Introduction
The neurofibromatosis type 2 (NF2) tumor
suppressor gene was first known for its association with
neurofibromatosis type 2, a hereditary neoplasia syndrome
characterized by the occurrence of multiple intracranial tumors,
including schwannomas, meningiomas and ependymomas (1–4). Since
then, NF2 mutations have also been reported in colorectal
(5) and thyroid cancer (6), melanoma (7) and mesotheliomas (8), indicating a more general tumor
suppressive role of NF2 in various cell types. The human
NF2 gene comprises 17 exons, which encode a 595-amino acid
protein termed merlin (9). Merlin
protein exhibits high sequence homology to the
membrane-cytoskeleton associated ezrin/radixin/moesin family and is
implicated in the regulation of several fundamental biological
processes, including contact-dependent inhibition of proliferation,
cell-cell communication and cell-matrix interactions, all of which
are important for tumor initiation and progression (10).
Significant differences in types of NF2 gene
mutations have been demonstrated in neurofibromatosis type 2,
sporadic schwannomas and other tumor types (11). Nonsense and frameshift mutations are
expected to cause truncated gene products, leading to loss of
merlin expression from the mutated NF2 allele, whilst merlin
harbouring missense mutations are supposedly stably expressed but
exhibit increased degradation activity (12,13). Lack
of functional merlin has been hypothesized to trigger the
dysregulation of a wide variety of signaling cascades from the cell
surface to the nucleus, including (but not limited to) growth
factor receptors, Ras/Rac downstream effectors, phosphoinositide
3-kinase/protein kinase B and cullin 4-RING ubiquitin ligase DDB1
and CUL4-X-box (14–17). However, a consensus for the mainstream
mechanism, by which merlin loss contributes to uncontrolled
proliferation or dysregulation of the cell cycle pattern in
mutation-bearing cells during tumor formation/progression, has not
yet been reached.
An ever-increasing breadth of evidence has proposed
that tumor protein p53 (TP53), a classical tumor suppressor
gene, is associated with NF2 in several genetic and protein
studies (18–21). Loss of functional
NF2-TP53-double mutant mice exhibited an increased
predisposal to neoplasms compared with each of the single mutant
mice (18). In a cohort of patients
with sporadic meningioma (19), loss
of heterozygosity of NF2 coupled with TP53
polymorphism increased the risk of tumor progression. A positive
association between the two tumor suppressor products has been
identified in glioma cell lines and meningiothelial meningiomas
(20). Furthermore, merlin and p53
downstream p21 were revealed to regulate each other in Schwann
cells and schwannomas (21). However,
little is currently known regarding the contribution of p53 to the
decreased tumor suppression in the absence of merlin. This appears
to be important for the clarification of mechanisms responsible for
merlin-deficient pathogenesis, and particularly the development in
potential areas to augment current modalities of treatment.
A more recent study (7) has shown that merlin loss caused by
NF2 mutations in sporadic colorectal carcinomas was
associated with an advanced tumor-stage, establishing the role of
merlin as a tumor suppressor in colorectal cancer progression. The
present study aimed to elucidate the exact role of p53 in the
merlin regulation of cellular proliferation, using human colon
carcinoma HCT116 cell lines expressing p53 or not expressing
p53.
Materials and methods
Cell lines and cell culture
The HCT116 wild-type for p53 (p53wt) and
HCT116 p53-null (HCT116/379.2, p53−/−) cell lines were
purchased from the Shanghai Institute of Cell Biology, Chinese
Academic of Science (Shanghai, China). The two cell types were
grown in McCoy's 5A medium (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
and 1% penicillin/streptomycin at 37°C in a humidified incubator
with 5% CO2.
NF2/merlin overexpression and
5-ethynyl-20-deoxyuridine (EdU) incorporation assay
For merlin overexpression experiments, the
recombinant pcDNA-NF2 or empty plasmid vector control
(ViGene Biosciences, Inc., Rockville, MD, USA) were transfected
into indicated HCT116 cell lines using Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc), according to the
manufacturer's protocol. On day 2 after transfection, cultures in
each of the experiment groups and control groups were examined for
the expression of NF2/merlin. EdU (Guangzhou RiboBio Co.,
Ltd., Guangzhou, China) labeling was used to investigate the effect
of merlin overexpression on DNA synthesis, which is an indicator of
cellular proliferation. Briefly, indicated cells were seeded onto
96-well plates at 8×103 cells/well, transfected with
NF2-expressing or empty vector at 37°C for 48 h, and then
exposed to 50 µl EdU for an additional 24 h at 37°C. The cells of
each well were then washed with PBS, fixed with 4% formaldehyde at
room temperature for 20 min and incubated with 0.3% Triton X-100
for 20 min at room temperature. Subsequently, cultures were reacted
with 80 µl of 1X Apollo® reaction cocktail (Guangzhou RiboBio Co.,
Ltd.) at room temperature for 20 min. The DNA contents of cells in
each well were stained with 10 µg/ml DAPI (Beyotime Institute of
Biotechnology, Haimen, China) at room temperature for 15 min.
Images were captured under LAS AF software V4.3 (Leica
Microsystems, Inc., Buffalo Grove, IL, USA). In total 3 fields of
view were randomly selected from each analysis; EdU-positive cells
were identified from the fluorescent images. Cells were counted
with ImageJ v1.43 software (National Institutes of Health,
Bethesda, MD, USA). Quantification of EdU incorporating-cells was
calculated as follows: EdU-positive cell numbers (red dots)/total
numbers (blue dots) ×100%. A total of ≥3 random images were
captured from each well and counted by 3 individuals. Experiments
were repeated 3 times.
NF2/merlin-knockdown
The pGC-FU-green fluorescence protein (GFP)
lentivirus vector, containing an independent open reading frame for
GFP, was used to produce small interfering RNA molecules that
inhibit expression of target genes of infected cells. Short hairpin
RNAs (shRNAs) were synthesized, annealed and inserted into the
lentivirus vector. Briefly, sense and antisense primer containing
the sense siRNA (small interfering RNA) sequence, 9 bp loop
sequence, antisense siRNA sequence and RNA polymerase III
terminator sequence were created with AgeI and EcoRI restriction
sites on the 5′ and 3′ ends, respectively. Subsequently pGCsiL-U6
(GeneChem Co., Ltd., Shanghai, China) was linearized using by
digestion with AgeI and EcoRI, these primers were annealed and
inserted into pGCsiL-U6 downstream of the U6 RNA polymerase III
promoter following the manufacturer's protocol. In order to obtain
optimized conditions, four candidate shRNAs (sh) targeting
different NF2 gene coding sequences (Table I) were designed using the siDESIGN
Center tool (http://dharmacon.gelifesciences.com) and synthesized
by GeneChem Co., Ltd.. In preliminary experiments, two shRNAs (sh1
and sh2) were revealed to exhibit ideal merlin knockdown effects
(data not shown) and were therefore utilized in the subsequent
investigations. A nonsense shRNA was also constructed as control
using the target sequence 5′-TTCTCCGAACGTGTCACGT-3′ (GeneChem Co.,
Ltd.). Two lentivirus-mediated shRNAs against NF2
(Lv-NF2-shRNAs) and a control shRNA (Lv-Con-shRNA) were used
to transfect HCT116 cell lines at a multiplicity of infection of
10. To screen the target for the most effective type of viral
transfection, the percentage of GFP-positive cells in total cell
numbers was evaluated under the fluorescence microscope (×100
magnification; ≥4 fields of view) 3 days after transduction.
 | Table I.Target sequences of four candidate
shRNAs. |
Table I.
Target sequences of four candidate
shRNAs.
| shRNA | Target
sequences |
|---|
| shRNA1a |
5′-GGAAGCAACCCAAGACGTTCA-3′ |
| shRNA2a |
5′-GCTCTGGATATTCTGCACAAT-3′ |
| shRNA3 |
5′-ACTTCAAAGATACTGACAT-3′ |
| shRNA4 |
5′-TTCGTGTTAATAAGCTGAT-3′ |
Immunofluorescence of merlin
protein
Briefly, cells were seeded onto 24-well plates with
a density of 1×104 cells/well, cultured at 37°C for 48
h, fixed with 4% formaldehyde at room temperature for 30 min,
washed 3 times with PBS and then permeabilized with 0.3% Triton
X-100 at room temperature for 20 min. Following incubation in
blocking buffer (0.05% BSA; Beyotime Institute of Biotechnology) at
room temperature for 1 h, coverslips were incubated with a rabbit
polyclonal anti-merlin antibody (dilution, 1:400; cat. no.
HPA003097; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C
for 1 h, incubated at 37°C for 1 h with red fluorescence-tagged
goat anti-rabbit secondary antibody (dilution, 1:400; cat. no.
SAB4600407; Sigma-Aldrich; Merck KGaA), and then counterstained
with DAPI.
Cellular proliferation and colony
formation
The effect of merlin knockdown on cellular
proliferation was determined by Cell Counting Kit-8 (CCK-8) from
Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). Briefly,
cells were seeded onto 96-well plates (4×103 cells/well)
and grown at 37°C for 0, 24, 48 or 72 h. At each specified time
point, 10 µl/well CCK-8 solutions were added and cells were
incubated for an additional 4 h at 37°C. Absorbance of wells was
measured using a SpectraMax190 microplate reader (Molecular
Devices, LCC, Sunnyvale, CA, USA), and absorbance values (optical
density) obtained from three aliquots were averaged to produce a
single value representing cell viability. The absorbance was then
expressed in numerical values, which were finally subjected to
statistical analysis. Colony formation experiments were also
performed to confirm the effect of merlin knockdown on cellular
proliferation. Briefly, cells were seeded onto 6-cm dishes at a
density of 500 cells per dish and cultured at 37°C for 1 week. At
the end of the incubation period, numbers of visible cell colonies
were estimated with Wright Giemsa staining (Beyotime Institute of
Biotechnology).
Cell cycle analysis
Propidium iodide (PI) staining was conducted to
examine the effect of merlin knockdown on cell-cycle distributions.
Briefly, 8×105 cells were seeded onto 6-cm dishes at
37°C for 18 h prior to analysis. Cells were then trypsinized,
washed and fixed in 1 ml 70% ethanol at 4°C overnight, followed by
centrifugation at 1,811 × g for 5 min at room temperature,
incubation with 100 µg/ml RNase and staining with 50 µg/ml PI for
15 min in the dark at room temperature. The DNA content of cells
was analyzed using a Cell Lab Quanta™ SC flow cytometer (Beckman
Coulter, Inc., Brea, CA, USA).
Protein immunoblotting analysis
The harvested cells were lysed with
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) and centrifuged at 16,060 × g for 15 min at 4°C.
Subsequently, the supernatant was removed and total cellular
protein concentration was assessed using a Bio-Rad protein assay
kit (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. Protein samples were separated by 8%
SDS-PAGE and transferred to polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were then separately
incubated with primary antibodies as follows: Mouse anti-human
β-actin monoclony antibody (dilution, 1:2,000; cat. no. A5441) was
obtained from Sigma-Aldrich (Merck KGaA); rabbit anti-human merlin
(dilution, 1:1,000; cat. no. sc-331), mouse anti-human p53
(dilution, 1:1,000; cat. no. sc-126), rabbit anti-human p21
(dilution, 1:1,000; cat. no. sc-397), rabbit anti-human cyclin D1
(dilution, 1:1,000; cat. no. sc-753), rabbit anti-human cyclin E1
(dilution, 1:1,000; cat. no. sc-481), rabbit anti-human CDK2
(dilution, 1:1,000; cat. no. sc-163) and rabbit anti-human CDK4
(dilution, 1:1,000; cat. no. sc-260) polyclonal antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Primary antibody binding was detected by horseradish
peroxidase-conjugated goat anti-mouse (dilution, 1:2,000; cat. no.
A0216; Beyotime Institute of Biotechnology) and anti-rabbit
(dilution, 1:2,000; cat. no. A0208; Beyotime Institute of
Biotechnology) secondary antibodies, and visualized using the ECL
plus system (GE Healthcare Life Sciences, Chalfont, UK).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
RNA extraction from 1×106 cells was
performed using TRIzol reagent (Takara Bio, Inc.), according to
standard methodology (22), and
stored at −80°C. The yield and quality of extracted RNA was
determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.). All RNA samples were quantified
spectrophotometrically at 260 and 280 nm. High quality RNA with
A260/A280 ratios of ~2.0 was used to produce cDNA by reverse
transcription by PrimerScript™ RT reagent kit (Takara Bio, Inc.)
according to the manufacturer's protocol. PCR amplification was
performed using SYBR® Premix Ex Taq™ (Takara Bio, Inc.). The PCR
protocol was as follows: 95°C for 30 sec, followed by 40 cycles of
95°C for 5 sec and 60°C for 34 sec, with a final dissociation
stage. Amplification and quantitative PCR measurements were
performed using the CFX96 RT-PCR Detection System (Bio-Rad
Laboratories, Inc.). The relative expression of the human
NF2 gene was normalized to β-actin as endogenous
housekeeping gene, and values were obtained by the comparative
threshold cycle method (2−ΔΔCq) (22). The PCR primer sequences were
constructed by Sangon Biotech Co., Ltd. (Shanghai, China) and
listed as follows: NF2 forward, 5′-ACCGTTGCCTCCTGACATAC-3′
and reverse, 5′-TTCAAGGCCTCGATTTCTGT-3′; β-actin forward,
5′-ACCGAGCGCGGCTACAG-3′ and reverse, 5′-CTTAATGTCACGCACGATTTCC-3′.
Experiments were repeated 3 times with similar results.
Statistical analysis
Unless otherwise stated, all data are presented as
the mean ± standard deviation from at least three independent
experiments. Student's t-test and one-way ANOVA were used to
determine differences between groups. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were conducted using MS Excel software 2013 (Microsoft
Corporation, Redmond, WA, USA) and SPSS v17.0 statistics software
(SPSS, Inc., Chicago, IL, USA).
Results
Anti-proliferative effects of
NF2/merlin expression are affected by p53 status in HCT116
cells
Previous studies have reported that the expression
of wild-type merlin in normal Schwann cells, schwannoma and
mesothelioma cells can inhibit cellular proliferation and arrest
cell growth at the G0/G1 phase (21,23,24). The
present study first attempted to investigate whether p53 performs a
role in modulating the cellular proliferation suppressive threshold
to excess merlin. Wild-type and p53-null HCT116 cell lines were
transfected with the NF2-expressing vectors (empty vectors
as control) under the same condition, and exhibited elevated
transcription levels of NF2 2 days after transfection, as
described by RT-PCR analysis. Additionally, merlin protein levels
were increased >2-fold, to a similar degree in the two cell
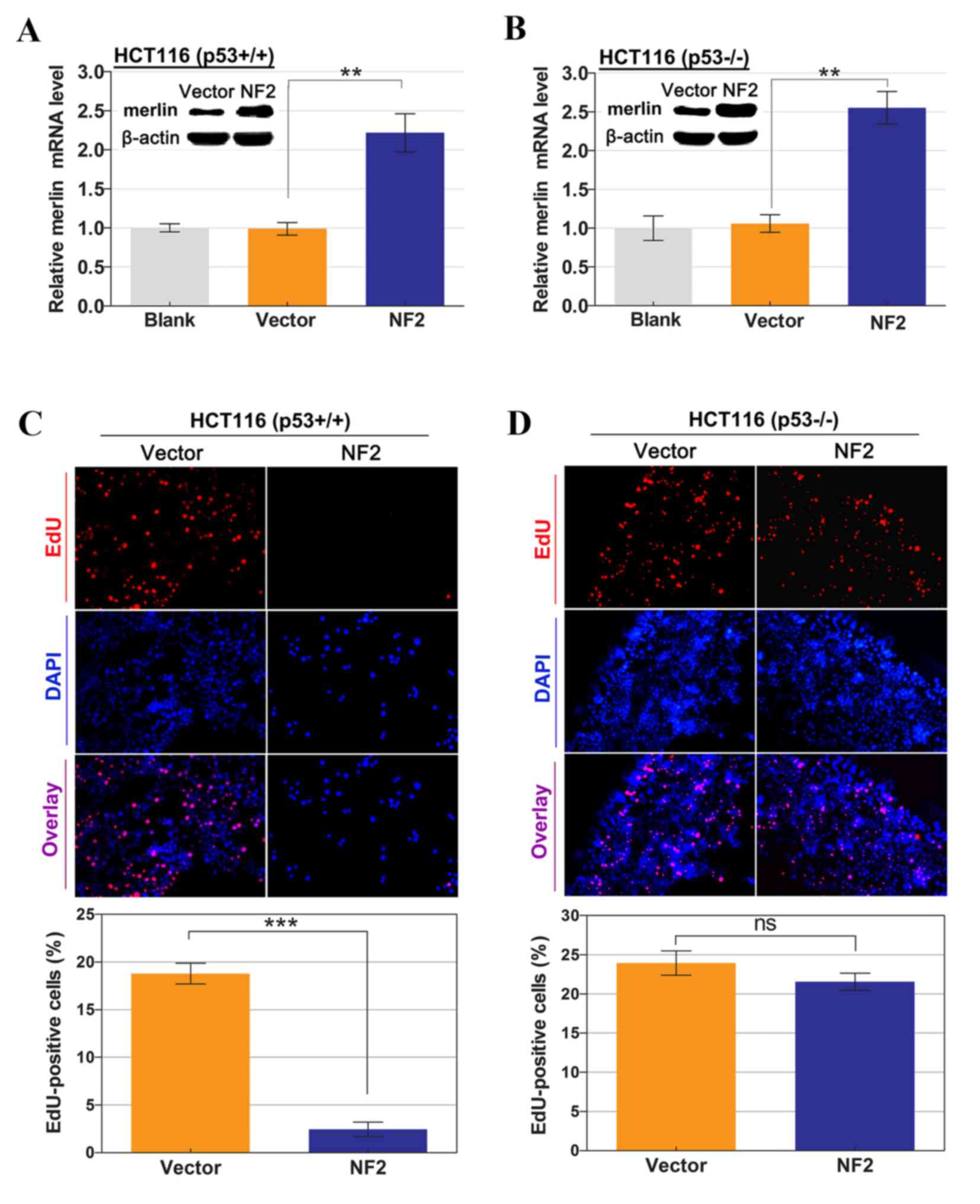
types (Fig. 1A and B). Considering
the short duration of overexpression effect in the transient
transfection model, EdU staining was conducted to detect the newly
synthesized DNA, thereby determining the proliferation activities.
As is demonstrated in Fig. 1C and D,
the percentage of EdU-positive cells in the S phase of the cell
cycle was significantly lower in HCT116 p53wt cells with
the NF2-expressing vector compared with those with empty
vector control (18.8±2.5 vs. 2.4±1.7%; P<0.001). However,
transfection of the NF2-expressing vector did not confer
significant growth inhibition in HCT116 p53−/− cells
compared with the control (23.9±3.8 vs. 21.6±2.7%; P=0.238). These
results indicated that p53 may perform an essential role in
mediating the inhibitory effect of merlin expression on cellular
proliferation.
Enhancement of cellular proliferation
is induced by merlin depletion in a p53-dependent manner
The loss of merlin is one of a number of molecular
changes that occur in tumor suppressor genes during the malignant
progression of colorectal cancer (7).
For HCT116 cell lines, no detectable NF2 mutations were
observed through direct PCR sequencing of genome DNA (data not
shown), indicating that they may possess a wild-type active form of
merlin. Knockdown of merlin was then performed using
lentivirus-mediated shRNA transfection into HCT116 cultures
(wild-type and p53-null), to mimic tumor progression in
vitro.
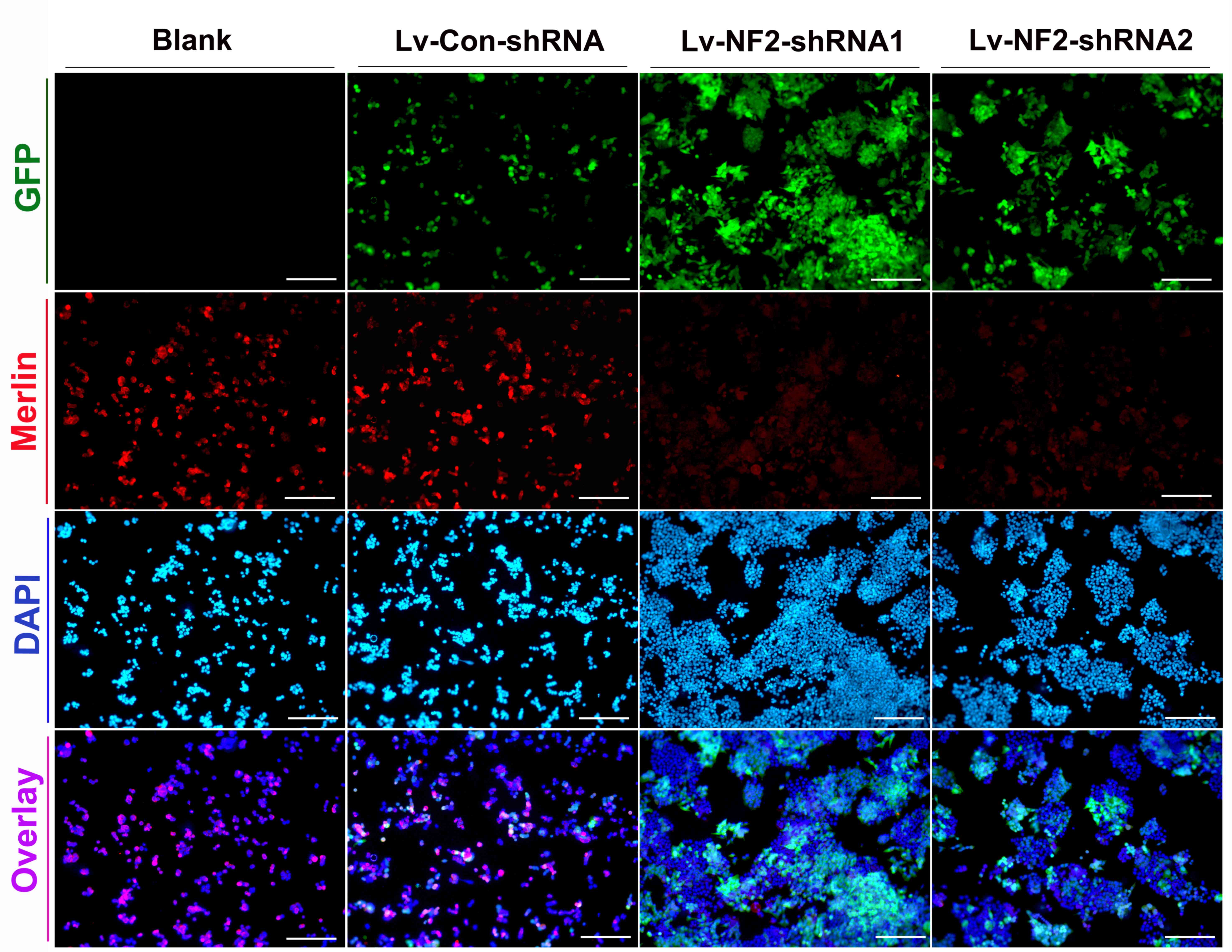
To superficially evaluate the transduction
efficacies of lentiviral vectors and the degree of merlin knockdown
on HCT116 cultures, fluorescence of lentiviral GFP and merlin
protein was observed under the microscope, comparing NF2
shRNA-transfected cultures (experiment group) with non-transfected
cultures (blank control) and control shRNA-transfected cultures
(negative control). As id demonstrated in Fig. 2, lentiviral vectors in the each of
experiment groups and control groups had >80% transduction
efficiencies, and fluorescence degree of merlin was decreased by
>50% in experiment groups compared with the controls. Similar
results were achieved from HCT116 p53−/− cells under the
same transfection conditions (data not shown).
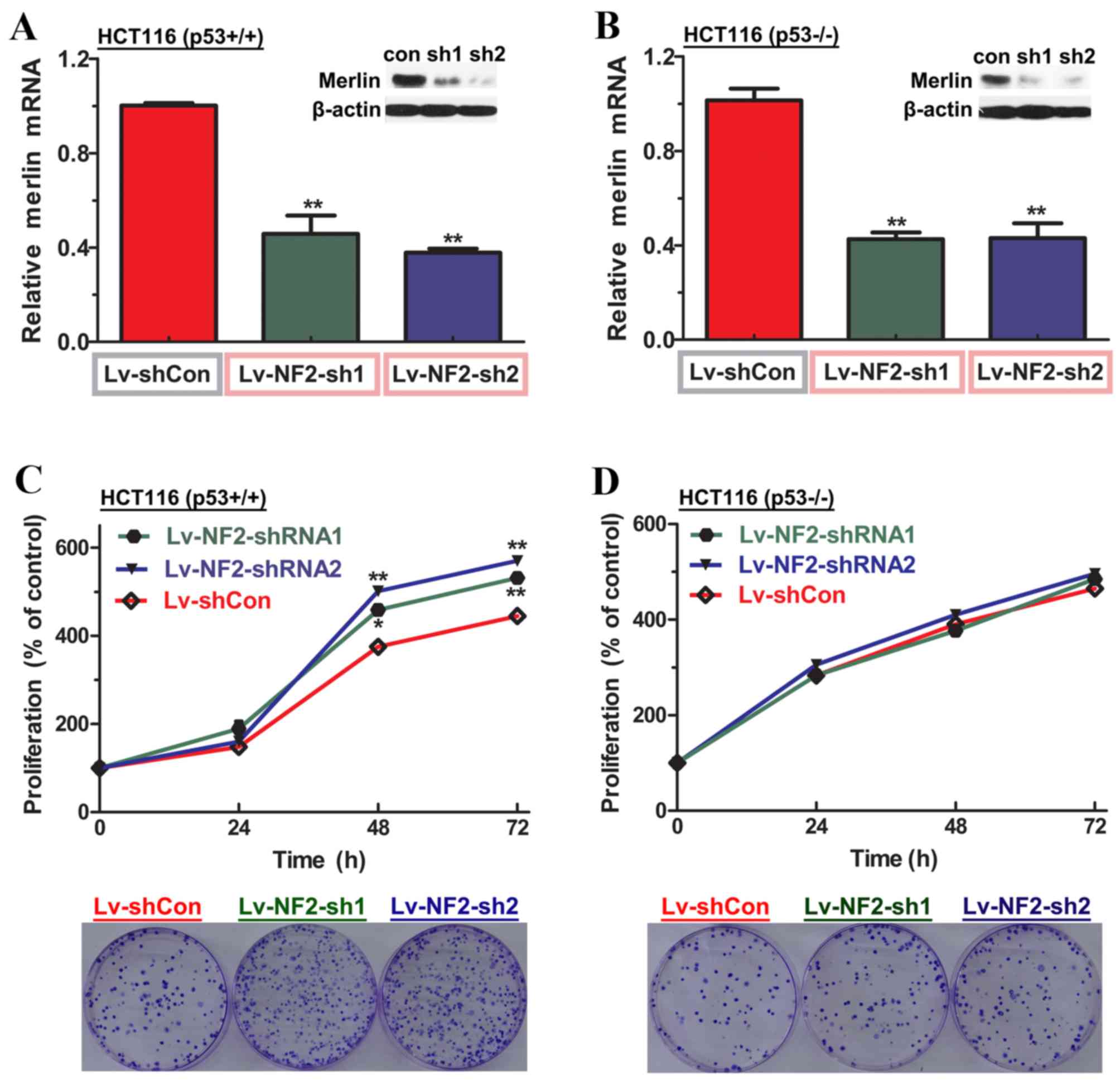
The extent of reduction in merlin expression was
then assessed in each of the experimental groups in the indicated
HCT116 cell lines compared with negative controls, and all
experimental groups demonstrated >50% reduction in mRNA level
and >80% reduction in protein level (Fig. 3A and B). Since uncontrolled
proliferation is one of the characteristics of malignancies
(6–7),
the effect of merlin knockdown on cellular proliferation in HCT116
cell lines, expressing p53 or not, was then examined. In line with
observations from merlin overexpression experiments, the CCK-8
assay demonstrated that merlin-knockdown significantly enhanced
cellular proliferation rates in HCT116 p53wt cells, but
not p53−/− cells 2 days after seeding (Fig. 3C and D). Notably, the aforementioned
results were confirmed by the colony formation assay, indicating
that the numbers of colonies were evidently increased only in
merlin-knockdown p53wt cells. This finding supports the
hypothesis that merlin deficiency triggers uncontrolled cellular
proliferation in a p53-dependent manner.
Knockdown of merlin abrogates
p53-invovled G0/G1 checkpoint, thus promoting cell cycle
progression
Merlin and p53 tumor suppressor products share
overlapping roles in regulating the G0/G1 checkpoint and thereby
controlling G1/S transition (21,25,26). The
presence of uncontrolled proliferation in wild-type HCT116
triggered by merlin depletion may be attributed to the
dysregulation of cell cycle progression. Based on these points, it
was then investigated whether merlin knockdown affected cell cycle
pattern in HCT116 cell lines and, if so, whether this was affected
by p53 status.
On day 3 post-transfection of lentiviral shRNAs,
indicated HCT116 cell lines in each of the experiment groups and
control groups were harvested and divided into two parts: One part
was subjected to western blotting aiming to verify the presence of
efficient merlin silencing and if successful (data not shown), the
remaining part was subjected to the analysis for cell cycle
pattern. It was identified that merlin-targeted shRNAs (sh1 and
sh2) conferred significant decreases in the percentage of cells in
the G0/G1 phase and increases in the percentage of cells in the S
phase in HCT116 p53wt cells, but not in HCT116
p53−/− cells, as compared with their control groups
(Fig. 4). Considering the critical
role of p53 in regulating the G0/G1 checkpoint, these observations
indicated that loss of merlin may abrogate p53-dependent G0/G1
checkpoint, thereby contributing to the transition of cell cycle
between the G1 and S phase, which is critical for cellular
proliferation.
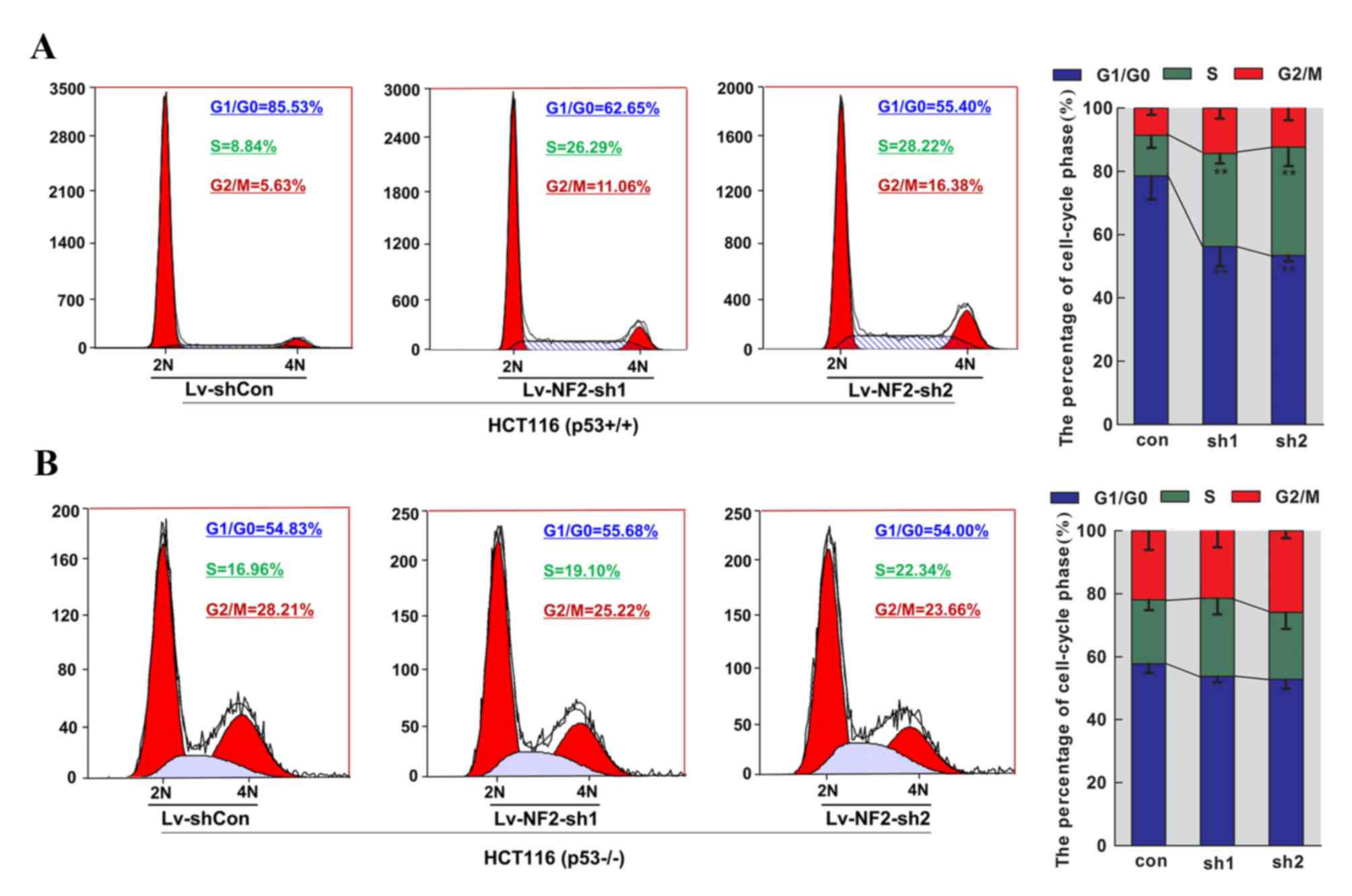 | Figure 4.Alteration in the cell cycle pattern
of HCT116 cell lines following transfection of merlin-targeted
lentiviral shRNAs. Two cell types, (A) HCT116 p53wt and
(B) p53−/− cells, treated with control shRNA (Con) or
merlin-targeted shRNAs (sh1 and sh2) for 72 h, were harvested and
subjected to flow cytometric analysis of DNA content subsequent to
propidium idodide staining. Representative micrographs and
quantification of proportions of cells in specific cell-cycle phase
(G0/G1, S or G2/M) were obtained from three independent
experiments. Merlin knockdown rendered significantly reduced
percentages of cells in the G1 phase (between 78.5±7.3 and 56.3±6.3
and 53.4±1.9% for sh1 and sh2, respectively) and increased
percentages of cells in the S phase (between 12.8±3.9 and 29.4±3.2
and 34.2±5.9% for sh1 and sh2, respectively) in HCT116
p53wt cells. Two similar results from three independent
experiments were used to plot a histogram with deviation bars.
**P<0.01 compared with controls. sh, short hairpin;
P53wt, wild-type for p53; NF2, neurofibromatosis;
p53−/−, p53-null; Con, control. |
Cell cycle progression by NF2
silencing involves p53 regulation of G1/S transition genes
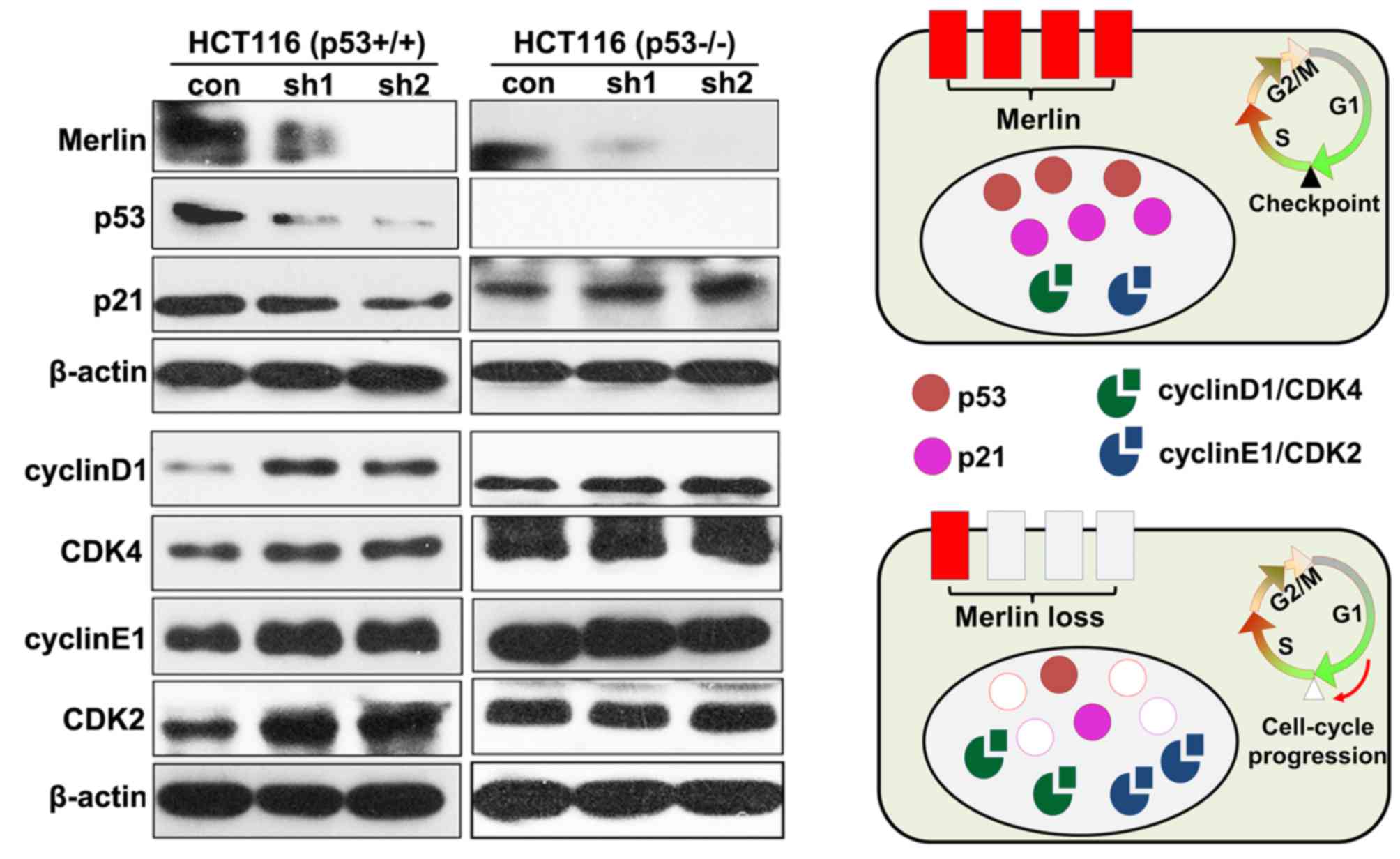
To investigate the molecular mechanisms by which
merlin knockdown contributes to cell cycle progression, the
expression of molecules involved in the G1/S transition of the cell
cycle was evaluated. It has been widely accepted that G1- to
S-phase progression is tightly regulated by p53 downstream p21
protein in association with cyclin-dependent kinases (CDKs 2, 4, 6)
and their essential activating coenzymes (cyclins D and E)
(1,21,26,27). As is
demonstrated in Fig. 5, the
downregulation of p53-p21 cascade signaling, followed by the
upregulation of cyclin D1/CDK4 and cyclin E1/CDK2 complexes were
observed in the HCT116 p53wt cell line compared with the
control. This was comparable to observations in our previous study
(21), in which merlin-deficient
schwannomas exhibited reduced p21 protein levels and heightened
expression of cyclin D1/CDK4 and cyclin E1/CDK2 complexes. As
expected, no significant changes in protein levels of any of these
cell-cycle regulators were observed in HCT116 p53−/−
cells upon merlin knockdown, which is in line with the results from
the cell cycle analysis. In summary, the present results indicate
that merlin loss may induce the downregulation of p53, and thus the
dysregulation of cell-cycle regulatory proteins, including p21,
cyclinD1/CDK4 and cyclin E1/CDK2 complexes. These cell-cycle
regulatory proteins may mediate p53-dependent G1/S cell cycle
progression (Fig. 5), and thereby
perform a role in the initiation or development of merlin-deficient
tumors.
Discussion
In a literature review study (28), merlin was reported to interact with
numerous signaling components involved in cellular proliferation
and cell cycle distributions. Recently, it was demonstrated that
other pathways are associated with merlin, including p53/p21
signaling (19–21). There are similarities between merlin
and p53, such as that merlin functions as a general tumor
suppressor product to multiple cell types as p53 does, and notably,
their tumor suppressive functions are associated with cell cycle
control (25–27). The major difference is that they are
predicted to localize into different subcellular compartments
(29,30). Merlin is localized preferentially to
areas of membrane ruffles (29),
whereas p53 is a nuclear phosphoprotein that functions as a
sequence-specific transcription factor. Certain oncogenic
activities require signaling from the cell membrane to the nucleus
to initiate the cell's entrance into the cell cycle (30), raising the possibility that p53 may be
one of the crucial mediators throughout the signal transmission
path modulated by merlin. This theory was supported by the results
from overexpression experiments, in which a significant inhibitory
effect of overexpressed merlin on cellular proliferation was
observed only in HCT116 p53wt cells.
Merlin depletion in human/mouse normal Schwann cells
was demonstrated to increase cellular proliferation, and therefore
promote the tumorigenesis of schwannomas (31). In colorectal cancer, merlin
deficiencies due to NF2 mutations tend to result in
malignant progression (7). Notably,
the HCT116 cell lines used in the present study had no detectable
mutations in the NF2 gene through standard sequencing (data
not shown), which is why RNA interference was performed to knock
down merlin expression in merlin-abundant HCT116 cell lines in
order to mimic tumor progression in vitro. The present study
reported that knockdown of endogenous merlin resulted in enhanced
cellular proliferation in the p53wt cells but not in
p53−/− cells, indicating that merlin depletion may
trigger uncontrolled cellular proliferation, one of the
characteristics of malignancies (6,7), at least
in part through p53-dependent mechanisms.
Potential alterations in cell cycle patterns were
also investigated, which may provide increased understanding into
the effects of merlin knockdown on the cellular proliferation of
HCT116 cell lines. For HCT116 cells with wild-type p53, merlin
knockdown led to a significant decrease in the percentage of cells
in the G0/G1 phase, which was accompanied by increased intra-S
status. However, no significant changes for cell cycle
distributions were observed in HCT116 cells lacking p53, indicating
that loss of merlin tended to abrogate p53-involved G0/G1 arrest,
and thereby promote G1- to S-phase progression.
It is well-known that p53 is induced in response to
DNA damage, resulting in upregulation of p21, a potent
cyclin-dependent kinase inhibitor that targets cyclin D1/CDK4,6 and
cyclin E/CDK2 complexes, thereby halting cell cycle progression
either prior to S-phase entry or during the S-phase (19–21). Our
previous study (21) reported that
merlin loss was accompanied by repressed p21 expression and
elevated levels of cyclin D1, CDK4, cyclin E1 and CDK2 proteins in
schwannomas, in which p53 mutations are rarely reported (32). Accordingly, the present study
demonstrated the presence of dysregulation of these cell-cycle
regulators (p21 and cyclins/CDKs) only in the HCT116
p53wt cell line upon knockdown of merlin, indicating
that loss of merlin may trigger deregulated expression of G1/S
transition genes mainly through a p53-dependent manner. Thus, the
present study provides additional information regarding the causes
and detailed events of merlin-deficient cell cycle progression.
In conclusion, to the best of our knowledge, the
present study reported for the first time that loss of merlin
contributes to cellular proliferation and cell cycle progression
through p53-dependent mechanisms. p53 is a molecule that should be
investigated for its potential in targeted drug therapy for
merlin-deficient malignancies, although verifications remain to be
performed in more cell types whose tumorigenicity is associated
with NF2/merlin.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81371086 and 81670919 to
Zhaoyan Wang and no. 81570906 to Hao Wu).
References
|
1
|
Wang Z, Lu Y, Tang J, Wang H and Wu H: The
phosphorylation status of merlin in sporadic vestibular
Schwannomas. Mol Cell Biochem. 324:201–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Z, Wang Z, Sun L, Li X, Huang Q,
Yang T and Wu H: Mutation spectrum and differential gene expression
in cystic and solid vestibular schwannoma. Genet Med. 16:264–270.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen H, Zhang X, Zhang Z, Yang T, Wang Z
and Wu H: The role of NF2 gene mutations and pathogenesis-related
proteins in sporadic vestibular schwannomas in young individuals.
Mol Cell Biochem. 392:145–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lau YK, Murray LB, Houshmandi SS, Xu Y,
Gutmann DH and Yu Q: Merlin is a potent inhibitor of glioma growth.
Cancer Res. 68:5733–5742. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sheikh HA, Tometsko M, Niehouse L, Aldeeb
D, Swalsky P, Finkelstein S, Barnes EL and Hunt JL: Molecular
genotyping of medullary thyroid carcinoma can predict tumor
recurrence. Am J Surg Pathol. 28:101–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sekido Y: Genomic abnormalities and signal
transduction dysregulation in malignant mesothelioma cells. Cancer
Science. 101:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cačev T, Aralica G, Lončar B and
Kapitanović S: Loss of NF2/Merlin expression in advanced sporadic
colorectal cancer. Cell Oncol (Dordr). 37:69–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimizu K, Nagamachi Y, Tani M, Kimura K,
Shiroishi T, Wakana S and Yokota J: Molecular cloning of a novel
NF2/ERM/4.1 superfamily gene, ehm2, that is expressed in
high-metastatic K1735 murine melanoma cells. Genomics. 65:113–120.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sainz J, Huynh DP, Figueroa K, Ragge NK,
Baser ME and Pulst SM: Mutations of the neurofibromatosis type 2
gene and lack of the gene product in vestibular schwannomas. Hum
Mol Genet. 3:885–891. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stamenkovic I and Yu Q: Merlin, a ‘magic’
linker between the extracellular cues and intracelular signaling
pathways that regulate cell motility, proliferation, and survival.
Curr Protein Pept Sci. 11:471–484. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schroeder RD, Angelo LS and Kurzrock R:
NF2/merlin in hereditary neurofibromatosis 2 versus cancer:
Biologic mechanisms and clinical associations. Oncotarget. 5:67–77.
2014.PubMed/NCBI
|
|
12
|
Gutmann DH, Giordano MJ, Fishback AS and
Guha A: Loss of merlin expression in sporadic meningiomas,
ependymomas and schwannomas. Neurology. 49:267–270. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang C, Asthagiri AR, Iyer RR, Lu J, Xu
DS, Ksendzovsky A, Brady RO, Zhuang Z and Lonser RR: Missense
mutations in the NF2 gene result in the quantitative loss of merlin
protein and minimally affect protein intrinsic function. Proc Natl
Acad Sci USA. 108:pp. 4980–4985. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ammoun S, Flaiz C, Ristic N, Schuldt J and
Hanemann CO: Dissecting and targeting the growth factor-dependent
and growth factor-independent extracellular signal-regulated kinase
pathway in human schwannoma. Cancer Res. 68:5236–5245. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Curto M, Cole BK, Lallemand D, Liu CH and
McClatchey AI: Contact-dependent inhibition of EGFR signaling by
Nf2/Merlin. J Cell Biol. 177:893–903. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flaiz C, Ammoun S, Biebl A and Hanemann
CO: Altered adhesive structures and their relation to RhoGTPase
activation in merlin deficient Schwannoma. Brain Pathol. 19:27–38.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li W, You L, Cooper J, Schiavon G,
Pepe-Caprio A, Zhou L, Ishii R, Giovannini M, Hanemann CO, Long SB,
et al: Merlin/NF2 suppresses tumorigenesis by inhibiting the E3
ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 140:477–490.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robanus-Maandag E, Giovannini M, van der
Valk M, Niwa-Kawakita M, Abramowski V, Antonescu C, Thomas G and
Berns A: Synergy of Nf2 and p53 mutations in development of
malignant tumours of neural crest origin. Oncogene. 23:6541–6547.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang Z, Guo CL, Ahronowitz I,
Stemmer-Rachamimov AO, MacCollin M and Nunes FP: A role for the p53
pathway in the pathology of meningiomas with NF2 loss. J
Neurooncol. 91:265–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim H, Kwak NJ, Lee JY, Choi BH, Lim Y, Ko
YJ, Kim YH, Huh PW, Lee KH, Rha HK and Wang YP: Merlin neutralizes
the inhibitory effect of Mdm2 on p53. J Biol Chem. 279:7812–7818.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu H, Chen Y, Wang ZY, Li W, Li JQ, Zhang
L and Lu YJ: Involvement of p21 (waf1) in merlin deficient sporadic
vestibular schwannomas. Neuroscience. 170:149–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Poulikakos PI, Xiao GH, Gallagher R,
Jablonski S, Jhanwar SC and Testa JR: Re-expression of the tumor
suppressor NF2/merlin inhibits invasiveness in mesothelioma cells
and negatively regulates FAK. Oncogene. 25:5960–5968. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schulze KM, Hanemann CO, Müller HW and
Hanenberg H: Transduction of wild-type merlin into human schwannoma
cells decreases schwannoma cell growth and induces apoptosis. Hum
Mol Genet. 11:69–76. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lü J, Zou J, Wu H and Cai L: Compensative
shuttling of merlin to phosphorylation on serine 518 in vestibular
schwannoma. Laryngoscope. 118:169–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muranen T, Grönholm M, Renkema GH and
Carpén O: Cell cycle-dependent nucleocytoplasmic shuttling of the
neurofibromatosis 2 tumour suppressor merlin. Oncogene.
24:1150–1158. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arellano M and Moreno S: Regulation of
CDK/cyclin complexes during the cell cycle. Int J Biochem Cell
Biol. 29:559–573. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scoles DR: The merlin interacting proteins
reveal multiple targets for NF2 therapy. Biochim Biophys Acta.
1785:32–54. 2008.PubMed/NCBI
|
|
29
|
Lallemand D, Manent J, Couvelard A,
Watilliaux A, Siena M, Chareyre F, Lampin A, Niwa-Kawakita M,
Kalamarides M and Giovannini M: Merlin regulates transmembrane
receptor accumulation and signaling at the plasma membrane in
primary mouse Schwann cells and in human schwannomas. Oncogene.
28:854–865. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Planque N: Nuclear trafficking of secreted
factors and cell-surface receptors: New pathways to regulate cell
proliferation and differentiation and involvement in cancers. Cell
Commun Signal. 4:72006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ahmad Z, Brown CM, Patel AK, Ryan AF,
Ongkeko R and Doherty JK: Merlin knockdown in human Schwann cells:
Clues to vestibular schwannoma tumorigenesis. Otol Neurotol.
31:460–466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Monoh K, Ishikawa K, Yasui N, Mineura K,
Andoh H and Togawa K: p53 tumor suppressor gene in acoustic
neuromas. Acta Otolaryngol Suppl. 537:11–15. 1998.PubMed/NCBI
|