Introduction
Lung cancer is one of the leading causes of
cancer-associated mortality worldwide, accounting for >1 000,000
mortalities/year (1). Progress has
been made in the diagnosis and treatment of lung cancer over the
past decades (2). Concurrent
chemoradiotherapy has been used to control locally advanced
non-small cell lung cancer (NSCLC) (3). However, the improvements are modest,
leading to only 4–5% improvements in the five-year survival rates
for cancer stages I–III (4).
Therefore, the development of multimodal therapeutics with
increased effectiveness is required in order to improve the rate of
survival of patients with lung cancer.
Niclosamide, a salicylanilide derivative used for
the treatment of tapeworm infections, is safe, well tolerated,
inexpensive and readily available (5). A previous study identified niclosamide
as a potential anticancer agent in in vitro and in
vivo models (6). The inhibitory
effects of niclosamide has been reported in a number of human tumor
types, including breast cancer, prostate cancer, colon cancer,
ovarian cancer, multiple myeloma, acute myelogenous leukemia,
glioblastoma, head and neck cancer and lung cancer cells (7). In addition, niclosamide demonstrated
synergistic effects when combined with chemotherapeutic agents,
including oxaliplatin (8), Ara-C,
etoposide and daunorubicin, and temozolomide (9). Furthermore, niclosamide may be able to
reverse the resistance of human head and neck cancer and NSCLC
cells to erlotinib (10,11). The present study demonstrated that
niclosamide enhanced the effects of irradiation by inhibiting the
hypoxia inducible factor-1α (HIF-1α)/vascular growth factor (VEGF)
signaling pathway. These results suggest that niclosamide may be a
promising candidate for clinical evaluation as part of a combined
regimen for the treatment of NSCLC.
Materials and methods
Cell culture and treatments
Human lung cancer cell lines, A549 and H1299 were
purchased from the American Type Culture Collection (Manassas, VA,
USA) and maintained by our laboratory (Zumin Xu, Affiliated
Hospital of Guangdong Medical University) and cultured in RPMI-1640
(GE Healthcare; HyClone, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS; Biological Industries, Beit Haemek,
Israel), 100 U/ml penicillin and 100 U/ml streptomycin in a humid
atmosphere of 5% CO2 at 37°C.
Irradiation conditions
The human lung cancer cells were exposed to 4 MV
X-rays with various doses of radiation (0–8 Gy) at a dose rate of
0.6 Gy/min at room temperature using a linear accelerator (Elekta
AB, Stockholm, Sweden) and the source-skin-distance technique (100
cm) (12). The depth was set at 1 cm
to the bottom of the 6-well plate or 6-cm dishes.
Cell proliferation assay
Cells in the early log growth phase were trypsinized
and plated in a 96-well plate at a density of 1×104
cells/well. Following 24 h, the RPMI-1640 medium containing 10% FBS
was removed and replaced with fresh RPMI-1640 medium supplemented
with niclosamide at the indicated concentrations (0, 0.25, 0.5, 1,
2, 4, 8, 16 and 32 µM) for 24 or 48 h at 37°C in the presence of 1%
FBS. Cell density was evaluated using a Cell Counting kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan) assay,
according to the manufacturer's protocol. The absorbance of each
well was determined at 450 nm using a microplate reader. The
percentages of surviving cells from each group, relative to the
control (untreated group), were defined as the proliferation rate.
For these investigations, all experiments were repeated ≥3
times.
Colony formation assay
Cells in the early log phase were trypsinized and
plated in 6-well plates at 200, 400, 1,000, 2,000 and 4,000
cells/well, and cultured overnight at 37°C in order to allow for
cell attachment. Subsequently, cells were treated with or without
niclosamide (0.8 µMol/l) in 37°C CO2 incubator for 24 h,
prior to irradiation with the exposure dose corresponding to 0, 2,
4, 6 or 8 Gy. The cells were incubated in 37°C CO2
incubator for 10 days to allow for the formation of colonies. Cells
were fixed and stained with 0.5% crystal violet (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) at room temperature for 15
min, and colonies containing >50 cells were counted under a
light microscope. Survival curves were determined using the click
multi-target model with GraphPad Prism 5.0 software (GraphPad
Software Inc., La Jolla, CA, USA). Each point on the survival curve
represents the mean surviving cell fraction of ≥3 independent
experiments.
Cell apoptosis analysis
The number of apoptotic cells was determined by flow
cytometry using the Annexin V-fluorescein isothiocyanate (FITC)
Apoptosis Detection kit (Kaiji Biotech Development Co., Ltd.,
Nanjing, China), as previously described (13). Cells in the early log phase were
trypsinized at 37°C and plated in 6-well plates at 2×105
cells per/well. Following 24 h, the cells were treated with
niclosamide (0.8 µMol/l) alone or combined with 6 Gy radiation (10
min exposure) at room temperature. Cells were then collected,
washed three times with PBS, added to 1 ml ethanol (70%) and fixed
for 2 h at 4°C. Subsequently, the cells were washed with PBS 3
times and the supernatant was removed following centrifugation at
800 × g for 10 min at room temperature. A total of 500 µl Annexin
V-binding buffer, 5 µl Annexin V-FITC and 5 µl propidium iodide
(PI) was then added and incubated at room temperature in the dark
for 15 min. The rate of cell apoptosis was determined using flow
cytometry (BD Biosciences, Franklin Lakes, NJ, USA) according to
the manufacturer's protocol.
Western blot analysis
Following treatment with niclosamide and/or
radiation (6 Gy), cells for immunoblotting were prepared as
previously described (12). The PVDF
membrane was then incubated with the appropriate primary antibody
at 4°C overnight, including anti-HIF-1α (cat. no. 14179S),
anti-VEGF (cat. no. sc-152) (both 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) or anti-β-actin (cat. no.
bsm-33139M, 1:1,500; Boster Biological Technology, Ltd., Wuhan,
China). The protein of interest was detected following incubation
with a goat anti-rabbit (cat. no. A0208, 1:1,500; Beyotime
Institute of Biotechnology, Haimen, China) or anti-mouse (cat. no.
A0216, 1:1,500; Beyotime Institute of Biotechnology, Haimen, China)
IgG-horseradish peroxidase conjugated secondary antibody
(dilution,) at 4°C for 2 h. The band intensities were evaluated
using ImageJ software version 1.41 (National Institutes of Health,
Bethesda, MD, USA). Data are presented as the relative protein
level normalized to β-actin, and the ratio of control samples was
expressed as 1.0.
Statistical analysis
All data are expressed as the mean ± standard
deviation. For comparisons between two groups, the Student's t-test
method was used. One-way analysis of variance was used for
comparison between ≥2 groups. SPSS version 13.0 was used for all
statistical analyses (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Niclosamide inhibits the proliferation
of lung cancer cells in a dose- and time-dependent manner
The effects of niclosamide on the proliferation of
H1299 and A549 cells were determined using a CCK-8 assay.
Increasing the treatment concentration of niclosamide and extending
the treatment time from 24 to 48 h resulted in a significant
reduction in viability of H1299 and A549 cells (Fig. 1). The 50% inhibitory concentration
(IC50) of niclosamide on A549 cells was 3.368 µM at 24 h
and 0.99 µM at 48 h. The IC50 of niclosamide on H1299
cells was 1.383 µM at 24 h and 0.836 µM at 48 h. To investigate the
radiosensitization effect in human lung cancer cells, a niclosamide
concentration of 0.8 µM was used in subsequent experiments.
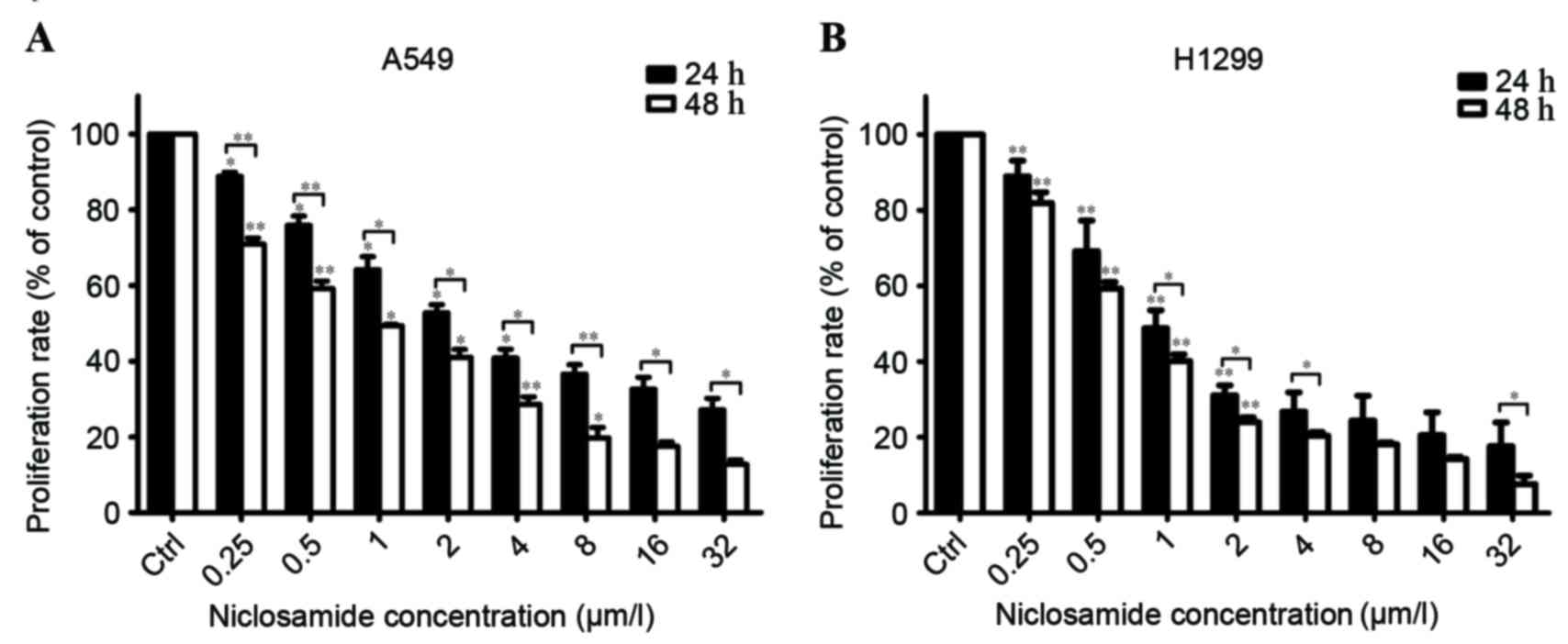 | Figure 1.Niclosamide inhibits the proliferation
of human lung cancer cells. Human lung cancer cell lines, (A) A549
and (B) H1299, were treated with niclosamide at various
concentrations (0, 0.25, 0.5, 1, 2, 4, 8, 16 and 32 µM) for 24 or
48 h, and the proliferation rates were then determined using a Cell
Counting kit-8. Niclosamide significantly inhibited the viability
of A549 and H1299 cell lines in a dose- and time-dependent manner.
All data are presented as the mean ± SD from three independent
experiments. Cell proliferation in untreated control cells was
assigned as 100%. *P<0.05; **P<0.01 vs. the control cells;
the bars represent SD. SD, standard deviation. |
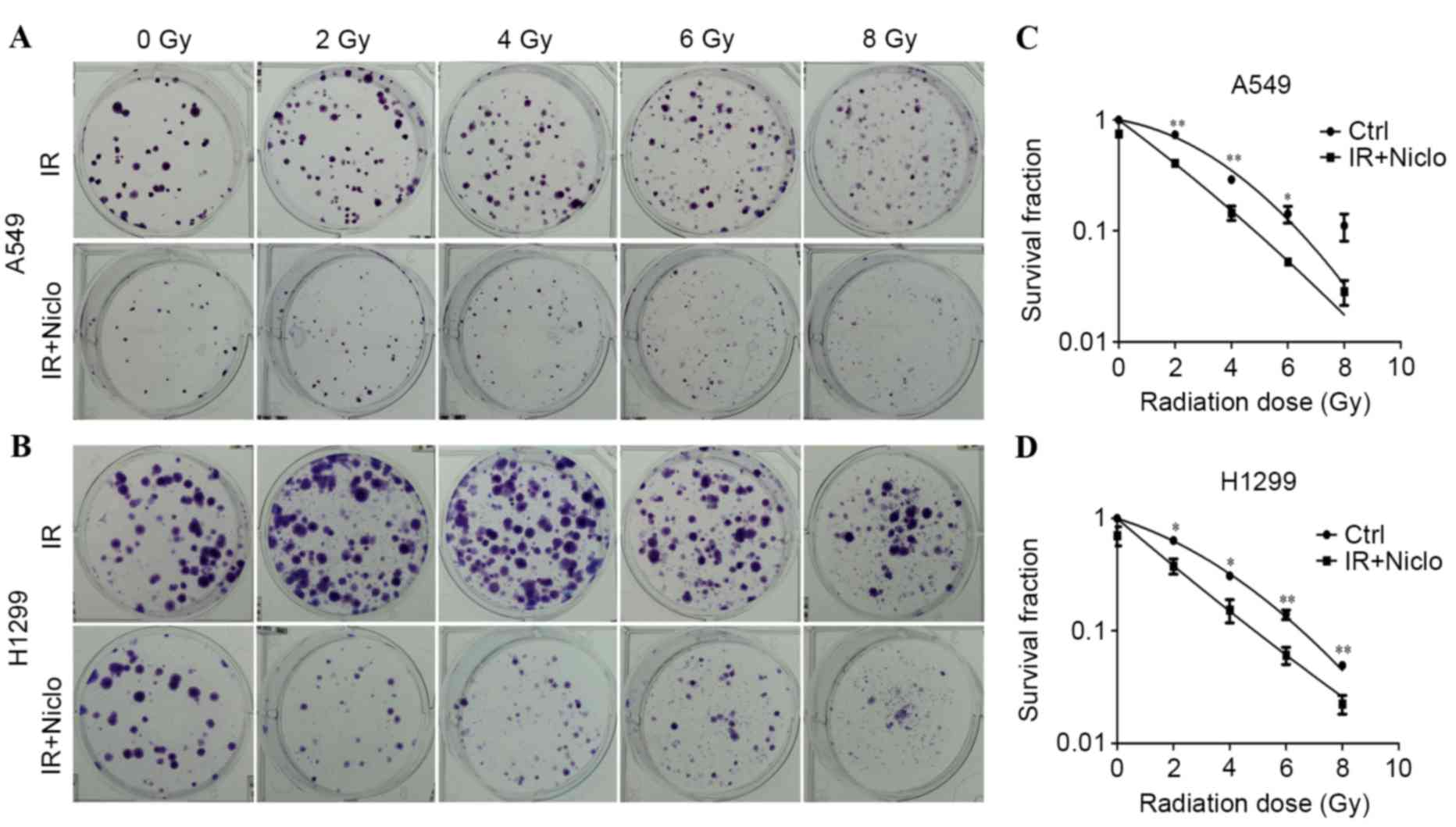
Niclosamide enhances the radiation
response in human lung cancer cells
A colony formation assay was performed in order to
evaluate whether niclosamide increased the radiosensitivity of lung
cancer cells. Cells were pretreated with 0.8 µMol/l niclosamide
prior to radiation exposure at 0, 2, 4, 6 or 8 Gy, and the
surviving colonies were counted following a 10-day incubation. The
radiation sensitivity was evaluated as the surviving fraction of
cells at a clinically relevant dose of 2 Gy, and sensitization
enhancement ratios (SERs) were determined. The result of the
clonogenic survival assay revealed that niclosamide enhanced the
radiosensitivity of A549 and H1299 cells, and the SER was 2.323 and
1.684, respectively (Fig. 2).
Therefore, the results indicate that niclosamide sensitized the
cytotoxic effect of radiation treatment in lung cancer cells.
Niclosamide enhances radiation-induced
apoptosis in lung cancer cells
In order to investigate whether niclosamide enhanced
the radiation response of lung cancer cells by inducing apoptosis,
A549 and H1299 cells were pretreated with 0.8 µMol/l niclosamide
for 24 h, prior to the administration of 6 Gy radiation and a 36-h
incubation. Subsequently, apoptotic cells were detected using PI
and Annexin V flow cytometry. The results reveal that treatment
with niclosamide and radiation significantly increased cell
apoptosis compared with cells exposed to radiation alone (A549,
P<0.0001; H1299, P<0.0001). The apoptotic rate of the
control, niclosamide, radiation and niclosamide combined with
radiation treatment in A549 cells were 6.5±1.0, 61.5±14.1, 19.8±1.9
and 91.3±1.52%, respectively. The apoptotic rate of the control,
niclosamide, radiation and niclosamide combined with radiation
treatment in H1299 cells were 4.1±1.8, 61.2±1.45, 14.2±2.1 and
93.8±1.53%, respectively (Fig.
3).
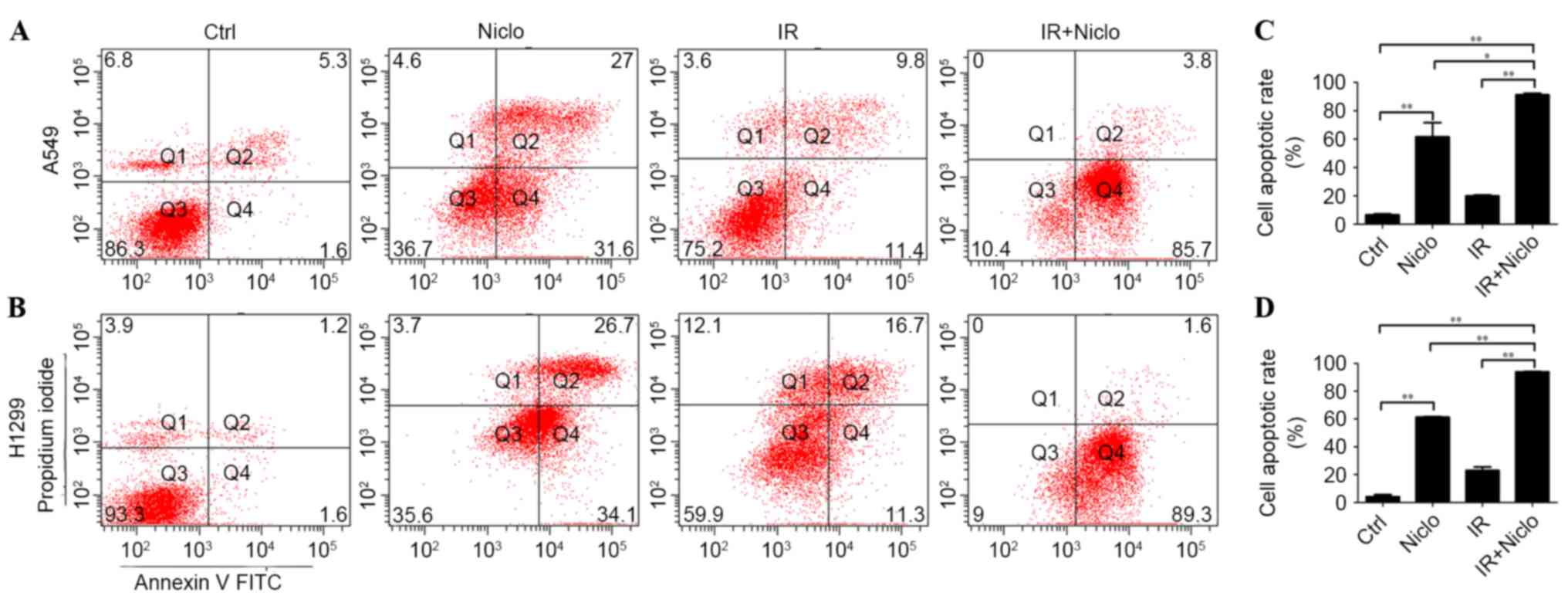 | Figure 3.Effect of niclosamide combined with
irradiation on the apoptosis of lung cancer cells. Cells were
treated with niclosamide (0.8 µm), radiation or pretreated with
niclosamide (0.8 µm) for 24 h, followed by 6 Gy radiation. Cells
were harvested and stained with Annexin V-PI and cell apoptosis was
detected by flow cytometry. The apoptotic rate of combined
treatments was significantly higher compared with the niclosamide
or irradiation treatment groups. Representative images of flow
cytometric analysis of the various treatments of (A) A549 and (B)
H1299 cells. (C) Apoptotic rate of A549 cells treated with control,
IR, Niclo and Niclo combined with IR were 6.5±1.0, 61.5±14.1,
19.8±1.9 and 91.3±1.52%, respectively. (D) Apoptotic rate of H1299
cells treated with control, IR, Niclo and Niclo combined with IR
were 4.1±1.8, 61.2±1.45, 14.2±2.1 and 93.8±1.53%, respectively.
Data are presented as the mean ± standard deviation from three
independent experiments. *P<0.05; **P<0.01. Ctrl, control;
Niclo, niclosamide; IR, irradiation; FITC, fluorescein
isothiocyanate; PI, propidium iodide. |
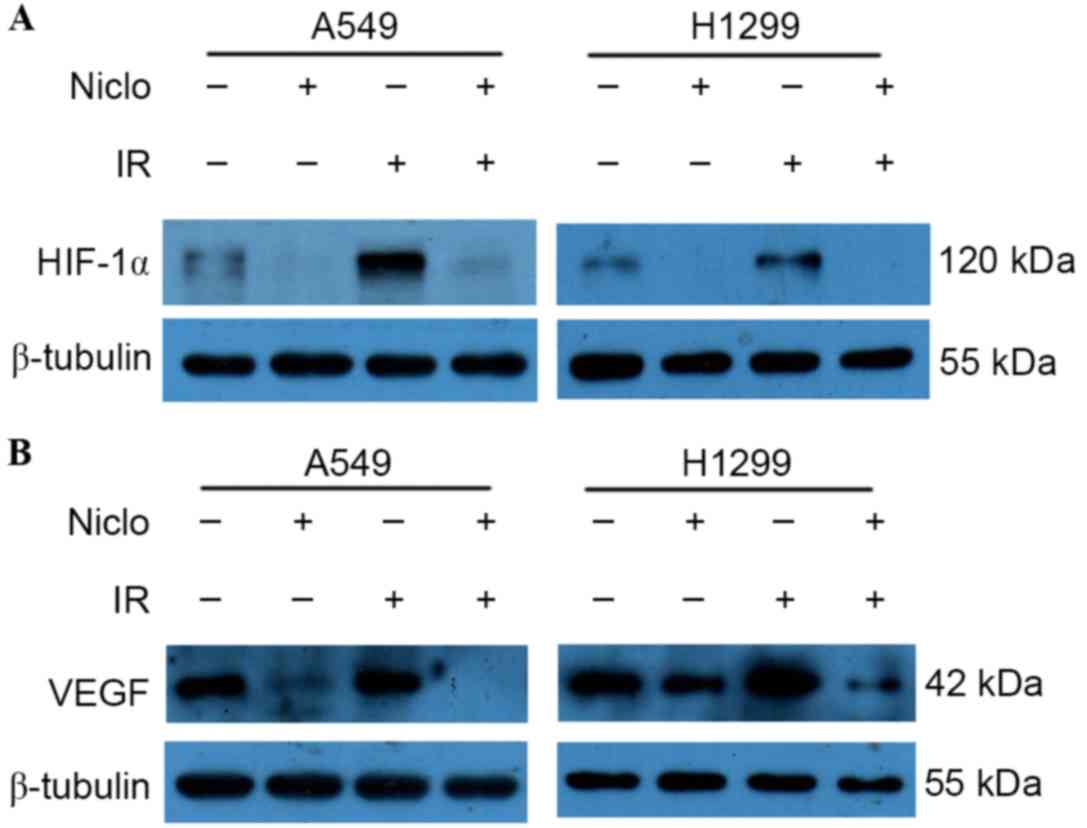
Niclosamide inhibits radiation-induced
HIF-1α/VEGF expression in lung cancer cells
Western blot analysis was performed in order to
detect the effects of radiation and/or niclosamide (0.8 µMol/l)
treatment on the expression levels of HIF-1α and VEGF. The HIF-1α
and VEGF proteins were detected, and niclosamide alone was able to
decrease the expression of HIF-1α and VEGF proteins. However, the
expression of HIF-1α and VEGF proteins was remarkably increased
following irradiation, and niclosamide combined with irradiation
significantly downregulated the radiation-induced HIF-1α (Fig. 4A) and VEGF (Fig. 4B) upregulation observed in A549 and
H1299 cells. These results suggest that niclosamide may induce
radiosensitizing effects via the downregulation of the HIF-1α/VEGF
signaling pathway.
Discussion
In the present study, it was observed that
niclosamide inhibited the proliferation of lung cancer cells in a
dose- and time-dependent manner. Niclosamide pretreatment followed
by irradiation may be able to enhance the radiosensitivity of lung
cancer cell lines by suppressing the HIF-1α/VEGF signaling
pathway.
Previous studies have identified niclosamide as a
potential antitumor agent, and further investigations suggested
that niclosamide may target a number of signaling pathways,
including nuclear factor-κ B, reactive oxygen species (9), Wnt/β-catenin (8), Notch (14), mammalian target of rapamycin complex 1
(15) and signal transducer and
activator of transcription 3 (STAT3) (16). Furthermore, previous studies have
investigated the effect of niclosamide on the response of human
lung cancer cells to radiation. You et al (17) demonstrated that ionizing radiation
induced the phosphorylation of Janus-associated kinase-2 and STAT3
in numerous types of human lung cancer cells, whereas niclosamide
reduced STAT3 nuclear localization in radioresistant lung cancer
cells. In addition, it was revealed that inhibition of STAT3 by
niclosamide may overcome radioresistance in lung cancer, suggesting
that niclosamide could potentially improve the outcome of lung
cancer, particularly for patients with resistance to radiotherapy.
Lee et al (18) demonstrated
that niclosamide enhanced cell death following the radiation
exposure of H1299 cells by activation of the p38 mitogen-activated
protein kinase/c-Jun signaling axis. The present study also
observed the radiosensitizing effects of niclosamide on H1299 and
A549 NSCLC cell lines. The SERs of H1299 and A549 were 1.684 and
2.323, respectively (Fig. 2). These
results suggest that niclosamide may be a potential radiation
sensitizer for lung cancer.
Hypoxia commonly occurs in solid tumors (19). Hypoxia activates a number of signaling
pathways including HIFs, which may lead to radiation resistance in
a number of cancer subtypes (20).
HIFs are heterodimeric basic helix-loop-helix Per-Arnt-Sim family
transcription factors composed of α and β subunits (21). When dimerized, HIFs bind to hypoxia
responsive elements in the promoter or enhancer regions of target
genes. The HIF-1α is regulated by oxygen tension. Under normal
oxygen levels, HIF-1α subunits are less abundant at the protein
level due to prolyl hydroxylase mediated degradation, which
decreases the half life of HIF-1α to 5–10 min despite continued
transcription of HIF-1α mRNA. Under hypoxia, HIF-1α is stable and
no longer degraded; therefore, HIF proteins are able to rapidly
alter signaling pathways involved in cellular metabolism,
angiogenesis and survival (22).
HIF-1α serves an important role in the response to hypoxia and
transcriptional activation of genes including VEGF, in order
to promote the resistance of cancer cells to radiotherapy (23). However, whether niclosamide enhances
the radiosensitivity of lung cancer by suppressing the HIF-1α/VEGF
signaling pathway requires further investigation. Therefore, the
present study detected the impact of niclosamide and/or radiation
treatment on the expression levels of HIF-1α and VEGF in lung
cancer cells. The results demonstrated that irradiation may induce
the expression of HIF-1α and VEGF in A549 and H1299 cells. However,
niclosamide downregulated the irradiation-induced expression of
HIF-1α (Fig. 4A) and VEGF (Fig. 4B). The present study suggested that
niclosamide may enhance the radiosensitivity of human lung cancer
cells by suppressing the HIF-1α/VEGF signaling pathway.
Niclosamide was previously identified as a potent
STAT3 inhibitor (16). STAT3 acts as
a signal transducer and transcription factor (24). STAT3 protein has been revealed to bind
to the HIF-1α promoter in vitro and in vivo under
hypoxia (25). In addition, STAT3 was
considered to be a novel regulator of HIF-1α activity by
participating in the transcriptional unit with HIF-1α and p300,
which leads to hypoxia-mediated transcriptional activation of HIF-1
target genes, including VEGF (26). Furthermore, the inhibition of STAT3
displayed an antitumor effect and promoted chemosensitivity and
radiosensitivity in nasopharyngeal carcinoma and head and neck
squamous cell carcinoma by modulating HIF-1α expression (27,28).
Therefore, niclosamide may inhibit HIF-1α/VEGF expression via the
STAT3 signaling pathway.
In conclusion, the present study demonstrated that
niclosamide exhibited a strong radiosensitizing potential in lung
cancer cells by reducing the expression of HIF-1α and VEGF. These
findings suggest that niclosamide may be a novel potent
radiosensitizer and a promising candidate for clinical evaluation
as part of a combined regimen for the treatment of NSCLC.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (grant no. 81201736) and the
Natural Science Foundation of Guangdong Province (grant no.
2015A030310460).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minguet J, Smith KH and Bramlage P:
Targeted therapies for treatment of non-small cell lung
cancer-Recent advances and future perspectives. Int J Cancer.
138:2549–2561. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Rourke N, Roqué I, Figuls M, Farré
Bernadó N and Macbeth F: Concurrent chemoradiotherapy in non-small
cell lung cancer. Cochrane Database Syst Rev: CD002140. 2010.
View Article : Google Scholar
|
|
4
|
Johnson DH, Schiller JH and Bunn PA Jr:
Recent clinical advances in lung cancer management. J Clin Oncol.
32:973–982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Merschjohann K and Steverding D: In vitro
trypanocidal activity of the anti-helminthic drug niclosamide. Exp
Parasitol. 118:637–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Li PK, Roberts MJ, Arend RC, Samant
RS and Buchsbaum DJ: Multi-targeted therapy of cancer by
niclosamide: A new application for an old drug. Cancer Lett.
349:8–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan JX, Ding K and Wang CY: Niclosamide,
an old antihelminthic agent, demonstrates antitumor activity by
blocking multiple signaling pathways of cancer stem cells. Chin J
Cancer. 31:178–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Osada T, Chen M, Yang XY, Spasojevic I,
Vandeusen JB, Hsu D, Clary BM, Clay TM, Chen W, Morse MA and Lyerly
HK: Antihelminth compound niclosamide downregulates Wnt signaling
and elicits antitumor responses in tumors with activating APC
mutations. Cancer Res. 71:4172–4182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin Y, Lu Z, Ding K, Li J, Du X, Chen C,
Sun X, Wu Y, Zhou J and Pan J: Antineoplastic mechanisms of
niclosamide in acute myelogenous leukemia stem cells: Inactivation
of the NF-kappaB pathway and generation of reactive oxygen species.
Cancer Res. 70:2516–2527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li R, Hu Z, Sun SY, Chen ZG, Owonikoko TK,
Sica GL, Ramalingam SS, Curran WJ, Khuri FR and Deng X: Niclosamide
overcomes acquired resistance to erlotinib through suppression of
STAT3 in non-small cell lung cancer. Mol Cancer Ther. 12:2200–2212.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li R, You S, Hu Z, Chen ZG, Sica GL, Khuri
FR, Curran WJ, Shin DM and Deng X: Inhibition of STAT3 by
niclosamide synergizes with erlotinib against head and neck cancer.
PLoS One. 8:e746702013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Chen X, Yu Y, Wang Z, Zuo Y, Li S,
Yang D, Hu S, Xiang M, Xu Z and Yu Z: Metformin inhibits the growth
of nasopharyngeal carcinoma cells and sensitizes the cells to
radiation via inhibition of the DNA damage repair pathway. Oncol
Rep. 32:2596–2604. 2014.PubMed/NCBI
|
|
13
|
Xu Z, Zuo Y, Wang J, Yu Z, Peng F, Chen Y,
Dong Y, Hu X, Zhou Q, Ma H, et al: Overexpression of the regulator
of G-protein signaling 5 reduces the survival rate and enhances the
radiation response of human lung cancer cells. Oncol Rep.
33:2899–2907. 2015.PubMed/NCBI
|
|
14
|
Wang AM, Ku HH, Liang YC, Chen YC, Hwu YM
and Yeh TS: The autonomous notch signal pathway is activated by
baicalin and baicalein but is suppressed by niclosamide in K562
cells. J Cell Biochem. 106:682–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fonseca BD, Diering GH, Bidinosti MA,
Dalal K, Alain T, Balgi AD, Forestieri R, Nodwell M, Rajadurai CV,
Gunaratnam C, et al: Structure-activity analysis of niclosamide
reveals potential role for cytoplasmic pH in control of mammalian
target of rapamycin complex 1 (mTORC1) signaling. J Biol Chem.
287:17530–17545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ren X, Duan L, He Q, Zhang Z, Zhou Y, Wu
D, Pan J, Pei D and Ding K: Identification of niclosamide as a new
small-molecule inhibitor of the STAT3 signaling pathway. ACS Med
Chem Lett. 1:454–459. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
You S, Li R, Park D, Xie M, Sica GL, Cao
Y, Xiao ZQ and Deng X: Disruption of STAT3 by niclosamide reverses
radioresistance of human lung cancer. Mol Cancer Ther. 13:606–616.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SL, Son AR, Ahn J and Song JY:
Niclosamide enhances ROS-mediated cell death through c-Jun
activation. Biomed Pharmacother. 68:619–624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rademakers SE, Span PN, Kaanders JH, Sweep
FC, van der Kogel AJ and Bussink J: Molecular aspects of tumour
hypoxia. Mol Oncol. 2:41–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meijer TW, Kaanders JH, Span PN and
Bussink J: Targeting hypoxia, HIF-1, and tumor glucose metabolism
to improve radiotherapy efficacy. Clin Cancer Res. 18:5585–5594.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gu YZ, Hogenesch JB and Bradfield CA: The
PAS superfamily: Sensors of environmental and developmental
signals. Annu Rev Pharmacol Toxicol. 40:519–561. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jaakkola P, Mole DR, Tian YM, Wilson MI,
Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji
M, Schofield CJ, et al: Targeting of HIF-alpha to the von
Hippel-Lindau ubiquitylation complex by O2-regulated prolyl
hydroxylation. Science. 292:468–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghattass K, Assah R, El-Sabban M and
Gali-Muhtasib H: Targeting hypoxia for sensitization of tumors to
radio- and chemotherapy. Curr Cancer Drug Targets. 13:670–685.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li B and Huang C: Regulation of EMT by
STAT3 in gastrointestinal cancer (Review). Int J Oncol. 50:753–767.
2017.PubMed/NCBI
|
|
25
|
Niu G, Briggs J, Deng J, Ma Y, Lee H,
Kortylewski M, Kujawski M, Kay H, Cress WD, Jove R and Yu H: Signal
transducer and activator of transcription 3 is required for
hypoxia-inducible factor-1alpha RNA expression in both tumor cells
and tumor-associated myeloid cells. Mol Cancer Res. 6:1099–1105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung JE, Lee HG, Cho IH, Chung DH, Yoon
SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK and Chung MH: STAT3 is
a potential modulator of HIF-1-mediated VEGF expression in human
renal carcinoma cells. FASEB J. 19:1296–1298. 2005.PubMed/NCBI
|
|
27
|
Pan Y, Zhou F, Zhang R and Claret FX:
Stat3 inhibitor Stattic exhibits potent antitumor activity and
induces chemo- and radio-sensitivity in nasopharyngeal carcinoma.
PLoS One. 8:e545652013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adachi M, Cui C, Dodge CT, Bhayani MK and
Lai SY: Targeting STAT3 inhibits growth and enhances
radiosensitivity in head and neck squamous cell carcinoma. Oral
Oncol. 48:1220–1226. 2012. View Article : Google Scholar : PubMed/NCBI
|