Introduction
Autophagy is a highly conserved lysosomal pathway
that is crucial for maintaining cellular homeostasis and cell
survival in stress conditions (1). In
the autophagic process, degraded organelles and cytoplasmic
proteins are phagocytosed in autophagosomes, double-membrane
vesicles, which then fuse with lysosomes to convert them into
autolysosomes. Phagocytosis digests material in autolysosomes to
amino acids and molecular precursors for cell anabolism. In the
majority of cell types, autophagy occurs at low levels to maintain
homeostasis and facilitate differentiation and developmental
processes (2). Abnormalities of
autophagy may result in disease, including cancer,
neurodegeneration, infectious disease and heart disease (3). Emerging evidence suggests that autophagy
may contribute to tumor cell resistance to radiation and
chemotherapy (4).
Autophagy may be regulated by various oncogenes
(5). Wild-type tumor protein p53
serves a dual role in regulating autophagy depending on its
subcellular localization (6).
Autophagy may be induced by p53 via transcription-dependent or
independent pathways (7), whereas
inhibiting the activity of p53 has also been demonstrated to be
sufficient to activate autophagy (8).
Although activation of p53 is associated with
tumor-suppressive functions, including cell senescence, cell cycle
arrest, apoptosis and inhibition of angiogenesis (9), a recent study has illustrated that p53
can also promote cell survival (10).
With reference to its effect in autophagy, it is hypothesized that
p53 serves a significant role in cancer cell survival during
chemotherapy in specific conditions. In the present study, the role
of p53-induced autophagy in nutrient deprivation was explored and
the effect of p53 inhibition on chemosensitivity in
cholangiocarcinoma cells under nutrient-deprived conditions was
examined.
Materials and methods
Cell culture and reagents
Human cholangiocarcinoma cell lines QBC939 and RBE,
which possess wild-type p53 (11,12), were
obtained from the Tumor Immunology and Gene Therapy Center of the
Eastern Hepatobiliary Surgery Hospital (Shanghai, China). The cells
were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and supplemented with 10% fetal bovine
serum (Shanghai Excell Biology, Shanghai, China), 100 U/ml
penicillin and 100 mg/ml streptomycin [designated as the
full-nutrient (FN) medium] in a humidified incubator with 5%
CO2 at 37°C. The cells were treated with culture media
containing 120 µg/ml 5-fluorouracil (5FU) or 8 µg/ml cisplatin 24 h
after seeding. Nutrient deprivation was induced by growth in a
nutrient-free (NF) medium composed of Earle's balanced salt
solution (EBSS), which was purchased from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany). 5FU and cisplatin were purchased
from Qilu Pharmaceutical Co., Ltd. (Jinan, China). 3-methyladenine
(3MA, 10 mM) was obtained from Selleck Chemicals (Houston, TX,
USA), as the inhibitor of autophagy. Pifithrin-α (PFT-α) was
obtained from Sigma-Aldrich (Merck Millipore). PFT-α was dissolved
in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck Millipore).
Cell viability assay
The measurement of the percentage of viable cells
was assessed with a Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technology, Inc., Kumamoto, Japan), as previously described
(13). QBC939 and RBE cells were
seeded in a 96-well plate at 5×103 cells per well. When
cell density reached 80%, fresh medium containing 5FU or cisplatin
was added to the cells. Cell viability was measured using the CCK-8
assay subsequent to incubation with the drugs for 24 h. Absorbance
was measured at 450 nm with a microtiter plate reader.
Western blot analysis
Western blot analysis was performed as described
previously (14) using antibodies
specific for p53 (cat. no. P9249, Sigma-Aldrich; Merck
KGaA-Aldrich; Merck Millipore). QBC939 and RBE cells were cultured
in FN or NF media for 24 h. The harvested cells were washed with
PBS twice and lysed on ice for 30 min with whole cell extract lysis
buffer (Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Lysates
were centrifuged at 16,099 × g for 10 min at 4°C and the protein
concentration was determined by a bicinchoninic acid kit for
Protein Determination (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) according to the manufacturer's protocol. Cell lysates were
mixed with loading buffer and heated for 5 min at 100°C. Protein
samples were separated by SDS-PAGE and transferred onto
nitrocellulose membranes. The membranes were blocked in blocking
buffer (TBS, 0.1% Tween-20 and 5% skimmed milk powder) for 1 h and
then incubated overnight at 4°C with the specific p53 antibody
(dilution, 1:1,000). Following three washes in TBS/0.1% Tween-20,
the membranes were incubated with a horseradish
peroxidase-conjugated secondary antibody against rabbit IgG (cat.
no. sc-2005; dilution, 1:2,000; Santa Cruz Biotechnology, Inc.) for
1 h at room temperature. Following three further washes in TBS/0.1%
Tween-20, the membranes were detected by chemiluminescence using
Western Blotting Luminol Reagent (Santa Cruz Biotechnology,
Inc.).
Transient transfection
Microtubule-associated protein 1A/1B-light chain 3
(LC3) is a commonly used molecular marker for autophagy. Plasmids
expressing green fluorescent protein-tagged microtubule-associated
protein 1A/1B-light chain 3 (GFP-LC3; Shanghai, China Institute for
Biological Sciences, Shanghai, China) were transiently transfected
into cholangiocarcinoma cells as previously described (14). Cells were incubated with cisplatin or
fluorouracil (Qilu Pharmaceutical Co., Ltd.) for 3 days and
transfected with the GFP-LC3 plasmid. After 24 h, the cells were
fixed in 4% paraformaldehyde for 30 min and mounted for confocal
microscopy. GFP fluorescence was observed under a confocal
microscope (TCS SP8; Leica Microsystems, Inc., Buffalo Grove, IL,
USA). Autophagic cells were counted as those that exhibited
punctate fluorescence from GFP-LC3.
Cell apoptosis assay
Apoptosis detection by 4′,6-diamidino-2-phenylindole
dihydrochloride (DAPI) staining was performed as described
(14). QBC939 cells cultured with 5FU
or cisplatin were fixed in 4% formaldehyde for 20 min at room
temperature. Fixed cells were permeabilized with 0.5% Triton X-100
in PBS for 10 min at room temperature. After being washed with PBS,
cells were incubated with 1 µg/ml of DAPI for 10 min and then
washed three times in PBS. Cell morphology was observed with a
fluorescence microscope (Zeiss GmbH, Jena, Germany).
Photomicrographs were taken with a digital camera (Olympus
Corporation, Tokyo, Japan).
Transmission electron microscopy
Cells were fixed with 2.5% glutaraldehyde in
phosphate buffer and stored at 4°C until embedding. Cells were
post-fixed with 1% osmium tetroxide followed by increasing gradient
dehydration steps using ethanol and acetone. Cells were
subsequently embedded in araldite and ultrathin sections (50–60 nm)
were obtained, placed on uncoated copper grids and stained with 3%
lead citrate-uranyl acetate. Images were examined with a CM-120
transmission electron microscope (Philips Medical Systems B.V.,
Eindhoven, The Netherlands).
Small interfering RNA (siRNA)
transfection
Beclin-1 is a key protein in the process of
autophagy (14). The Stealth RNAi™
negative control duplex (cat. no. 12935-200) and siRNA duplex
oligoribonucleotides targeting human Beclin-1 (cat. no. 1299003)
were obtained from Invitrogen (Thermo Fisher Scientific, Inc.) and
siRNA-p53 (cat. no. 13750047) from Ambion (Thermo Fisher
Scientific, Inc.). The siRNA was transfected into
cholangiocarcinoma cells using siRNA Transfection Reagent (cat. no.
sc-29528; Santa Cruz, Biotechology, Inc.) according to the
manufacturer's protocol.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analysis between two groups was performed
using a Student's t-test, and multiple groups were compared by
one-way analysis of variance, using SPSS version 15.0 (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to represent a
statistically significant difference.
Results
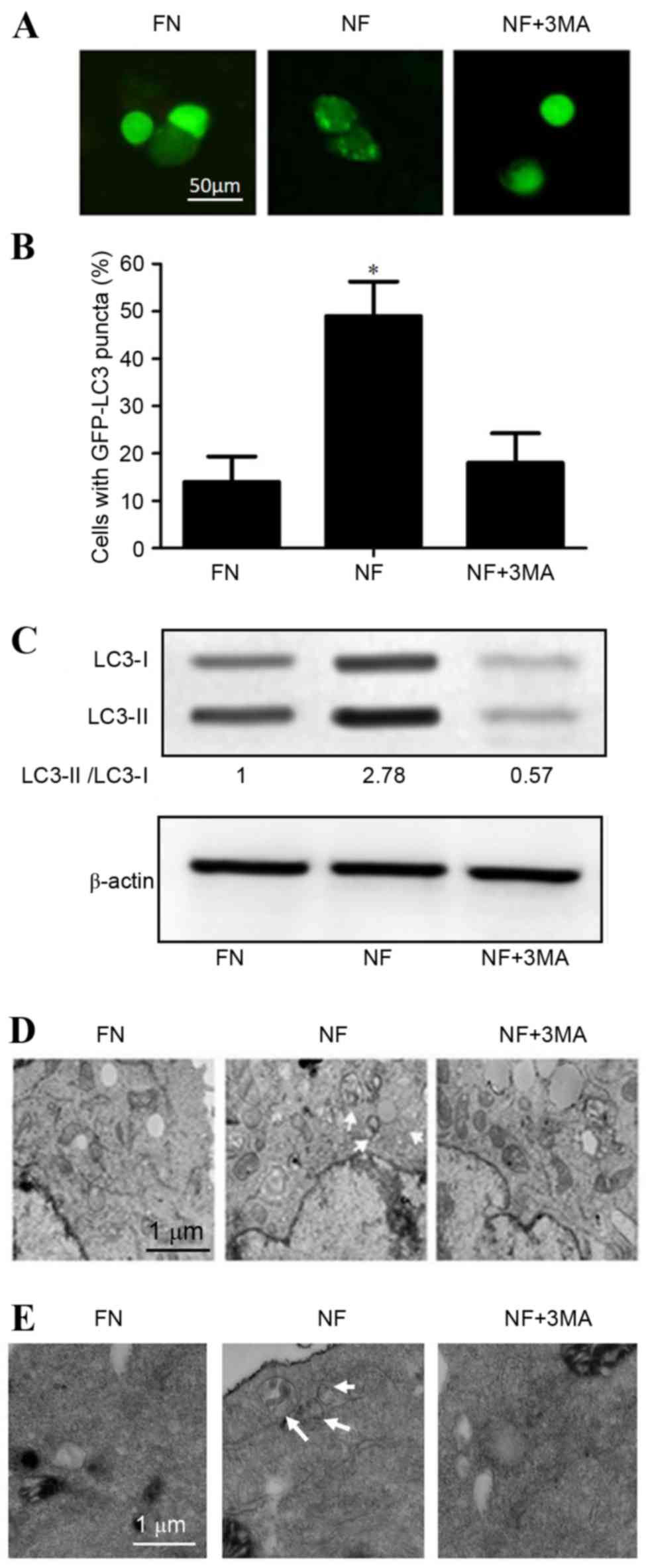
Nutrient-deprived conditions induce
autophagy in cholangiocarcinoma cells
To confirm the role of autophagy in
nutrient-deprived conditions, cholangiocarcinoma cell lines were
transfected with GFP-LC3 plasmids following culture for 12 h in FN
or NF medium. Fluorescence microscopy results revealed that the NF
condition comprised a significantly higher percentage of cells
containing GFP puncta (P<0.05) compared with cells grown in the
FN medium, which predominantly exhibited a diffuse GFP
distribution. Treatment with the autophagy inhibitor
3-methyladenine (3MA) decreased the proportion of cells containing
GFP-LC3 puncta among the NF medium-treated QBC939 cells compared
with NF treatment alone (48.27–19.24%; Fig. 1A and B).
Western blot analysis of LC3 revealed that the
protein expression level of LC3-II was significantly increased in
the nutrient-deprived condition in RBE cells (P<0.05; Fig. 1C). Furthermore, electron microscopy
analysis demonstrated that the number of observable autophagosomes
in EBSS-treated cholangiocarcinoma cells increased (Fig. 1D and E). Taken together, these
observations suggest that autophagy may be activated by
nutrient-deprived conditions in cholangiocarcinoma cells.
Inhibition of autophagy enhances the
sensitivity to chemotherapy of cholangiocarcinoma cells in a
nutrient-deprived condition
The rate of cell death after chemotherapy in
different nutritional conditions was investigated. QBC939 and RBE
cells were cultured in FN or NF medium and treated with 120 µg/ml
5FU or 8 µg/ml cisplatin for 12 h. As presented in Fig. 2A, the cell death rate of
chemotherapy-treated cholangiocarcinoma cells was significantly
reduced in the cells growing in a nutrient-deprived environment
(P<0.05), which demonstrated the greater resistance to
chemotherapy of cells in nutrient-deprived conditions.
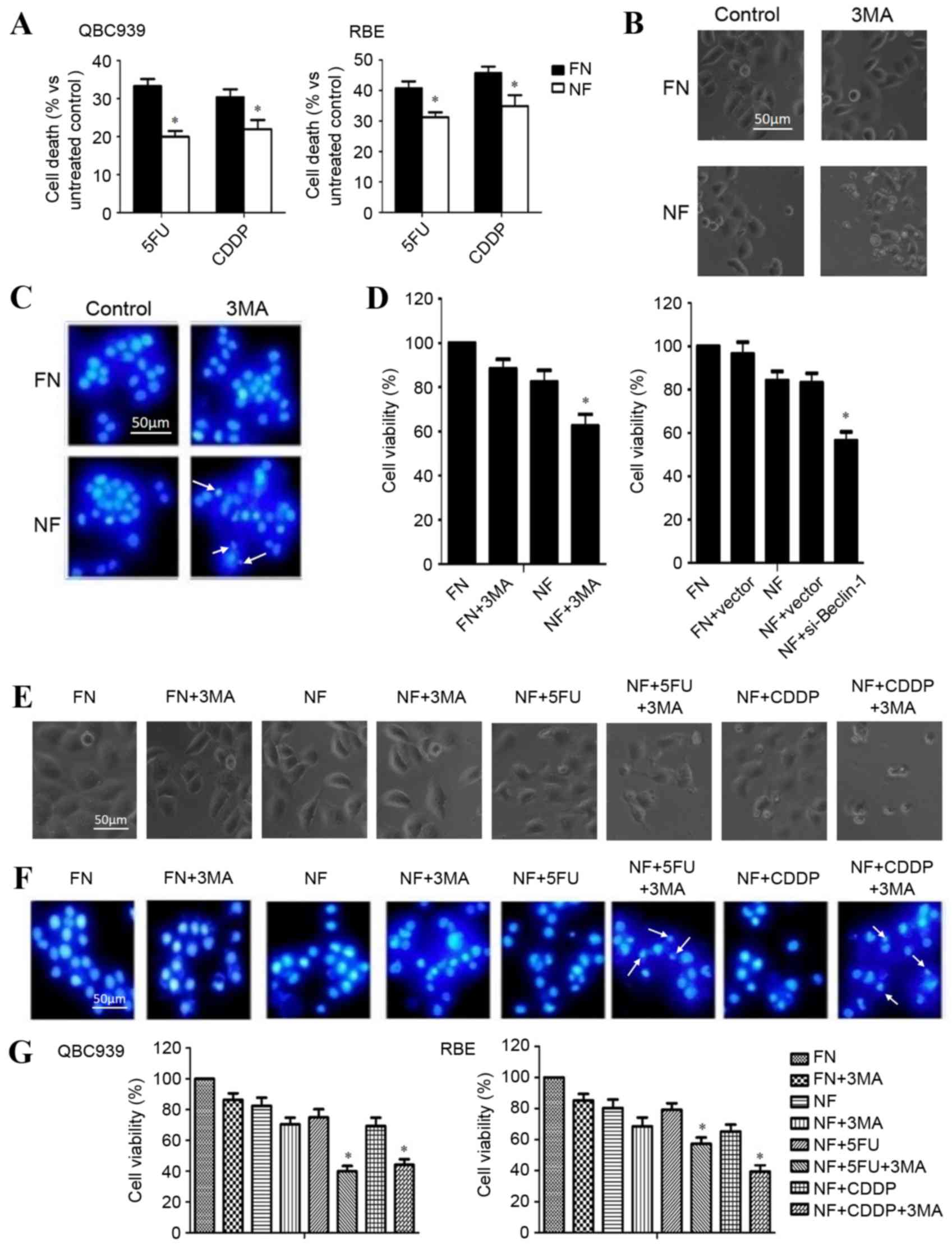 | Figure 2.Inhibition of autophagy enhanced the
chemosensitivity of cholangiocarcinoma cells in nutrient-deprived
conditions. (A) QBC939 and RBE cells were cultured in FN or NF
media, then treated with 5FU or CDDP for 12 h. Cell viability was
determined by a CCK-8 assay. Data are presented as the mean + SD of
≥3 independent experiments. (B) QBC939 cell morphological changes
were detected by inverted phase contrast microscopy following
treatment with the autophagy inhibitor 3MA. Representative images
are presented. (C) Nuclei were visualized with DAPI staining
following treatment with 3MA or a control. Representative images
are presented. Arrows indicate apoptotic bodies. (D) Cell viability
was evaluated using a CCK-8 assay following treatment with 3MA, or
transfection with a plasmid containing si-Beclin-1. (E) QBC939
cells were treated with 3MA for 1 h, then cells were incubated with
5FU or CDDP as previously described. Morphological changes were
detected by inverted phase contrast microscopy. Representative
images are presented. (F) Nuclei were visualized with DAPI staining
following treatment of the cells with 3MA with or without 5FU or
CDDP in each condition. Representative images are presented. Arrows
indicate apoptotic bodies. (G) QBC939 and RBE cells were treated
with 3MA then incubated with 5FU or CDDP, as previously described.
Cell viability was determined by a CCK-8 assay. The viability of
the untreated cells was regarded as 100%. Data are presented as the
mean ± SD of ≥3 independent experiments. *P<0.05, compared with
the FN and NF groups. FN, full-nutrient; NF, nutrient-free; 5FU,
5-fluorouracil (120 µg/ml); CDDP, cisplatin (8 µg/ml); CCK-8, Cell
Counting Kit-8; SD, standard deviation; 3MA, 3-methyladenine (10
mM); DAPI, 4′, 6-diamidino-2-phenylindole dihydrochloride;
si-Beclin-1, small interfering RNA against Beclin-1. |
To determine whether the inhibition of autophagy
enhanced the chemosensitivity of cholangiocarcinoma cells, QBC939
cells were cultured in FN or NF media, treated with autophagy
inhibitor 3MA and observed for morphological changes with a phase
contrast microscope. A marked increase in cell death was observed
in the nutrient-deprived group. The dead cells showed typical
apoptotic changes, including marked rounding, shrinkage and
detachment from the culture dish (Fig.
2B). Similar effects were further confirmed by DAPI staining,
which facilitated the visualization of apoptotic bodies in the
3MA-treated and nutrient-deprived cells (Fig. 2C). Additionally, a CCK-8 assay
revealed that the death rate increased following 3MA and
Beclin-1-siRNA treatment in nutrient-deprived conditions (Fig. 2D).
QBC939 cells were treated with 3MA for 1 h and then
incubated with 5FU or cisplatin for 12 h. The rate of cell death
was significantly increased in the combination groups (3MA plus
CDDP or 5FU), compared with the 5FU or CDDP group, as assessed by
cell morphology (Fig. 2E), DAPI
staining (Fig. 2F) or CCK-8 assay
(Fig. 2G; P<0.05). These results
suggest that the inhibition of autophagy contributed to the
increased chemosensitivity of cholangiocarcinoma cells during
nutrient deprivation.
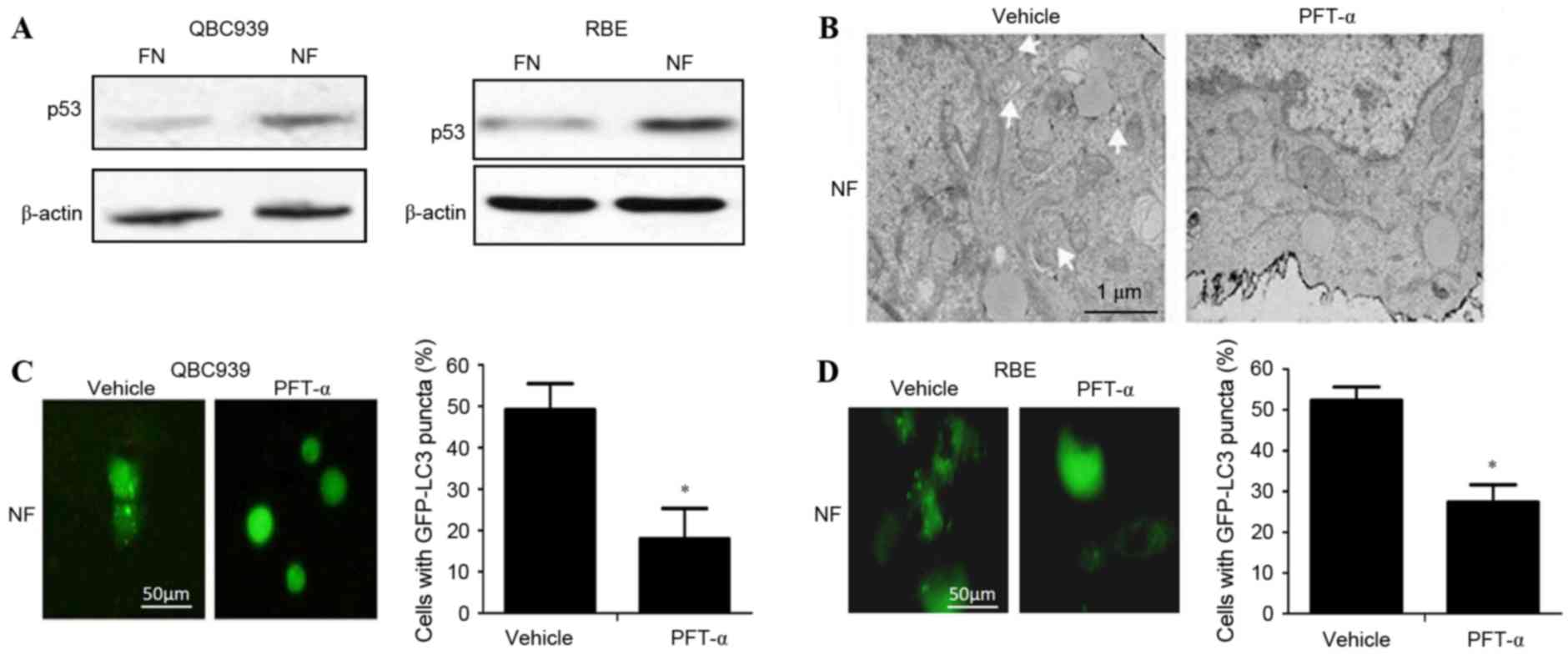
p53 activates autophagy in
cholangiocarcinoma cells in nutrient-deprived environments
To determine whether p53 activated autophagy in
cholangiocarcinoma cells in nutrient-deprived conditions, the
expression level of p53 in cholangiocarcinoma cells cultured in FN
and NF media was investigated. The level of p53 protein in the NF
groups was significantly increased compared with the FN groups
(P<0.05; Fig. 3A). Following
administration of the p53-inhibitor PFT-α, the activation of
autophagy was decreased in nutrient-deprived cholangiocarcinoma
cells as indicated by a significant decrease in the numbers of
visible autophagosomes on TEM (Fig.
3B) and GFP-LC3 puncta on fluorescence microscopy (Fig. 3C and D; P<0.01). Taken together,
these data suggest that p53 can activate autophagy in
cholangiocarcinoma cells in nutrient-deprived conditions.
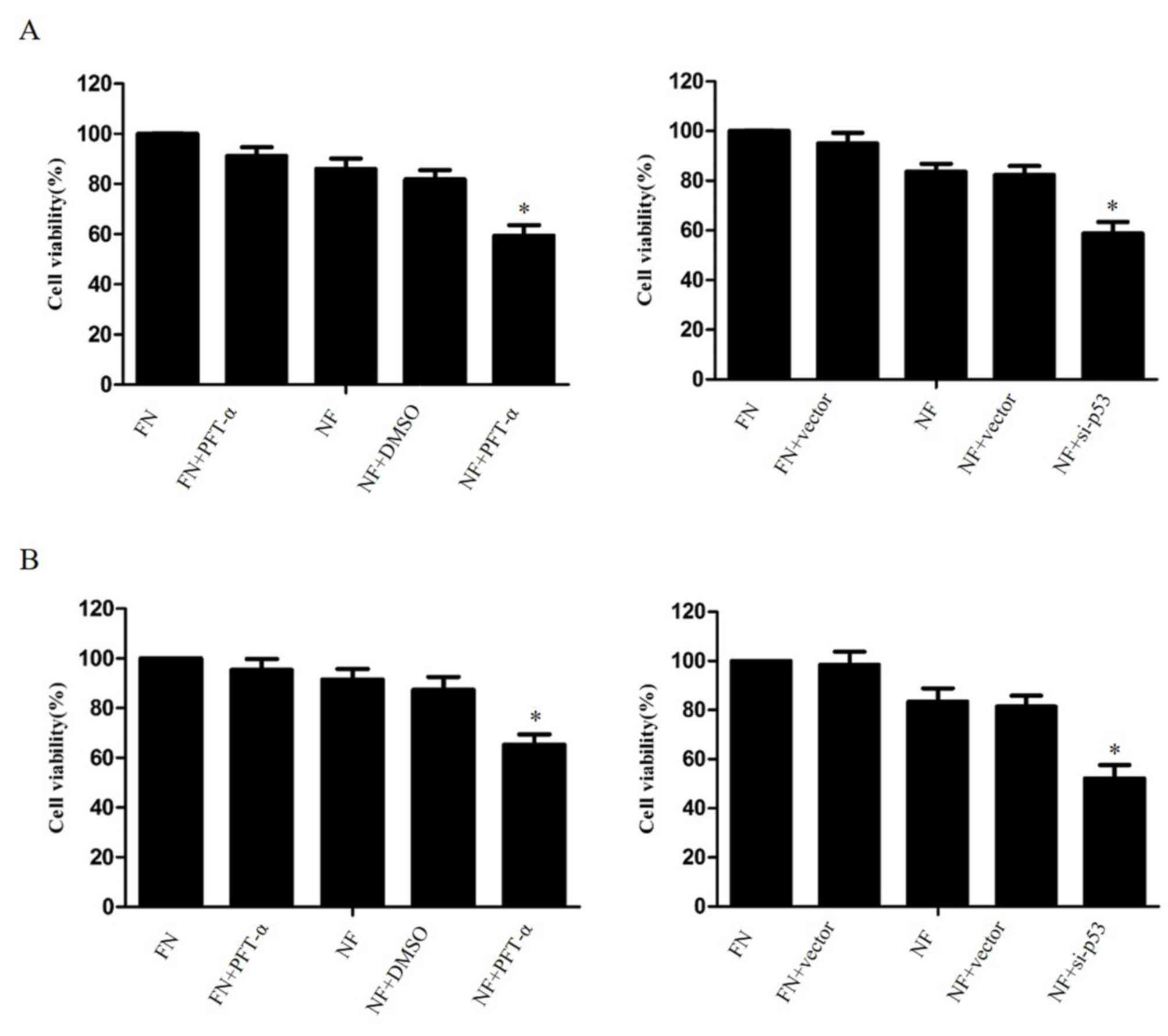
Inhibition of p53 enhances
chemosensitivity in cholangiocarcinoma cells during nutrient
deprivation
The effect of p53 inhibition on the survival of
nutrient-deprived cholangiocarcinoma cells was investigated. The
cell lines QBC939 and RBE were treated with 20 µM PFT-α or
transfected with p53-siRNA (6231; Cell Signaling Technology, USA),
then cultured for 24 h in FN or NF medium. A CCK-8 assay revealed
that the suppression of p53 significantly increased the cell death
rate of cholangiocarcinoma cells in nutrient-deprived conditions,
compared with DMSO-treated NF cells (P<0.05; Fig. 4).
QBC939 cells were treated with 20 µM PFT-α for 1 h
and then cultured in NF medium combined with 5FU or cisplatin for
24 h. As shown in Fig. 5A and B,
cell-death morphology increased markedly when PFT-α was combined
with 5FU or cisplatin in nutrient-deprived conditions compared with
cells treated only with 5FU or cisplatin. This result was confirmed
by CCK-8 assays (P<0.05; Fig. 5C and
D). Taken together, these results indicate that p53 inhibition
may increase the chemosensitivity of cholangiocarcinoma cells
during nutrient deprivation.
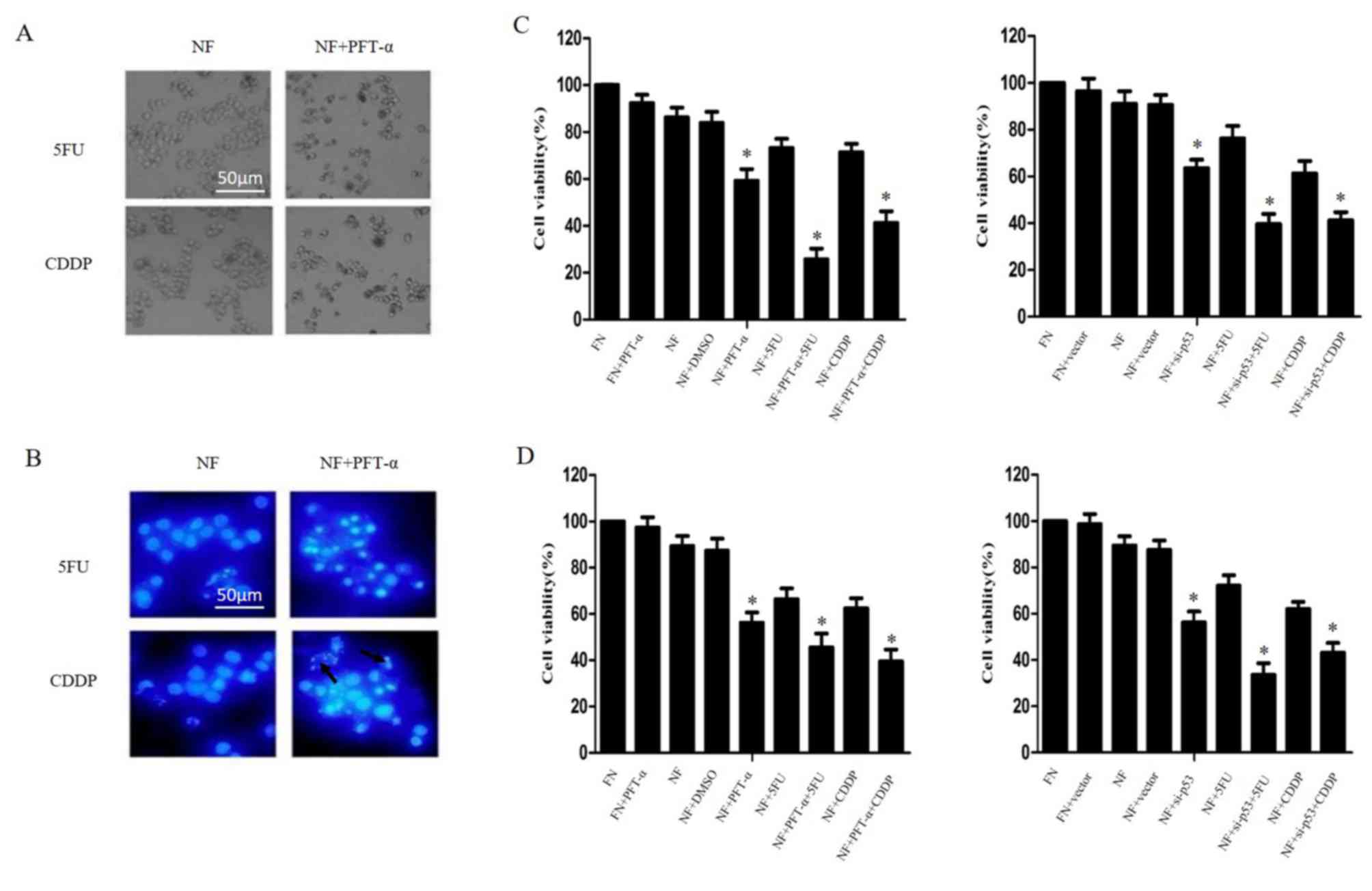 | Figure 5.Inhibition of p53 enhanced
chemosensitivity in cholangiocarcinoma cells in nutrient deprived
conditions. QBC939 and RBE cells were treated with PFT-α for 1 h or
transfected with plasmids containing siRNA against p53, then
cultured in NF or FN media with 5FU or CDDP treatment for 24 h. (A)
QBC939 cell morphology was detected by inverted phase contrast
microscope. Representative images are presented. (B) QBC939 cell
nuclei were visualized with DAPI staining. Representative images
are presented. Arrows indicate apoptotic bodies. The viability of
(C) QBC939 and (D) RBE cells was determined by Cell Counting Kit-8
assay. The viability of the untreated cells was regarded as 100%.
Data are presented as the mean ± standard deviation of ≥3
independent experiments. *P<0.05, compared with the FN and NF
groups. PFT-α, 20 µM of pifithrin-α; siRNA, small interfering RNA;
NF, nutrient-free; FN, full-nutrient; 5FU, 5-fluorouracil (120
µg/ml); CDDP, cisplatin (8 µg/ml); DAPI,
4′,6-diamidino-2-phenylindole dihydrochloride. |
Discussion
Autophagy is one of the crucial catabolic reactions
of cells to stimulation or stress (15). Previous studies have demonstrated that
p53 serves a complicated role in autophagy (16–18). In
the present study, the results indicated that p53 is associated
with the induction of autophagy during nutrient deprivation;
inhibition of p53 was observed to result in the deactivation of
autophagy and increased chemosensitivity in nutrient-deprived
cholangiocarcinoma cells.
The effect of p53, a well-studied tumor suppressor,
on autophagy is controversial (16).
In the present study, it was demonstrated that the level of p53 was
increased in cholangiocarcinoma cells in nutrient-deprived
conditions; autophagy induced by nutrient deprivation was inhibited
by PFT-α, a p53 suppressor, demonstrating the importance of p53 to
the activation of autophagy in nutrient-deprived cholangiocarcinoma
cells. These data are consistent with a previous study that
demonstrated that p53 regulates the autophagy protein LC3 in order
to mediate cancer cell survival during prolonged starvation
(17).
A number of studies have shown that autophagy
contributes to chemoresistance and that the inhibition of autophagy
enhances chemosensitivity in cancer cells (19–21).
However, these studies were performed in environments with nutrient
availability. In normal conditions during tumor development,
ischemic or innutritious environments are ubiquitous. The effect of
inhibited autophagy on the efficacy of chemotherapy during
nutrient-deprived conditions remains unclear. In the present study,
it was demonstrated that the inhibition of p53 contributes to the
inhibition of autophagy and an increased chemotherapy-induced cell
death rate in nutrient-deprived cholangiocarcinoma cells; this
indicates that autophagy is responsible for chemoresistance in
cholangiocarcinoma cells during nutrient-deprivation. To the best
of our knowledge, this is one of only a small number of reports
that inhibition of p53 led to increased chemosensitivity.
Under normal circumstances, cells sustain a certain
level of autophagy in order to maintain cellular homeostasis. When
cells are confronted with adverse conditions, including nutrient
deprivation, autophagy is activated to facilitate cell survival
(22). Factors that affect the
autophagic process are of crucial importance in managing a
variation in condition. Studies by our group have shown that the
inhibition of autophagy increases chemosensitivity in
nutrient-deprived carcinoma cells (18). Therefore, it is hypothesized that p53
inhibition may increase cell death in conditions where autophagy is
serving an important role in cell survival.
In certain conditions, particularly where apoptosis
is inhibited, autophagy contributes to chemotherapy-induced cell
death, and the strict definition of autophagic cell death needs to
conform to specific requirements (23). Furthermore, the difference between
‘autophagic cell death’ and ‘autophagy with cell death’ remains
controversial (24). It has become
clear that the effect of autophagy is not only to promote cell
death; it may also protect cells by allowing them to adapt to
adverse conditions (25). Emerging
evidence has indicated that autophagy is addictive in certain types
of Ras-driven tumors (26). Autophagy
is also known as type II-programmed cell death and the relationship
between autophagy and apoptosis is as yet inconclusive; it may take
the form of interdependence or interconversion under certain
conditions.
In the present study, the morphology of the cells
was observed under the fluorescence microscope following DAPI
staining, and morphological changes to the nuclei could be
observed. The results demonstrated that the inhibition of autophagy
could increase the chemosensitivity of cholangiocarcinoma cells and
increase the rate of tumor cell death, and that the mechanism for
cell death was apoptosis. In this circumstance, inhibition of
autophagy is a feasible therapeutic approach. The data suggest that
cholangiocarcinoma cells may gain tolerance to chemotherapy through
the activation of autophagy. Accordingly, inhibiting autophagy
could be a novel method to improve the efficiency of traditional
chemotherapies. However, autophagy-targeting strategies for cancer
will require additional clinical trial testing before they can be
fully realized.
In conclusion, the data reveals that p53 contributes
to cell survival in nutrient-deprived conditions and that the
inhibition of p53 increases the chemosensitivity of
cholangiocarcinoma cells. Although mutations to p53 are detected in
up to 50% of all human tumors (27,28), the
remainder of tumors maintain the expression of wild-type p53. This
implies that tumor cell survival may benefit from the functional
status of p53 in specific situations (17). In response to minor stress, p53 could
facilitate cellular homeostasis (29,30).
Although p53 is better known for its role in the induction of
apoptosis, further study by our group may contribute to expanding
the understanding of the effect of p53 on cancer therapy. These
results elucidate the role of autophagy in cancer formation and
progression. Further studies on the molecular mechanism by which
autophagy promotes chemoresistance should be considered, and may
contribute to the development of therapy against cholangiocarcinoma
or other types of cancer.
Glossary
Abbreviations
Abbreviations:
|
3MA
|
3-methyladenine
|
|
5FU
|
5-fluorouracil
|
|
CCK-8
|
Cell Counting Kit-8
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
dihydrochloride
|
|
DMSO
|
dimethyl sulfoxide
|
|
EBSS
|
Earle's balanced salt solution
|
|
GFP
|
green fluorescent protein
|
|
LC3
|
microtubule-associated protein
1A/1B-light chain 3
|
|
PFT-α
|
pifithrin-α
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Lin WJ and Kuang HY: Oxidative stress
induces autophagy in response to multiple noxious stimuli in
retinal ganglion cells. Autophagy. 10:1692–1701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
White E, Mehnert JM and Chan CS:
Autophagy, metabolism and cancer. Clin Cancer Res. 21:5037–5046.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hale AN, Ledbetter DJ, Gawriluk TR and
Rucker EB III: Autophagy: Regulation and role in development.
Autophagy. 9:951–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Livesey KM, Tang D, Zeh HJ and Lotze MT:
Autophagy inhibition in combination cancer treatment. Curr Opin
Investig Drugs. 10:1269–1279. 2009.PubMed/NCBI
|
|
5
|
Ávalos Y, Canales J, Bravo-Sagua R,
Criollo A, Lavandero S and Quest AF: Tumor suppression and
promotion by autophagy. Biomed Res Int. 2014:6039802014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang J, Di J, Cao H, Bai J and Zheng J:
p53-mediated autophagic regulation: A prospective strategy for
cancer therapy. Cancer Lett. 363:101–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
White EJ, Martin V, Liu JL, Klein SR, Piya
S, Gomez-Manzano C, Fueyo J and Jiang H: Autophagy regulation in
cancer development and therapy. Am J Cancer Res. 1:362–372.
2011.PubMed/NCBI
|
|
8
|
Borodkina AV, Shatrova AN, Deryabin PI,
Grukova AA, Nikolsky NN and Burova EB: Tetraploidization or
autophagy: The ultimate fate of senescent human endometrial stem
cells under ATM or p53 inhibition. Cell Cycle. 15:117–127. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Levine AJ and Oren M: The first 30 years
of p53: Growing ever more complex. Nat Rev Cancer. 9:749–758. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alexandrova EM and Marchenko ND: Mutant
p53 - heat shock response oncogenic cooperation: A new mechanism of
cancer cell survival. Front Endocrinol (Lausanne).
6:532015.PubMed/NCBI
|
|
11
|
Sun HW, Tang QB, Tang C and Zou SQ:
Effects of dendritic cells transfected with full length wild-type
p53 and modified by bile duct cancer lysates on immune response.
Hepatobiliary Pancreat Dis Int. 4:121–125. 2005.PubMed/NCBI
|
|
12
|
Wang Z, Tang X, Zhang Y, Qi R, Li Z, Zhang
K, Liu Z and Yang X: Lobaplatin induces apoptosis and arrests cell
cycle progression in human cholangiocarcinoma cell line RBE. Biomed
Pharmacother. 66:161–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takasu H, Sugita A, Uchiyama Y, Katagiri
N, Okazaki M, Ogata E and Ikeda K: c-Fos protein as a target of
anti-osteoclastogenic action of vitamin D, and synthesis of new
analogs. J Clin Invest. 116:528–535. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song J, Qu Z, Guo X, Zhao Q, Zhao X, Gao
L, Sun K, Shen F, Wu M and Wei L: Hypoxia-induced autophagy
contributes to the chemoresistance of hepatocellular carcinoma
cells. Autophagy. 5:1131–1144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parkhitko AA, Favorova OO and Henske EP:
Autophagy: Mechanisms, regulation, and its role in tumorigenesis.
Biochemistry (Mosc). 78:355–367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maiuri MC, Galluzzi L, Morselli E, Kepp O,
Malik SA and Kroemer G: Autophagy regulation by p53. Curr Opin Cell
Biol. 22:181–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scherz-Shouval R, Weidberg H, Gonen C,
Wilder S, Elazar Z and Oren M: p53-dependent regulation of
autophagy protein LC3 supports cancer cell survival under prolonged
starvation. Proc Natl Acad Sci USA. 107:pp. 18511–18516. 2010;
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo XL, Hu F, Zhang SS, Zhao QD, Zong C,
Ye F, Guo SW, Zhang JW, Li R, Wu MC and Wei LX: Inhibition of p53
increases chemosensitivity to 5-FU in nutrient-deprived
hepatocarcinoma cells by suppressing autophagy. Cancer Lett.
346:278–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ji MM, Wang L, Zhan Q, Xue W, Zhao Y, Zhao
X, Xu PP, Shen Y, Liu H, Janin A, et al: Induction of autophagy by
valproic acid enhanced lymphoma cell chemosensitivity through
HDAC-independent and IP3-mediated PRKAA activation. Autophagy.
11:2160–2171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan Y, Gao Y, Chen L, Gao G, Dong H, Yang
Y, Dong B and Chen X: Targeting autophagy augments in vitro and in
vivo antimyeloma activity of DNA-damaging chemotherapy. Clin Cancer
Res. 17:3248–3258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo XL, Li D, Hu F, Song JR, Zhang SS,
Deng WJ, Sun K, Zhao QD, Xie XQ, Song YJ, et al: Targeting
autophagy potentiates chemotherapy-induced apoptosis and
proliferation inhibition in hepatocarcinoma cells. Cancer Lett.
320:171–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Russell RC, Yuan HX and Guan KL: Autophagy
regulation by nutrient signaling. Cell Res. 24:42–57. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiong HY, Guo XL, Bu XX, Zhang SS, Ma NN,
Song JR, Hu F, Tao SF, Sun K, Li R, et al: Autophagic cell death
induced by 5-FU in Bax or PUMA deficient human colon cancer cell.
Cancer Lett. 288:68–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kroemer G and Levine B: Autophagic cell
death: The story of a misnomer. Nat Rev Mol Cell Biol. 9:1004–1010.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen S, Kepp O and Kroemer G: The end of
autophagic cell death? Autophagy. 8:1–3. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mancias JD and Kimmelman AC: Targeting
autophagy addiction in cancer. Oncotarget. 2:1302–1306. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vaughan C, Pearsall I, Yeudall A, Deb SP
and Deb S: p53: Its mutations and their impact on transcription.
Subcell Biochem. 85:71–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muller PA and Vousden KH: Mutant p53 in
cancer: New functions and therapeutic opportunities. Cancer Cell.
25:304–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chao CC: Mechanisms of p53 degradation.
Clin Chim Acta. 438:139–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J, Zhang C, Hu W and Feng Z: Tumor
suppressor p53 and its mutants in cancer metabolism. Cancer Lett.
356:197–203. 2015. View Article : Google Scholar : PubMed/NCBI
|