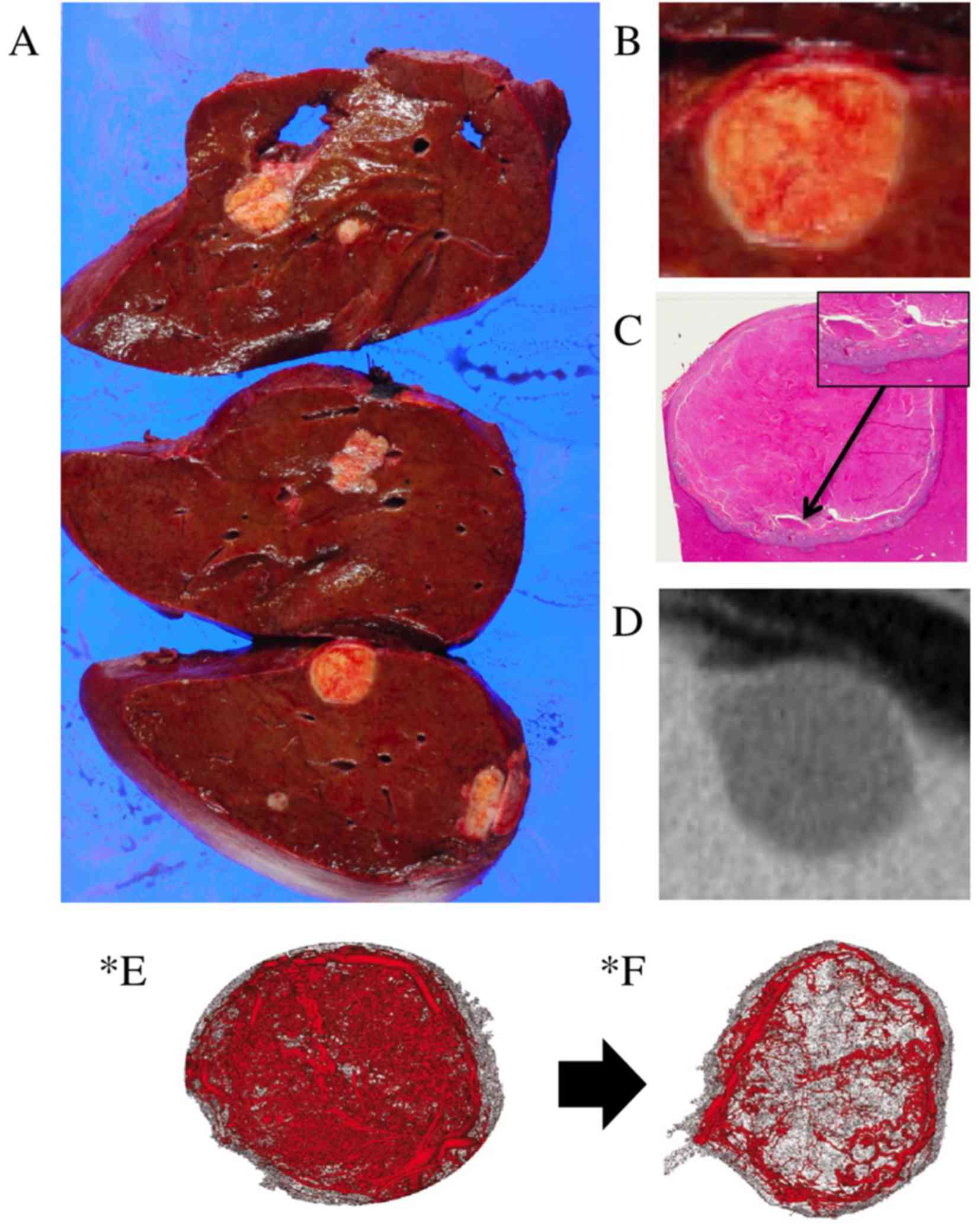
Fig. 5A-C show the pathological
features of the tumor, which consisted of necrosis, granulation and
fibrosis, with a clear borderline between the tumor tissue and the
non-tumor tissue. Fig. 5D shows the
radiological imaging of the tumor with morphological response,
which may have been a result of pathological change induced by
bevacizumab. While the tumor exhibited a major response according
to the tumor regression grade (19),
it was revealed to possess living tumor cells at the edge of the
necrotic tissue. The living tumor cells were distributed in a way
that was consistent with the area of vascular reconstruction of a
mouse xenograft model induced by the anti-vascular endothelial
growth factor (VEGF) antibody (Fig. 5E
and F) in a previous study (20).
Therefore, the pathological change may have been a result of the
antitumor effect of the bevacizumab that the patient received.
Discussion
The present study demonstrated that patients with
mCRC with liver metastasis showing OR/IR within 6 months of
chemotherapy were oncologically stable, which made the patients
candidates for rescue liver resection. Assessment of the
morphological response contributed to the selection of the
therapeutic strategy, which included surgical intervention for
patients with mCRC who underwent chemotherapy for their initially
unresectable tumors.
Assessment of morphological response has been
reported to be a good predictor of therapeutic outcomes for
patients undergoing chemotherapy (16–18,21,22),
whereas conventional size-based criteria, such as RECIST, may be
limited in assessing the response of the patient to biological
agents that exhibit a cytostatic mechanism of action. The present
data revealed that the prognostic advantage of an optimal
morphological response of patients with mCRC undergoing
chemotherapy with bevacizumab was consistent with results of
previous studies (16–18). Furthermore, the present study provides
evidence for the clinical application of morphological criteria in
selecting the therapeutic strategy for those patients who most
likely benefit from surgery.
Advances in combination chemotherapy with biological
agents have improved the response rates of patients and may reduce
the size of tumors (4). This allows
liver resection of patients with mCRC with borderline resectable,
as well as unresectable, liver metastasis. In the present study,
chemotherapy was used to treat the primary tumor prior to its
resection, which was then followed by a hepatectomy.
The limitation of this strategy includes the lack of
a clear definition of what constitutes a resectable liver tumor
(3). Technical considerations
pertaining to the resectability of the tumors may be overcome when
there is a reduction in the size of the tumor, although oncological
concerns may remain. Several guidelines produced by the European
Society for Medical Oncology (23)
and the National Comprehensive Cancer Network (24) outline the management of patients with
mCRC and liver metastasis. While these guidelines take into account
the number, location and distribution of the liver tumors, there is
no consensus on how to apply these characteristics to determine
whether surgery is indicated. Patient candidates for liver
resection are those unlikely to relapse within a short time period
following surgery; that is, the patients must possess oncologically
stable tumors. The present study was conducted to examine whether
morphological criteria are useful in the selection of patients with
oncologically stable tumors. The present data shows that the
patients who had an OR/IR presented with stable disease and a
median PFS period of 15.1 months. As such, they may be classified
as potential candidates for rescue liver resection.
While the decision for resection is clear for
patients with an OR/IR, those patients whose tumors had a complete
response following chemotherapy should also be considered. Benoist
et al (25) reported that
persistent macroscopic or microscopic residual disease, or early
recurrence in situ, was observed in 55 (83%) of 66 liver
metastases that were classified as having a CR on imaging.
Therefore, resectable tumors should be treated with surgical
intervention while they can be identified on imaging. The majority
of patients who showed a morphological response in the present
study were classified at the first or second assessment using CT
3–6 months after chemotherapy. The 6-month point is important, as
it is the time when a decision is made on whether surgical
intervention for rescue liver resection is appropriate for a
patient.
The morphological criteria was first reported in
2009 as a novel surrogate marker for the prognosis of patients with
mCRC undergoing chemotherapy, including bevacizumab (16), and was later validated surgically
(18) and medically (26) in treated populations. The reasons
behind why the morphological response has a predictive value for
prognosis in patients treated with bevacizumab should also be
considered. It has been reported that the pathological response
induced by bevacizumab is associated with patient OS (19). A change in morphology, as determined
by CT imaging, includes vascular reconstruction, which is believed
to be a response to treatment with bevacizumab. By comparison with
the pathological and morphological features of the tumor of the
representative optimal response case in the present study, living
tumor cells were revealed to be distributed within a vascular
reconstruction area that was induced by an anti-VEGF antibody in a
previous study (20). From the
present study, it was revealed that morphological response
correctly predicted the pathological change produced by the
antitumor effect of bevacizumab, which meant that it had predictive
value in the prognosis of the patients treated with
bevacizumab.
Although the definition of resectable liver
metastases has changed, it has been estimated that 20–30% of
patients with liver metastases are potential candidates for liver
resection (27,28). Recent phase III trials have shown that
the increased use of liver resection as a treatment option has
significantly impacted the survival of the CRC population (1,2). The liver
resection rate in more recent phase III trials has been between 10
and 14% for patients who underwent liver resection (1,29). This
means that 10–16% of patients with mCRC remained excluded from
surgical treatment. For these patients, the loss of opportunity to
be considered for liver resection means they may become candidates
for rescue liver resection by assessment of their morphological
response.
In conclusion, the present findings provided
evidence for physicians to consider previously un-resectable mCRC
patients as candidates for surgical treatment. However, it is
important to interpret the present results within the context of
the study limitations, such as retrospective analysis and selected
population, and additional studies may be undertaken prior to
definitive guidelines for their clinical application being
made.
Acknowledgements
The present study was supported in part by a
grant-in-aid awarded to the post graduate students from Jichi
Medical University, a grant-in-aid from the Ministry of Education,
Culture, Sports, Science and Technology (grant no. JP16K10514), and
the JKA Foundation through its promotion funds from the Keirin Race
(grant no. 27-1-068).
References
|
1
|
Venook AP, Niedzwiecki D, Lenz HJ,
Innocenti F, Mahoney MR, O'Neil BH, Shaw JE, Polite BN, Hochster
HS, Atkins JN, et al: CALGB/SWOG 80405: Phase III trial of
irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin
(mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients
(pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma
of the colon or rectum (MCRC). J Clin Oncol. 32 Suppl:52014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siriwardena AK, Mason JM, Mullamitha S,
Hancock HC and Jegatheeswaran S: Management of colorectal cancer
presenting with synchronous liver metastases. Nat Rev Clin Oncol.
11:446–459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kopetz S, Chang GJ, Overman MJ, Eng C,
Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM and
McWilliams RR: Improved survival in metastatic colorectal cancer is
associated with adoption of hepatic resection and improved
chemotherapy. J Clin Oncol. 27:3677–3683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fong Y, Fortner J, Sun RL, Brennan MF and
Blumgart LH: Clinical score for predicting recurrence after hepatic
resection for metastatic colorectal cancer: Analysis of 1001
consecutive cases. Ann Surg. 230:3093211999. View Article : Google Scholar
|
|
6
|
Gayowski TJ, Iwatsuki S, Madariaga JR,
Selby R, Todo S, Irish W and Starzl TE: Experience in hepatic
resection for metastatic colorectal cancer: Analysis of clinical
and pathologic risk factors. Surgery. 116:703–711. 1994.PubMed/NCBI
|
|
7
|
Kato T, Yasui K, Hirai T, Kanemitsu Y,
Mori T, Sugihara K, Mochizuki H and Yamamoto J: Therapeutic results
for hepatic metastasis of colorectal cancer with special reference
to effectiveness of hepatectomy: Analysis of prognostic factors for
763 cases recorded at 18 institutions. Dis Colon Rectum 46 (10
Suppl). 1–31. 2003.
|
|
8
|
Nordlinger B, Guiguet M, Vaillant JC,
Balladur P, Boudjema K, Bachellier P and Jaeck D: Surgical
resection of colorectal carcinoma metastases to the liver. A
prognostic scoring system to improve case selection, based on 1568
patients. Association Française de Chirurgie. Cancer. 77:1254–1262.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Viganò L, Capussotti L, Majno P, Toso C,
Ferrero A, De Rosa G, Rubbia-Brandt L and Mentha G: Liver resection
in patients with eight or more colorectal liver metastases. Br J
Surg. 102:92–101. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei AC, Greig PD, Grant D, Taylor B,
Langer B and Gallinger S: Survival after hepatic resection for
colorectal metastases: A 10-year experience. Ann Surg Oncol.
13:668–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yi JH, Kim H, Jung M, Shin SJ, Choi JS,
Choi GH, Baik SH, Min BS, Kim NK and Ahn JB: Prognostic factors for
disease-free survival after preoperative chemotherapy followed by
curative resection in patients with colorectal cancer harboring
hepatic metastasis: A single-institute, retrospective analysis in
Asia. Oncology. 85:283–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Antoch G, Kanja J, Bauer S, Kuehl H,
Renzing-Koehler K, Schuette J, Bockisch A, Debatin JF and
Freudenberg LS: Comparison of PET, CT, and dual-modality PET/CT
imaging for monitoring of imatinib (STI571) therapy in patients
with gastrointestinal stromal tumors. J Nucl Med. 45:357–365.
2004.PubMed/NCBI
|
|
13
|
Stroobants S, Goeminne J, Seegers M,
Dimitrijevic S, Dupont P, Nuyts J, Martens M, Van den Borne B, Cole
P, Sciot R, et al: 18FDG-Positron emission tomography for the early
prediction of response in advanced soft tissue sarcoma treated with
imatinib mesylate (Glivec). Eur J Cancer. 39:2012–2020. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blanke CD, Demetri GD, von Mehren M,
Heinrich MC, Eisenberg B, Fletcher JA, Corless CL, Fletcher CD,
Roberts PJ, Heinz D, et al: Long-term results from a randomized
phase II trial of standard-versus higher-dose imatinib mesylate for
patients with unresectable or metastatic gastrointestinal stromal
tumors expressing KIT. J Clin Oncol. 26:620–625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC, et al: New guidelines to evaluate the
response to treatment in solid tumors. european organization for
research and treatment of cancer, national cancer institute of the
United States, national cancer institute of Canada. J Natl Cancer
Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
16
|
Chun YS, Vauthey JN, Boonsirikamchai P,
Maru DM, Kopetz S, Palavecino M, Curley SA, Abdalla EK, Kaur H,
Charnsangavej C and Loyer EM: Association of computed tomography
morphologic criteria with pathologic response and survival in
patients treated with bevacizumab for colorectal liver metastases.
Jama. 302:2338–2344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishioka Y, Shindoh J, Yoshioka R, Gonoi
W, Abe H, Okura N, Yoshida S, Oba M, Hashimoto M, Watanabe G, et
al: Radiological morphology of colorectal liver metastases after
preoperative chemotherapy predicts tumor viability and
postoperative outcomes. J Gastrointest Surg. 19:1653–1661. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shindoh J, Loyer EM, Kopetz S,
Boonsirikamchai P, Maru DM, Chun YS, Zimmitti G, Curley SA,
Charnsangavej C, Aloia TA and Vauthey JN: Optimal morphologic
response to preoperative chemotherapy: An alternate outcome end
point before resection of hepatic colorectal metastases. J Clin
Oncol. 30:4566–4572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klinger M, Tamandl D, Eipeldauer S, Hacker
S, Herberger B, Kaczirek K, Dorfmeister M, Gruenberger B and
Gruenberger T: Bevacizumab improves pathological response of
colorectal cancer liver metastases treated with XELOX/FOLFOX. Ann
Surg Oncol. 17:2059–2065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Connor JP, Carano RA, Clamp AR, Ross J,
Ho CC, Jackson A, Parker GJ, Rose CJ, Peale FV, Friesenhahn M, et
al: Quantifying antivascular effects of monoclonal antibodies to
vascular endothelial growth factor: Insights from imaging. Clin
Cancer Res. 15:6674–6682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi H, Charnsangavej C, Faria SC,
Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA and
Benjamin RS: Correlation of computed tomography and positron
emission tomography in patients with metastatic gastrointestinal
stromal tumor treated at a single institution with imatinib
mesylate: Proposal of new computed tomography response criteria. J
Clin Oncol. 25:1753–1759. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shindoh J, Chun YS, Loyer EM and Vauthey
JN: Non-size-based response criteria to preoperative chemotherapy
in patients with colorectal liver metastases: The morphologic
response criteria. Curr Colorectal Cancer Rep. 9:198–202. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmoll HJ, Van Cutsem E, Stein A,
Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde
CJ, Balmana J, Regula J, et al: ESMO consensus guidelines for
management of patients with colon and rectal cancer. a personalized
approach to clinical decision making. Ann Oncol. 23:2479–2516.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
NCCN Clinical Practice Guidelines in
Oncology: Colon Cancer. http://www.nccn.org/professionals/physician_gls/f_guidelines.aspJanuary
1–2016
|
|
25
|
Benoist S, Brouquet A, Penna C, Julié C,
El Hajjam M, Chagnon S, Mitry E, Rougier P and Nordlinger B:
Complete response of colorectal liver metastases after
chemotherapy: Does it mean cure? J Clin Oncol. 24:3939–3945. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshita H, Hosokawa A, Ueda A, Ando T,
Kajiura S, Kato H, Kawabe H, Tomizawa G, Horikawa N, Yabuhita K, et
al: Predictive value of optimal morphologic response to first-line
chemotherapy in patients with colorectal liver metastases.
Digestion. 89:pp. 43–48. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garden OJ, Rees M, Poston GJ, Mirza D,
Saunders M, Ledermann J, Primrose JN and Parks RW: Guidelines for
resection of colorectal cancer liver metastases. Gut. 55 Suppl
3:iii1–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stangl R, Altendorf-Hofmann A, Charnley RM
and Scheele J: Factors influencing the natural history of
colorectal liver metastases. Lancet. 343:1405–1410. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schwartzberg LS, Rivera F, Karthaus M,
Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS and Go WY: PEAK: A
randomized, multicenter phase II study of panitumumab plus modified
fluorouracil, leucovorin and oxaliplatin (mFOLFOX6) or bevacizumab
plus mFOLFOX6 in patients with previously untreated, unresectable,
wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol.
32:2240–2247. 2014. View Article : Google Scholar : PubMed/NCBI
|