Introduction
Mastectomy for the treatment of locally advanced
breast cancer (LABC) may cause large skin defects that cannot be
closed directly. Even if the defect were closed directly using
over-tension, the blood circulation to the edge of the skin would
be disturbed and prevent wound healing (1). Similar difficulties are observed in
cases of mastectomy for the treatment of malignant phyllodes
tumors, which invade a wide are of the skin and may cause the
sternum or rib to be exposed. Skin grafting is the primary
treatment for coverage of these types of skin defects. However,
skin grafting has a poor aesthetic outcome and is not well received
at cortical bone sites (2,3). To overcome these limitations, various
flaps have been developed, including myocutaneous, fasciocutaneous
and cutaneous flaps (2,4–7).
Breast-conserving surgery (BCS) for LABC causes a
relatively large skin defect. If the skin is closed directly,
breast deformity or dislocation of the nipple-areolar complex may
occur (8). Oncoplastic surgical
techniques are necessary to achieve good cosmetic outcomes from BCS
(8,9).
The current study reports the application of a
rhomboid flap for the coverage of skin defects after mastectomy or
BCS in 11 female patients, which achieved good outcomes. The total
number of surgeries for primary and recurrent breast cancer and
malignant phyllodes tumor was 400–500/year at Saitama Cancer
Center. Among them, 3–4 cases required rhomboid flap coverage. This
method improved the patients' quality of life, as it enabled short
surgery duration and hospital stays, efficient wound repair, and
allowed for adjuvant therapy administration without delay. This
present case report details the methods and describes the outcomes
of the application of this technique.
Case report
Materials and methods
Patients
Between September 2011 and December 2013,
reconstructive operations using a rhomboid flap were performed in
11 female patients who underwent mastectomy or BCS including skin
resection, at Saitama Cancer Center (Saitama, Japan). The current
study was approved by the Ethics Committee of Saitama Cancer Center
and all patients provided written informed consent. The patients
were aged 41–82 years old. A total of 9/11 patients underwent
mastectomy and 2/11 patients underwent BCS including skin
resection. Out of the 9 patients who underwent a mastectomy, 6/9
were diagnosed with LABC, 2/9 were diagnosed with a malignant
phyllodes tumor, and 1/9 was diagnosed with ipsilateral breast
cancer recurrence with an inflammation of the skin. The 2 patients
who underwent BCS including skin resection were diagnosed with
LABC. All patients with primary LABC had noninflammatory skin
invasion [T4b; Union for International Cancer Control
tumor-node-metastasis classification (10)] and an advanced tumor stage [stage
IIIb/IIIc/IV (10)]. Although 2
patients had distant metastases, (both underwent mastectomy, one
patient was diagnosed with LABC and the other was diagnosed
malignant phyllodes tumor), they were in generally in good health
and considered to be suitable candidates for surgery. No patients
had any severe comorbidity, including diabetes, heart failure or
asthma, and all patients were non-smokers. After the surgery, the
size of the skin defect, the total surgery time and complication
rate were analyzed. The clinicopathological characteristics,
surgical data, and preoperative and postoperative treatment of the
patients are presented in Table
I.
 | Table I.Clinicopathological characteristics of
patients included in the present study (n=11). |
Table I.
Clinicopathological characteristics of
patients included in the present study (n=11).
| Patients no. | Age, years | Type of tumor | Clinical staging | Type of surgery | Skin resection size,
cm | Type of axillary
surgery | Bone exposure | Length of surgery,
min | Wound
complication | Preoperative systemic
therapy | Postoperative
systemic therapy | Postoperative
radiation therapy |
|---|
| 1 | 70 | Breast cancer | T4bN0M0 Stage
IIIB | Mastectomy | 12×8 | ALND | Rib/sternum | 171 | – | ET | ET | CW/SC |
| 2 | 79 | Recurrent breast
cancer |
| Mastectomy | 18×15 | – | Rib | 150 | – | – | – | – |
| 3 | 82 | Breast cancer | T4bN0M0 Stage
IIIB | Mastectomy | 7×6 | ALND | – | 143 | Partial flap
necrosis | ET | ET | – |
| 4 | 72 | Breast cancer | T4bN1M0 Stage
IIIB | BCS | 5×6 | ALND | – | 105 | – | CT | ET | BT/SC |
| 5 | 59 | Malignant phyllodes
tumor |
| Mastectomy | 13×15 | – | Rib | 134 | – | – | – | – |
| 6 | 60 | Breast cancer | T4bN3cM0 Stage
IIIC | Mastectomy | 11×11 | ALND | – | 173 | – | CT | ET | CW/SC |
| 7 | 55 | Malignant phyllodes
tumor |
| Mastectomy | 20×20 | – | Rib | 180 | – | – | – | – |
| 8 | 55 | Breast cancer | T4bN3cM0 stage
IIIC | Mastectomy | 23×13 | ALND | – | 208 | – | CT | ET | CW/SC |
| 9 | 66 | Breast cancer | T4bN1M0 stage
IIIB | BCS | 8×7 | ALND | – | 175 | – | CT/ET | ET | BT/SC |
| 10 | 41 | Breast cancer | T4bN1M1 stage
IV | Mastectomy | 12×10 | ALND | – | 172 | – | CT | CT | – |
| 11 | 78 | Breast cancer | T4bN0M0 stage
IIIB | Mastectomy | 8×7 | SLNB | – | 76 | – | ET | ET | – |
Surgical technique
All surgery was performed under general anesthesia
(desflurane inhalation 3%, remifentanil i.v. 0.25 µg/kg/min,
rocuronium i.v. 0.7 µg/kg/min) in a supine position. In the case of
mastectomy, a resection line was marked on the skin and the tumor
was resected. When axillary lymph node dissection was required,
another incision line was drawn from the edge of resection area
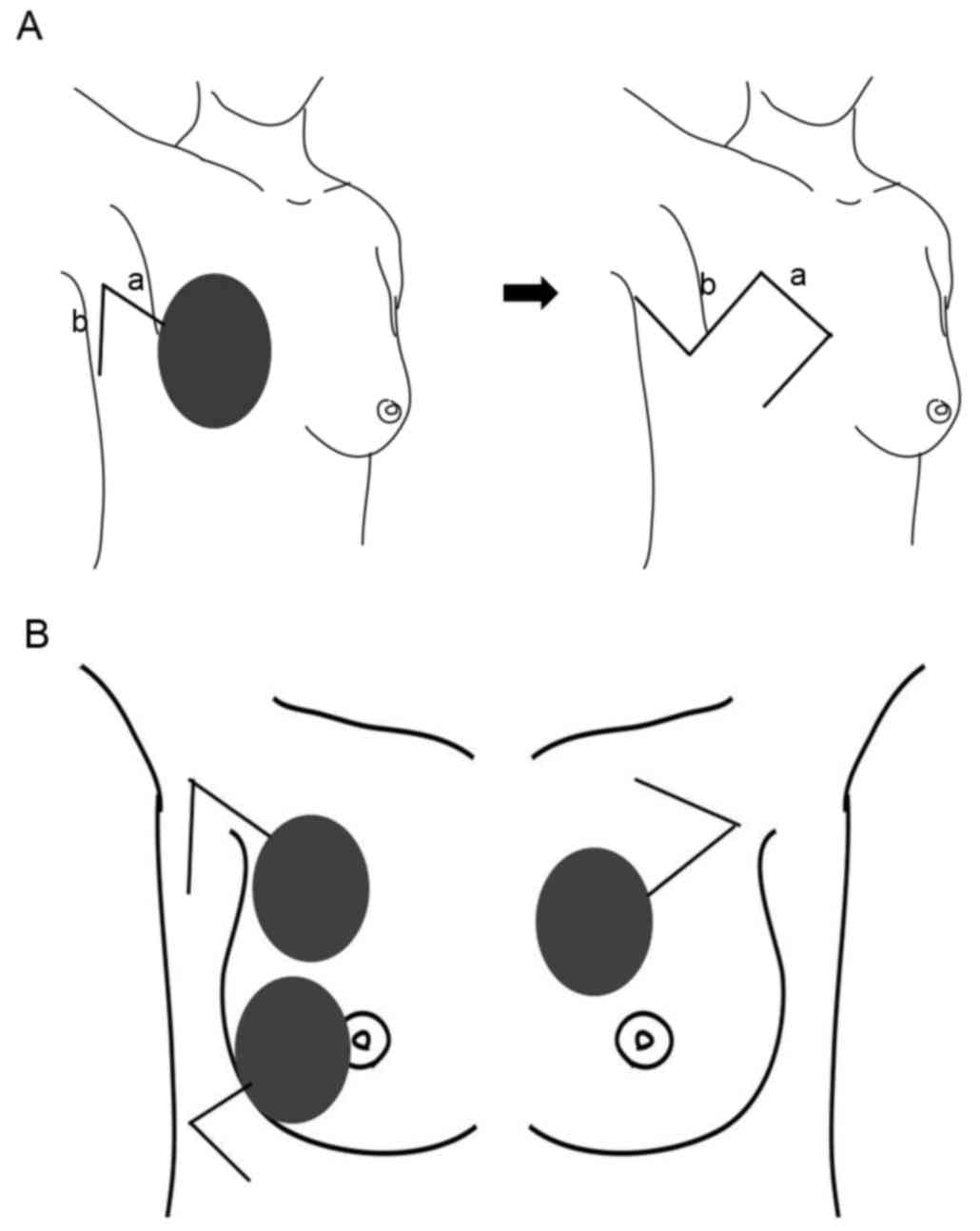
towards the axilla (line a; Fig. 1A).
After completing the mastectomy with or without axillary lymph node
dissection, another incision was made to create a rhomboid flap at
the lateral side of the chest, and the flap was elevated (line b;
Fig. 1A). By extending the length of
the incision, the size of the flap can be changed to fit the size
of the skin defect. Skin defects typically become slightly larger
compared with the marked incision line prior to tumor resection,
because the skin contracture caused by tumor invasion is released
after tumor resection. Therefore, the size of flap should be
estimated after tumor resection.
In the case of BCS, the location and design of the
flap is dependent on the location of the skin defect (Fig. 1B). If the skin defect is located
within the inner upper quadrant of the breast, the flap should be
fashioned towards the outer lateral side of the skin defect. If the
defect is located within the outer upper or outer lower quadrant of
the breast, the flap should be made laterally to the defect.
However, it is difficult to apply this flap for defects located
within the inner lower quadrant.
When a rhomboid flap is designed with a superior
pedicle within the axilla area, a dog-ear deformity is formed
within the axilla area, which is difficult to revise during the
surgery. When a rhomboid flap is designed with an inferior pedicle
within the axilla area, an easily revised dog-ear deformity is
formed at the front of the chest (Fig.
1A). If suitable perforator vessels from intercostal artery are
identified during the flap elevation, they can be included to
improve blood circulation to the flap.
Overall results
The overall results of the surgery are presented in
Table I. The range in size of the
skin defect was between 5×6 and 20×20 cm. A total of 7 patients
underwent axillary lymph node dissection and 4 patients had bone
exposure during the surgery (costal bone, 3 patients; costal bone
and sternal bone, 1 patient). The length of time of the surgery
ranged from 76–208 min (average time, 153.4 min). No wound
complications from the surgical procedures were recorded. Partial
necrosis was observed at the distant parts of the flap in 1
patient, which healed quickly with debridement and application of
an oil base ointment. A total of 8 patients underwent preoperative
systemic therapy (chemotherapy, 4 patients; endocrine therapy, 3
patients; chemotherapy and endocrine therapy, 1 patient). No
patients had received preoperative radiation therapy. Postoperative
systemic therapy was received by 8 patients (endocrine therapy, 7
patients; chemotherapy, 1 patient). A total of 5 patients underwent
postoperative radiation therapy (chest wall and supraclavicular
node, 3 patients; breast and supraclavicular node, 2 patients).
Representative cases
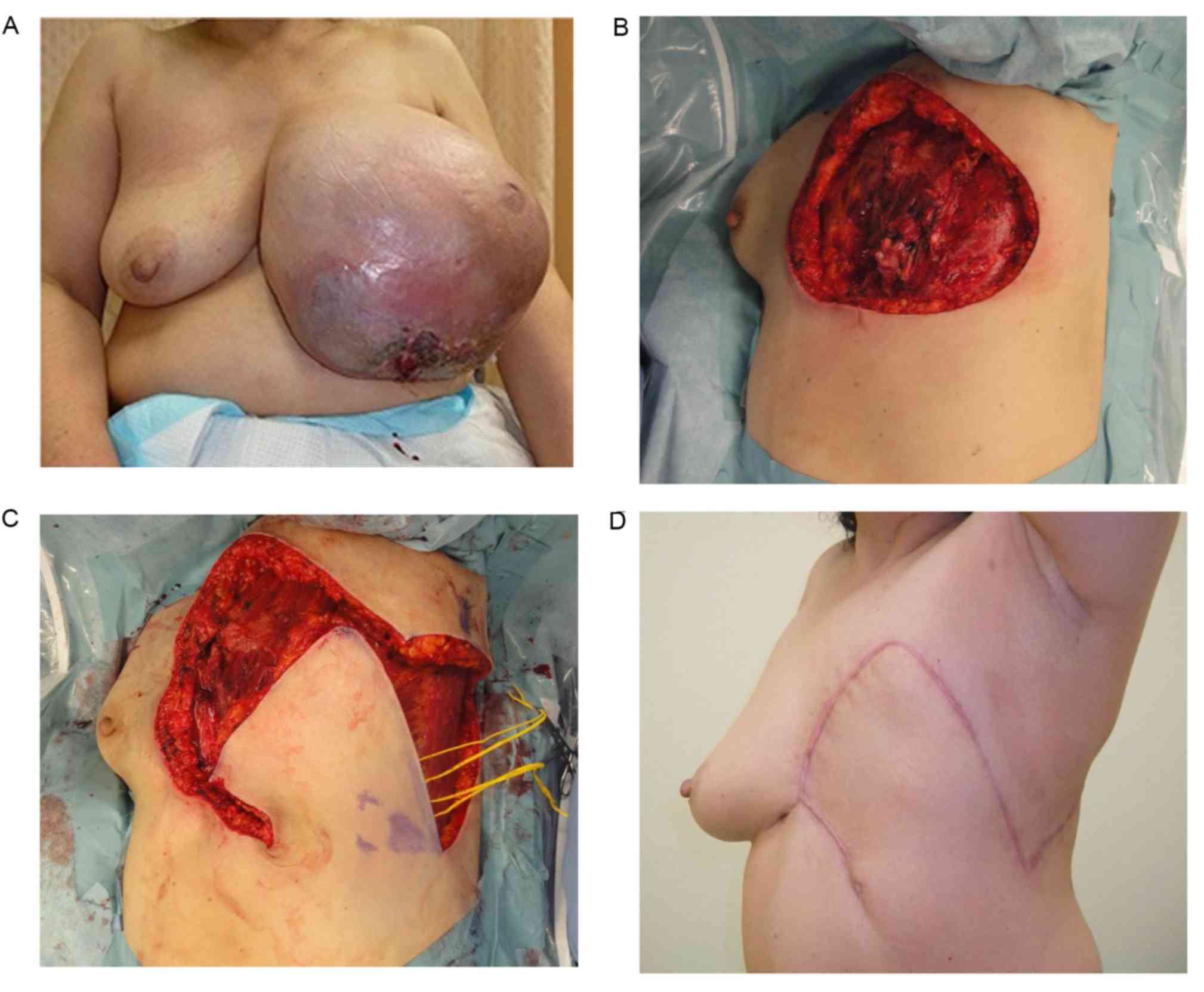
Case 1 (patient 7)
A 55-year-old woman was diagnosed with a malignant
phyllodes tumor at the Saitama Cancer Center (Table I). The patient first noticed a tumor
in their left breast several years prior to presenting, which had
rapidly increased in size in the last several months. The maximum
diameter of the tumor was 30 cm and the affected breast had an
ulcer that oozed continuously (Fig.
2A). No distant metastases were detected in preoperative
examinations. A mastectomy with axillary lymph node dissection was
performed under general anesthesia in the supine position. As a
result, a 20×20 cm skin defect and costal bone exposure occurred
after the tumor resection (Fig. 2B;
Table I). A rhomboid flap with an
inferior pedicle (15 cm limbs) was fashioned towards the lateral
side of the skin defect (Fig. 2C).
The flap covered the defect and the wound healed completely with no
complications, and there was no sign of local recurrence or
metastasis 4 months after surgery (Fig.
2D).
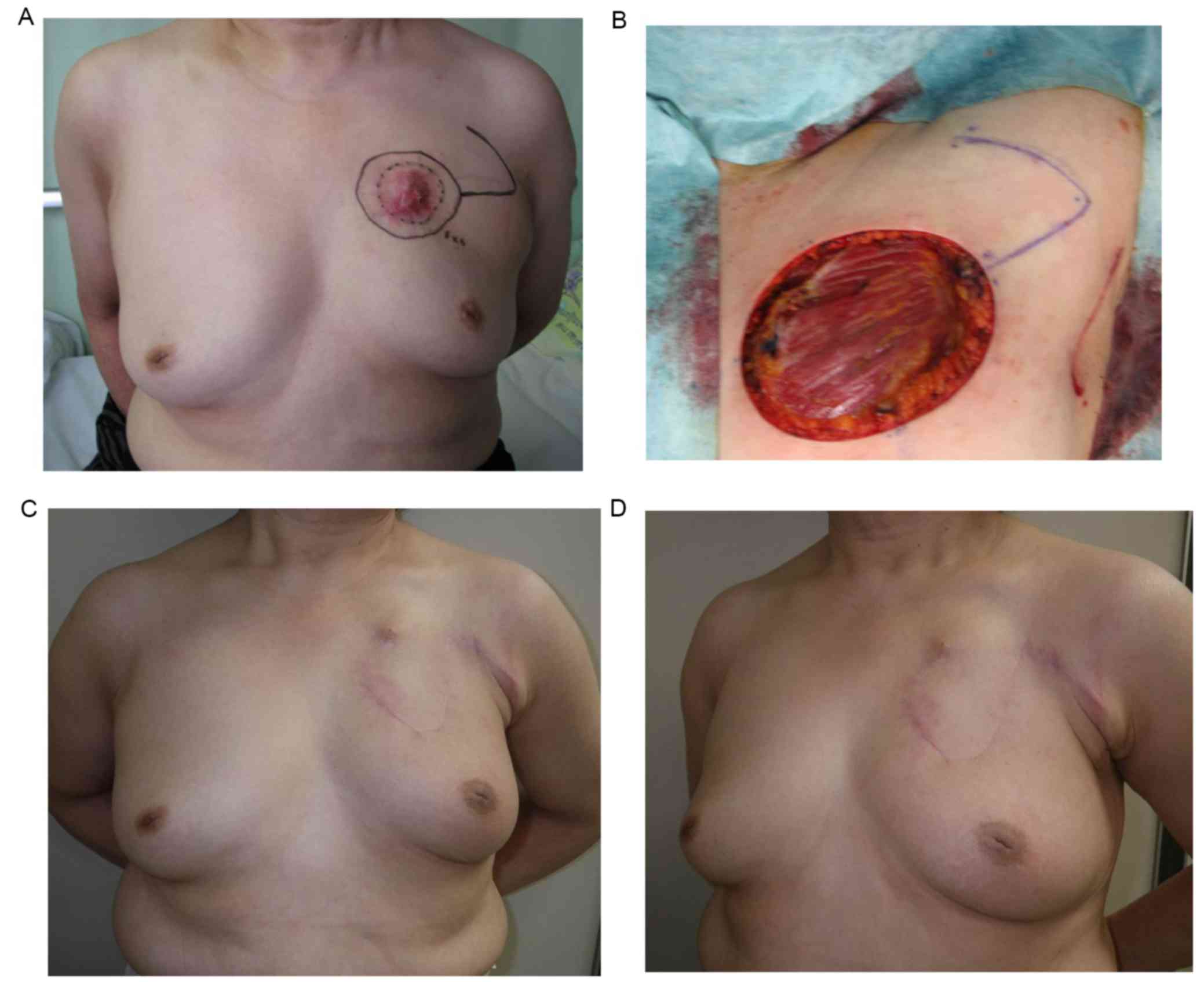
Case 2 (patient 9)
A 66-year-old woman was diagnosed with LABC (T4cN1M0
stage IIIB) after presenting to the Saitama Cancer Center (Table I). The patient underwent preoperative
systemic therapy (4 cycles triweekly doxorubicin 60
mg/m2 cyclophosphamide 600 mg/m2 followed by
4 cycles triweekly docetaxel 75 mg/m2 followed by 6
months aromatase inhibitor administration), to which the tumor
exhibited a partial response. A BCS including skin resection and
axillary lymph node dissection was performed. Due to the
contracture caused by the invasion of the tumor, the left side
nipple-areolar complex was located superior with respect to the
right side (Fig. 3A). The size of
skin resection was 8×7 cm. A rhomboid flap with 7 cm limbs was
fashioned towards the lateral side of the skin defect within the
axilla, revealing deep fascia of pectralis major muscle (Fig. 3B). The flap was elevated and the skin
defect was covered with the flap. Pathological examination,
performed as previously described (11), revealed ypT4bN0 invasive ductal
carcinoma with positive estrogen receptor, positive progesterone
receptor and negative human epidermal growth factor receptor 2
status (data not shown). The patient underwent postoperative
endocrine therapy and breast/supraclavicular node radiation
therapy. A total of 8 months after surgery there was no sign of
locoregional recurrence or metastasis. The malposition of the
nipple-areolar complex was corrected and the breasts were almost
symmetrical (Fig. 3C and D).
Discussion
There are regional differences in the incidence of
LABC due to accessibility to medical services (12). Due to advancements in systemic
therapy, the extent of surgical treatment required for LABC has
decreased (13). The volume of LABC
tumors can typically be decreased preoperatively with neoadjuvant
systemic therapy, which has a high response rate (13,14).
However, certain cases require a large-scale resection, producing a
large skin defect (15). The majority
of phyllodes tumors are benign and only a small number of cases are
diagnosed as malignant, characterized by a marked proliferation of
the stromal cells and metastatic potential (16). It is rare for flap coverage to be
required for skin defects after the resection of malignant
phyllodes tumors. However, if the tumor invades the surrounding
tissues an extended tumor resection is required (17,18).
Skin grafting is a useful technique in the
correction of skin defects; however, they have several
disadvantages, including the following: Cosmetic morbidities caused
by a mismatched color and/or texture of the grafted skin; scar
contracture due to thinner skin; and a poor response to grafting,
which is dependent on the condition of the recipient site. A number
of flap coverage techniques for skin defects after breast surgery
have been reported (2,4–7). Pedicled
myocutaneous flaps, including latissimus dorsi, external oblique
and rectus abdominis myocutaneous flaps, can cover large skin
defects with a stable blood flow, so they have been considered
useful methods. The application of cutaneous or fasciocutaneous
flaps, including any local flap, is another useful method as they
do not require the sacrifice of major muscles.
Although various methods of flap coverage have been
reported, there are few comparative studies between these methods.
Deo et al (19) reported that
fasciocutaneous flaps are more successful compared with
myocutaneous flap due to decreased blood loss during the surgery, a
shorter length of the surgery and a shorter hospital stay.
Conversely, Martella et al (20) suggested that there is no difference
between myocutaneous and fasciocutaneous flaps in regards to local
complications and the length of hospital stay required. Typically,
myocutaneous flaps provide enough tissue volume to cover the skin
defect and have a good blood supply (2,21).
Cutaneous and fasciocutaneous flaps do not require the loss of
major muscles and can be completed in a shorter length of time
(19). The flap method should be
considered and chosen on a case-by-cases basis according to the
size of the skin defect and the condition of the recipient/donor
sites.
The rhomboid flap, a type of transposition flap, has
been frequently used for >50 years since Dr Limberg's first
reports (22), and variable modified
methods have been reported (23–25). The
present study applied the rhomboid flap for coverage of skin
defects in patients with LABC or malignant phyllodes tumors after
mastectomy or BCS including skin resection, and achieved good
outcomes. The rhomboid flap can be elevated as a cutaneous or
fasciocutaneous flap, and the blood circulation to the flap is very
stable (26,27). The maximum skin defect size that can
be covered with the rhomboid flap depends on the flexibility of the
skin and the amount of subcutaneous fat tissue in the individual
(28). A maximum skin defect size of
20×20 cm was covered with the rhomboid flap in the present
study.
Due to advancements in neoadjuvant systemic therapy
for LABC, it is possible for patients with LABC to undergo BCS.
Touboul et al (29) reported
that BCS is not inferior compared with mastectomy for the treatment
of LABC after chemotherapy in regards to overall survival rates if
residual tumor sizes were small. However, the optimal local
management for patients with LABC remains undefined (14,29–31). This
warrant further study, particularly following recent advancements
in systemic therapy. For patients with LABC, oncoplastic breast
surgery is frequently required for cosmetic reasons, in addition to
wound management, due to the large amount of skin and soft tissue
resection in the majority of cases. Only a few studies have
investigated the safety of BCS with oncoplastic surgery compared
with BCS without oncoplastic surgery in patients with LABC
(32,33), so additional research in this area is
required.
Methods of BCS are classified into two groups as
follows: Volume displacement using glandular transposition and
volume replacement using autologous tissue (9,34,35). The method applied should be chosen
based on the size of the defect and the breast. The volume
replacement technique, which includes the rhomboid flap, is useful
for large defects and small breast sizes (9).
In conclusion, the rhomboid flap can be fashioned
quickly and easily, with a low risk of flap failure. The flap can
cover a relatively large skin defect, provide soft tissue volume
and achieve good cosmetic results. In addition, no special devices
are required to harvest the flap. Therefore, it is a good method
for the coverage of defects after malignant breast tumor
resection.
Acknowledgements
The authors would like to thank Dr Jeffrey D.
Meserve (The University of Texas, TX, USA) for his editorial
assistance.
References
|
1
|
Robertson SA, Jeevaratnam JA, Agrawal A
and Cutress RI: Mastectomy skin flap necrosis: Challenges and
solutions. Breast Cancer (Dove Med Press). 9:141–152.
2017.PubMed/NCBI
|
|
2
|
Persichetti P, Tenna S, Cagli B and
Scuderi N: Extended cutaneous ‘thoracoabdominal’ flap for large
chest wall reconstruction. Ann Plast Surg. 57:177–183. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mathes SJ: Mathes plastic surgery.
Saunders Elsevier; Philadelphia: pp. 293–316. 2006
|
|
4
|
Arnold PG and Pairolero PC: Chest-wall
reconstruction: An account of 500 consecutive patients. Plast
Reconstr Surg. 98:804–810. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bogossian N, Chaglassian T, Rosenberg PH
and Moore MP: External oblique myocutaneous flap coverage of large
chest-wall defects following resection of breast tumors. Plast
Reconstr Surg. 97:97–103. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Larson DL and McMurtrey MJ:
Musculocutaneous flap reconstruction of chest-wall defects: An
experience with 50 patients. Plast Reconstr Surg. 73:734–740. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Woo E, Tan BK, Koong HN, Yeo A, Chan MY
and Song C: Use of the extended V-Y latissimus dorsi myocutaneous
flap for chest wall reconstruction in locally advanced breast
cancer. Ann Thorac Surg. 82:752–755. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Emiroglu M, Sert I, Karaali C, Aksoy SO,
Ugurlu L and Aydin C: The effectiveness of simultaneous oncoplastic
breast surgery in patients with locally advanced breast cancer.
Breast Cancer. 23:463–470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haloua MH, Krekel NM, Winters HA, Rietveld
DH, Meijer S, Bloemers FW and van den Tol MP: A systematic review
of oncoplastic breast-conserving surgery: Current weaknesses and
future prospects. Ann Surg. 257:609–620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sobin L, Gospodarowicz M and Wittekind C:
TNM Classification of malignant tumours seventh edition. John Wiley
& Sons. Ltd; New Jersey: pp. 181–193. 2009
|
|
11
|
Hoda SA, Brogi E, Koerner FC and Rosen PP:
Rosen's Breast Pathology fourth edition. Lippincott Williams &
Wilkins; Philadelphia: pp. 413–467. 2014
|
|
12
|
Sinacki M, Badzio A, Wełnicka-Jaśkiewicz
M, Bogaerts J, Piccart MJ, Therasse P, Smith IE, Hatschek T and
Jassem J: Pattern of care in locally advanced breast cancer: Focus
on local therapy. Breast. 20:145–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eroglu A and Aydin F: Management of
non-inflammatory locally advanced breast cancer: Focus on surgical
approaches. Exp Oncol. 35:272–279. 2013.PubMed/NCBI
|
|
14
|
Sinacki M, Jassem J and van Tienhoven G:
Conservative local treatment versus mastectomy after induction
chemotherapy in locally advanced breast cancer: A randomised phase
III study (EORTC 10974/22002, LAMANOMA)-why did this study fail?
Eur J Cancer. 41:2787–2788. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arya R, Chow WT, Rozen WM, Patel NG,
Griffiths M, Shah S and Ramakrishnan VV: Microsurgical
reconstruction of large oncologic chest wall defects for locally
advanced breast cancer or osteoradionecrosis: A retrospective
review of 26 cases over a 5-year period. J Reconstr Microsurg.
32:121–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
AP J: Phyllodes tumors. Diseases of the
breast. 3rd. Lippincott Williams & Wilkins; Philadelphia: pp.
669–675. 2000
|
|
17
|
Rajesh A and Farooq M: Resection and
reconstruction following recurrent malignant phyllodes-Case report
and review of literature. Ann Med Surg (Lond). 16:14–18. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Banno A, Shimada A, Aga K, Harada H,
Kaburagi T, Seki H, Yasui N and Matsumoto H: Total mastectomy and
chest reconstruction for a rapidly progressing giant phyllodes
tumor with skin necrosis: A case report. Surg Case Rep. 1:822015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deo SV, Purkayastha J, Shukla NK and
Asthana S: Myocutaneous versus thoraco-abdominal flap cover for
soft tissue defects following surgery for locally advanced and
recurrent breast cancer. J Surg Oncol. 83:31–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martella S, Caliskan M, Brenelli FP,
Rossetto F, De Oliveira H Aparecida, De Brito Lima LN, Chifu C,
Rodriguez-Fernandez J, Petit JY and Luini A: Surgical closure of
chest wall in noninflammatory locally advanced breast carcinoma
with ulceration of the skin. Breast J. 14:345–352. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pavletic MM, Kostolich M, Koblik P and
Engler S: A comparison of the cutaneous trunci myocutaneous flap
and latissimus dorsi myocutaneous flap in the dog. Vet Surg.
16:283–293. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Limberg A: Mathematical principles of
local plastic procedures on the surface of the human body. Medgis;
Leningrad: 1946
|
|
23
|
Akin M, Leventoglu S, Mentes BB, Bostanci
H, Gokbayir H, Kilic K, Ozdemir E and Ferahkose Z: Comparison of
the classic Limberg flap and modified Limberg flap in the treatment
of pilonidal sinus disease: A retrospective analysis of 416
patients. Surg Today. 40:757–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Quaba AA and Sommerlad BC: ‘A square peg
into a round hole’: A modified rhomboid flap and its clinical
application. Br J Plast Surg. 40:163–170. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yucel E, Tezcan L, Yilmaz OC and Akin ML:
‘Flag Excision and Flap’ Procedure: A novel modification for
off-midline closure after pilonidal sinus excision. Indian J Surg.
77 Suppl 3:S1191–S1195. 2015. View Article : Google Scholar
|
|
26
|
Azab AS, Kamal MS and el Bassyoni F: The
rationale of using the rhomboid fasciocutaneous transposition flap
for the radical cure of pilonidal sinus. J Dermatol Surg Oncol.
12:1295–1299. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blake BP, Simonetta CJ and Maher IA:
Transposition flaps: Principles and locations. Dermatol Surg. 41
Suppl 10:S255–S264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thorne CH: Grabb & Smith's Plastic
Surgery. 6th. Lippincott Williams & Wilkins; Philadelphia: pp.
122007
|
|
29
|
Touboul E, Buffat L, Lefranc JP, Blondon
J, Deniaud E, Mammar H, Laugier A and Schlienger M: Possibility of
conservative local treatment after combined chemotherapy and
preoperative irradiation for locally advanced noninflammatory
breast cancer. Int J Radiat Oncol Biol Phys. 34:1019–1028. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
De Lena M, Varini M, Zucali R, Rovini D,
Viganotti G, Valagussa P, Veronesi U and Bonadonna G: Multimodal
treatment for locally advanced breast cancer. Result of
chemotherapy-radiotherapy versus chemotherapy-surgery. Cancer Clin
Trials. 4:229–236. 1981.PubMed/NCBI
|
|
31
|
Perloff M, Lesnick GJ, Korzun A, Chu F,
Holland JF, Thirlwell MP, Ellison RR, Carey RW, Leone L, Weinberg
V, et al: Combination chemotherapy with mastectomy or radiotherapy
for stage III breast carcinoma: A Cancer and Leukemia Group B
study. J Clin Oncol. 6:261–269. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chauhan A, Sharma MM and Kumar K:
Evaluation of surgical outcomes of oncoplasty breast surgery in
locally advanced breast cancer and comparison with conventional
breast conservation surgery. Indian J Surg Oncol. 7:413–419. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Silverstein MJ, Savalia N, Khan S and Ryan
J: Extreme oncoplasty: Breast conservation for patients who need
mastectomy. Breast J. 21:52–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Giacalone PL, Roger P, Dubon O, El Gareh
N, Rihaoui S, Taourel P and Daurés JP: Comparative study of the
accuracy of breast resection in oncoplastic surgery and
quadrantectomy in breast cancer. Ann Surg Oncol. 14:605–614. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaur N, Petit JY, Rietjens M, Maffini F,
Luini A, Gatti G, Rey PC, Urban C and De Lorenzi F: Comparative
study of surgical margins in oncoplastic surgery and quadrantectomy
in breast cancer. Ann Surg Oncol. 12:539–545. 2005. View Article : Google Scholar : PubMed/NCBI
|