Introduction
Acute myeloid leukemia (AML) is a heterogeneous
group of malignant hematopoietic disorders characterized by
uncontrolled proliferation of clonally neoplastic cells and
accumulation in the bone marrow of blasts with an impaired
differentiation program which are inhibited at various maturation
steps and are resistant to cell death (1). Intensification of chemotherapy has
resulted in remission in between 70 and 85% of patients with AML;
however, post-remission relapses occur frequently (2,3).
The phosphoinositide 3-kinase (PI3K)/protein kinase
B (Akt) signaling pathway is a key mediator of cell viability,
proliferation and apoptosis. Its constitutive activation has been
implicated in the pathogenesis and progression of a variety of
neoplasias (4). The PI3K/Akt axis is
activated in AML (5–8). The disease-free survival and overall
survival times have been demonstrated to be significantly decreased
in cases of AML with upregulated PI3K/Akt signaling pathway protein
expression (9). The PI3K/Akt
signaling pathway is crucial to diverse physiological processes
that include cell cycle progression, differentiation,
transcription, translation and apoptosis (10,11). The
PI3K/Akt signaling pathway is targeted for genomic aberrations
including amplification, mutation and rearrangement more frequently
than any other signaling pathway in human cancer, with the possible
exception of the p53 and retinoblastoma signaling pathways. There
is convincing evidence that the alterations of the PI3K/Akt
signaling pathway are associated with tumor progression and
resistance to radiation and systemic therapies in humans (12,13).
LY294002 is an inhibitor of PI3K, which has been used extensively
to investigate the role of the PI3K/Akt signaling pathway in normal
and transformed cells (14,15). Inactivation of PI3K using LY294002 has
been demonstrated to lead to the dephosphorylation of Akt at
Thr308 and Ser473, consequently inducing
specific G1 phase arrest in cell proliferation and
finally to apoptosis (16,17). PI3K inhibitors also exhibit antitumor
activity in vitro and in vivo in a variety of tumor
types (18–20). Therefore, in the present study, it was
investigated whether LY294002 was able to increase the sensitivity
of AML cells to PHI.
Isothiocyanates are naturally sourced compounds
typically isolated from plants of the Brassicaceae family,
including broccoli, cabbage, and radish. Isothiocyanates are best
known for their antioxidative, anticancer chemotherapeutic,
chemopreventive, and anti-angiogenic properties (21,22). PHI,
a synthetic isothiocyanate, is able to induce cell growth arrest
and apoptosis in a number of types of tumor cell by inhibiting
histone deacetylation, histone methylation and DNA methylation, and
by remodeling chromatins (23–27).
In the present study, the rationale for the combined
inhibition of PHI and LY294002 in HL-60 cells was investigated.
Although the effect of LY294002 or PHI has been identified in a
number of types of human cancer, the synergistic effect of PHI and
LY294002 remains unclear.
Materials and methods
Reagents
PHI (LKT Laboratories, Inc., St. Paul, MN, USA),
>98% pure, was dissolved in 75% methanol to a stock
concentration of 5 mmol/l and stored at −20°C following positive
pressure filtration through a 0.22 µmol/l microporous membrane
filter. LY294002 (Cell Signaling Technology, Inc., Danvers, MA,
USA), >99% pure, was dissolved in dimethyl sulfoxide (DMSO) to a
stock concentration of 5 mmol/l. Antibodies against acetyl-histone
H3 (cat. no., 06-599) and acetyl-histone H4 (cat. no., 06-866) were
purchased from Upstate Biotechnology, Inc. (Lake Placid, NY, USA).
Antibodies against p-Akt (cat. no., 12694), p-mTOR (cat. no., 5536)
and p-p70S6K (cat. no., 9208) antibodies were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). β-actin antibody
(cat. no., sc-47778), goat anti-rabbit IgG-HRP (cat. no., sc-2004)
and goat anti-mouse IgG-HRP (cat. no., sc-2005) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). MTT (Sigma; Merck
KGaA, Darmstadt, Germany) was dissolved in PBS to a working
concentration of 5 mmol/l.
Cell culture
The human AML cell line HL-60 was obtained from the
China Center for Type Culture Collection (Beijing, China). Cells
were maintained in RPMI-1640 medium supplemented with 10%
heat-inactivated fetal bovine serum (both were obtained from Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and maintained at
37°C in a humidified atmosphere containing 5% CO2. Cells
in exponential growth phase were exposed to PHI and/or LY294002 at
various concentrations, as indicated for each assay. The control
cultures were supplemented with the methanol-containing medium and
DMSO-containing medium respectively.
Cell viability assay
MTT assay was used to analyze cell viability as
described previously (28). The
spectrophotometric absorbance of the samples was determined using
an Ultra Multifunctional microplate reader at 490 nm. Cells in
exponential growth phase (1.0×105 cells/ml in 100 µl) were cultured
in 96-well plates with various concentrations of PHI and LY294002
(0, 10, 20 and 40 µmol/l, respectively, or in combination at 20
µmol/l each). Cell viability was observed at 24, 48, 72 and 96 h.
The assay was conducted in triplicate. For evaluating the
synergistic effects of the drugs, HL-60 cells were treated with 10,
15, 20, 30 and 40 µmol/l PHI, and 10, 15, 20, 30 and 40 µmol/l
LY294002 for 72 h.
Analysis of cell apoptosis using flow
cytometry
HL-60 cells were seeded at 5.0×105 cells/ml in Petri
dishes and cultured with the aforementioned concentrations of PHI
or LY294002, or in combination, for 72 h. An annexin V-fluorescein
isothiocyanate (FITC)/PI double-fluorescence apoptosis detection
kit (Kaiji Biotech, Nanjing, China) was employed to quantify the
apoptosis of the HL-60 cells, according to the manufacturer's
protocol. Briefly, the suspended cells were pooled, washed twice
with ice-cold PBS and re-suspended in binding buffer (Kaiji
Biotech, Nanjing, China) to 106/ml. Subsequently, 0.2 ml of this
cell suspension was incubated with 5 µl Annexin V-FITC and 5 µl PI
Staining Solution for 10 min at room temperature in the dark. Then,
samples were analyzed using a flow cytometer (BD FACSCaliburTM, San
Jose, CA, USA) within 1 h. The experiment was performed in
triplicate.
Western blot analysis
The protein levels were established by Western blot
analysis as described previously (29). Total proteins were prepared from each
culture condition with a lysis buffer containing protease
inhibitors, and the lysed solution was centrifuged at 13,000 × g
for 20 min at 4°C. The protein content of the lysates was
determined using the Bradford protein assay. Equivalent amounts of
protein (20 µg) were separated were subjected to 12% SDS-PAGE and
then electrotransferred onto a polyvinylidene fluoride membrane
(EMD Millipore, Billerica, MA, USA). The membranes were blocked in
PBS containing 5% w/v skimmed dry milk at room temperature for 1 h,
and then incubated at 4°C overnight with the following antibodies:
Acetyl-histone H3, acetyl-histone H4, p-Akt, p-mTOR and p-p70S6K at
the recommended dilution (1:500). Additionally, the membranes were
incubated with HRP-conjugated goat anti-mouse (1:5,000) or goat
anti-rabbit secondary antibodies (1:5,000) at room temperature for
1 h. Finally, the membranes were exposed to X-ray film following
use of enhanced chemiluminescence reagents (Cell Signaling
Technology). Protein levels were quantified relative to β-actin as
a loading control by using Image-Pro Plus v.6.0 software (IPP;
Media Cybernetics, Bethesda, MD, USA), and this protocol was
repeated three times.
Analysis of the combined effects of
the drugs
CompuSyn software (version 2.0; ComboSyn, Inc.,
Paramus, NJ, USA) was used to evaluate the synergistic effects of
the combination of PHI and LY294002 using to the median-effect
method (30). An MTT assay was
performed to determine the fraction of cells affected (Fa). The
combination index (CI) was calculated using CompuSyn. In this
analysis, the combined effect was reported as synergistic,
antagonistic or additive when the CI value was <1, >1 and 1,
respectively.
Statistical analysis
Data were analyzed using SPSS statistical software
(version 13.0; SPSS, Inc., Chicago, IL, USA). Results are presented
as the mean ± standard deviation from multiple independent
experiments using a homogeneity test for variance and test of
normality. Results were evaluated by one-way analysis of variance
between groups. Multiple comparison between the groups was
performed using Student Newman-Keuls method. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of PHI and LY294002 on HL-60
cell viability
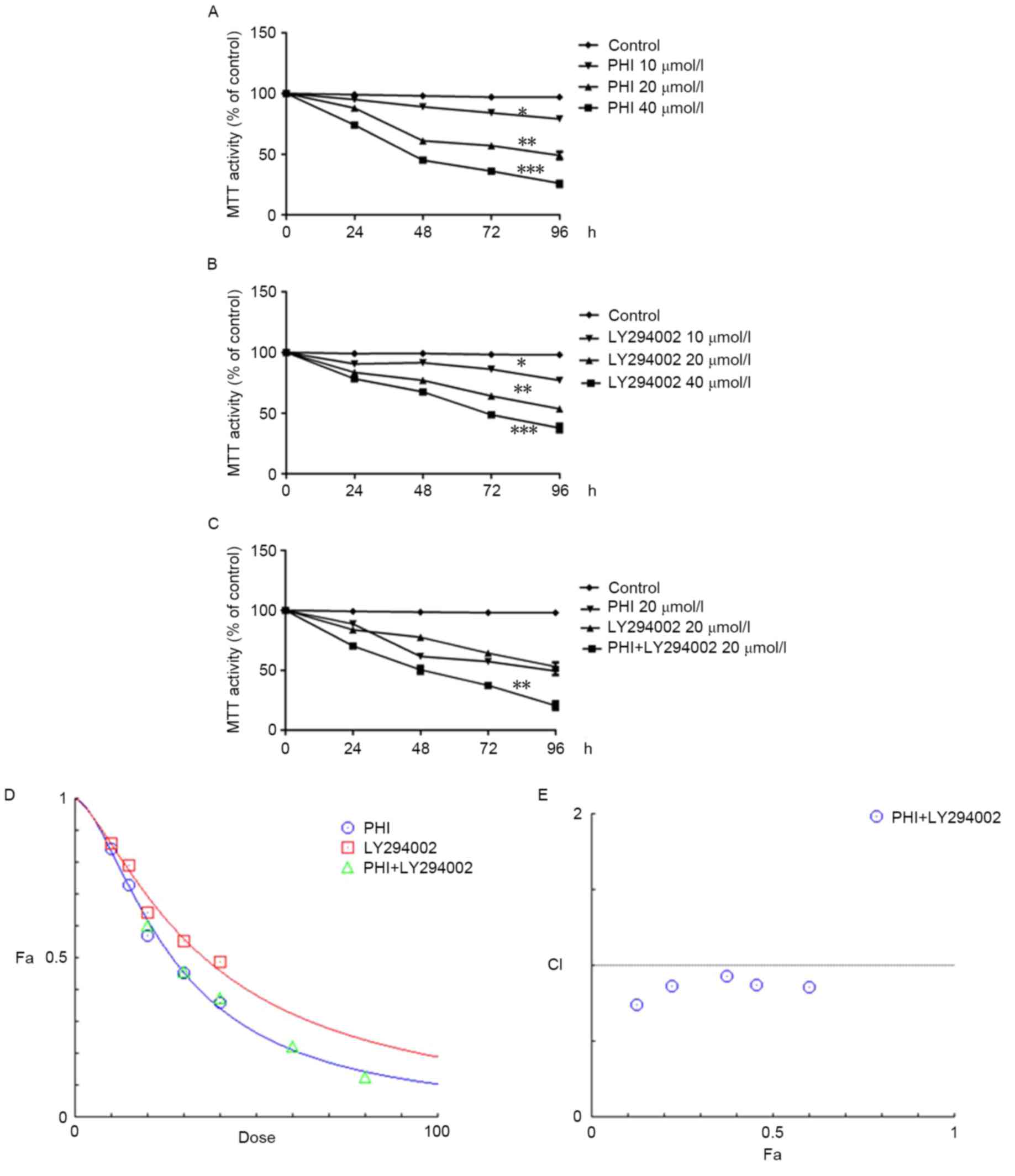
PHI and LY294002 were identified to inhibit
viability in HL-60 cells in a dose- and time-dependent manner. For
instance, the viability of HL-60 cells was 97.1±2.1, 84.3±2.5,
57.3±2.1 and 36.2±2.4% when treated with PHI at 0, 10, 20 and 40
µmol/l, respectively, for 72 h (Fig.
1A); the half-maximal inhibitory concentration
(IC50) was 26.19 µmol/l. Similarly, the viability of
HL-60 cells was 98.3±1.1, 86.2±2, 64.2±2.3 and 48.7±2.0% when
treated with LY294002 at 0, 10, 20 and 40 µmol/l, respectively, for
72 h (Fig. 1B); the IC50
was 36.44 µmol/l. The viability of HL-60 cells was 37.4±2.7% when
treated with PHI and LY294002 in combination at 20 µmol/l each for
72 h. However, the viability was 57.3±2.5 and 64.2±2.3% when
treated with PHI or LY294002, respectively, at 20 µmol/l separately
for 72 h (Fig. 1C). The combination
of PHI and LY294002 synergistically inhibited the viability of
HL-60 cells. HL-60 cells were treated with 10, 15, 20, 30 and 40
µmol/l PHI and 10, 15, 20, 30 and 40 µmol/l LY294002 separately or
in combination for 72 h (Fig. 1D).
The CI values were 0.85635, 0.87545, 0.93326, 0.86816 and 0.73989
when treated with a combination of PHI and LY294002 at 10, 15, 20,
30 and 40 µmol/l (Fig. 1E). The
combination of PHI and LY294002 at all concentrations exerted
synergistic inhibitory effects on HL-60 cell viability.
Effects of PHI and LY294002 on
apoptosis
Using flow cytometry, it was identified that the
number of cells undergoing apoptosis was increased in a
dose-dependent manner following exposure to PHI and/or LY294002 for
72 h. Exposure of HL-60 cells to PHI at 0, 10, 20 and 40 µmol/l for
72 h resulted in 1.28±0.43, 8.43±1.64, 15.40±2.48 and 26.4±3.22%
apoptosis, respectively. Exposure of HL-60 cells to LY294002 at 0,
10, 20 and 40 µmol/l for 72 h resulted in 1.28±0.43, 5.43±1.34,
12.4±1.52 and 18.80±2.52% apoptosis, respectively. PHI and LY294002
in combination at 20 µmol/l each led to 52.7±4.51% apoptosis
compared with 15.4±3.48% apoptosis for PHI alone and 12.4±1.52%
apoptosis for LY294002 alone, each at 20 µmol/l (Fig. 2).
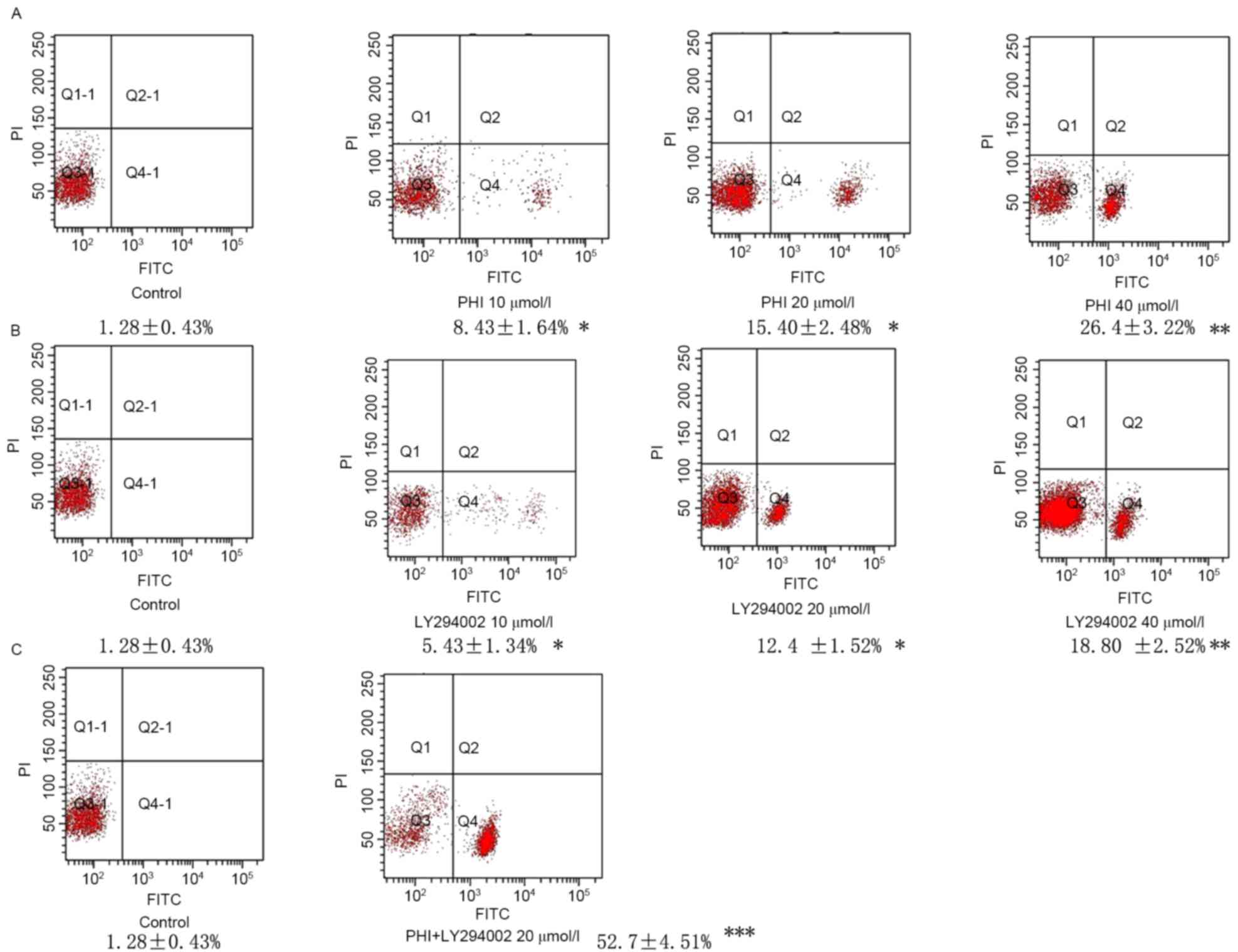 | Figure 2.PHI and LY294002 induce apoptosis in
HL-60 cells. Fluorescence signals from annexin V-FITC and PI are
reported on the x-axis and y-axis, respectively. Four
quadrants represent viable (lower left), necrotic (upper right),
early apoptotic (lower right) and late apoptotic (upper right)
cells. The rate of apoptosis of the cells is presented in the
figure. (A) Apoptosis was increased gradually following treatment
with PHI at 10, 20 and 40 µmol/l for 72 h in HL-60 cells vs.
untreated control. (B) Apoptosis was increased gradually following
treatment with LY294002 at 10, 20 and 40 µmol/l for 72 h in HL-60
cells vs. untreated control. (C) PHI and LY294002 in combination
significantly increased apoptosis in HL-60 cells vs. PHI or
LY294002 alone. *P<0.05, **P<0.01, ***P<0.001; FITC,
fluorescein isothiocyanate; PI, propidium iodide; Q, quadrant. |
LY294002 enhances the effect of PHI on
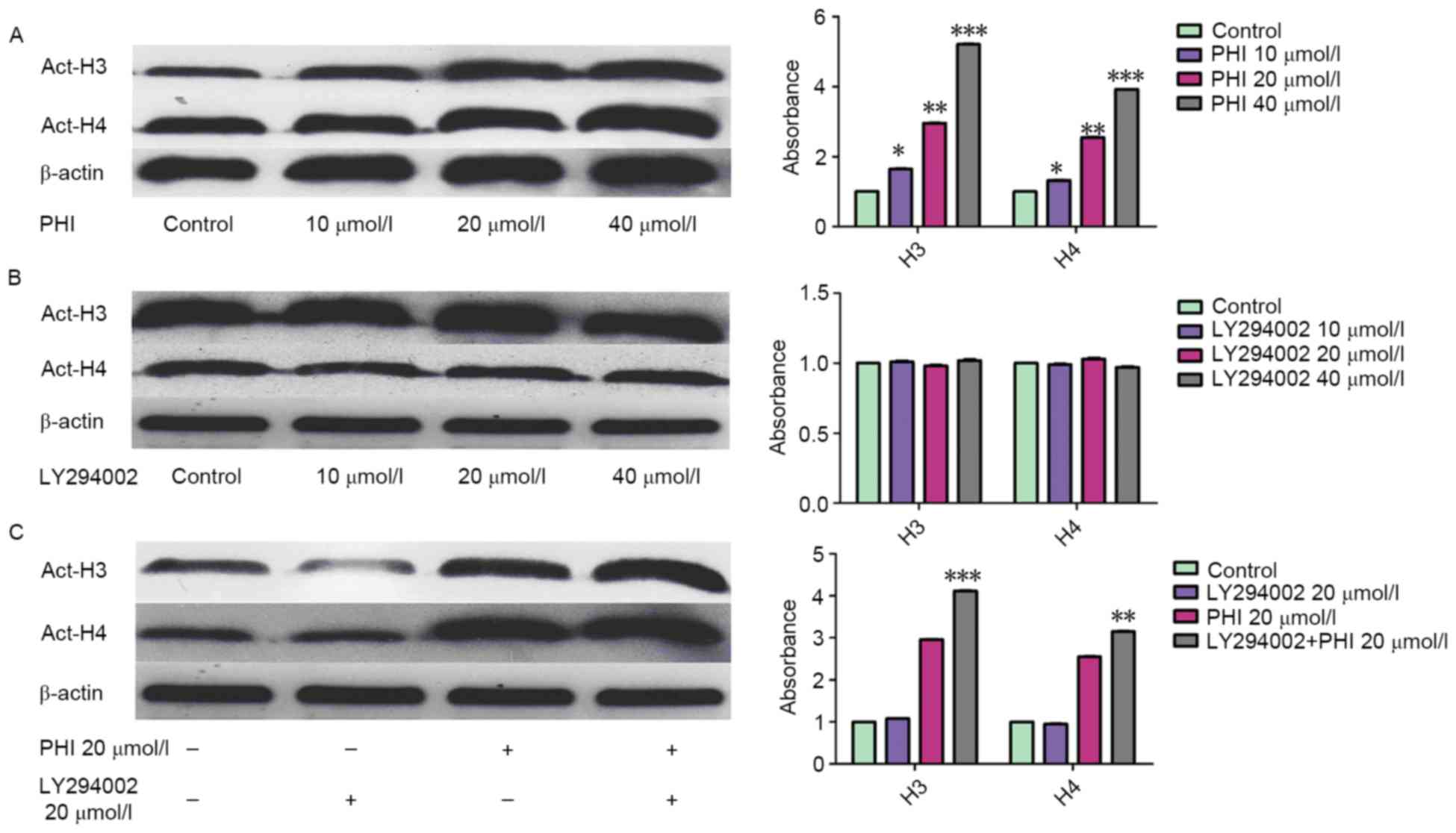
acetylation of histone H3 and H4 in HL-60 cells
Treatment with PHI led to an accumulation of
acetylated histone H3 and H4. However, LY294002 had no effect on
it. The acetylation of histone H3 and histone H4 was increased
markedly in dose- and time-dependent manner following exposure of
HL-60 cells to PHI (Fig. 3A).
Acetylated histone H3 was increased 1.65±0.08-, 2.96±0.14- and
5.21±0.24-fold by PHI at 10, 20 and 40 µmol/l, respectively, for 72
h compared with the control. Acetylated histone H4 was increased
1.32±0.06-, 2.55±0.12- and 3.92±0.18-fold by PHI at 10, 20 and 40
µmol/l, respectively, compared with the control. LY294002 was not
able to alter the level of acetylation of histone H3 and histone
H4. Acetylated histone H3 was increased 1.01±0.04-, 0.98±0.03- and
1.02±0.05-fold by LY249002 at 10, 20 and 40 µmol/l, respectively,
for 72 h compared with the control. Acetylated histone H4 was
increased 0.99±0.05-, 1.03±0.05- and 0.97±0.04-fold by LY249002 at
10, 20 and 40 µmol/l, respectively, for 72 h compared with the
control (P>0.05; Fig. 3B).
However, with in combination, LY294002 increased the effect that
PHI exerted on histone H3 and histone H4 acetylation. Acetylated
histone H3 and H4 were increased 2.96±0.14- and 2.55±0.12-fold by
PHI alone, 3.11±0.16- and 2.83±0.13-fold by PHI and LY294002 in
combination at 20 µmol/l each (Fig.
3C).
Synergistic effects of combined PHI
and LY294002 treatment on inhibiting the PI3K/Akt signaling pathway
in HL-60 cells
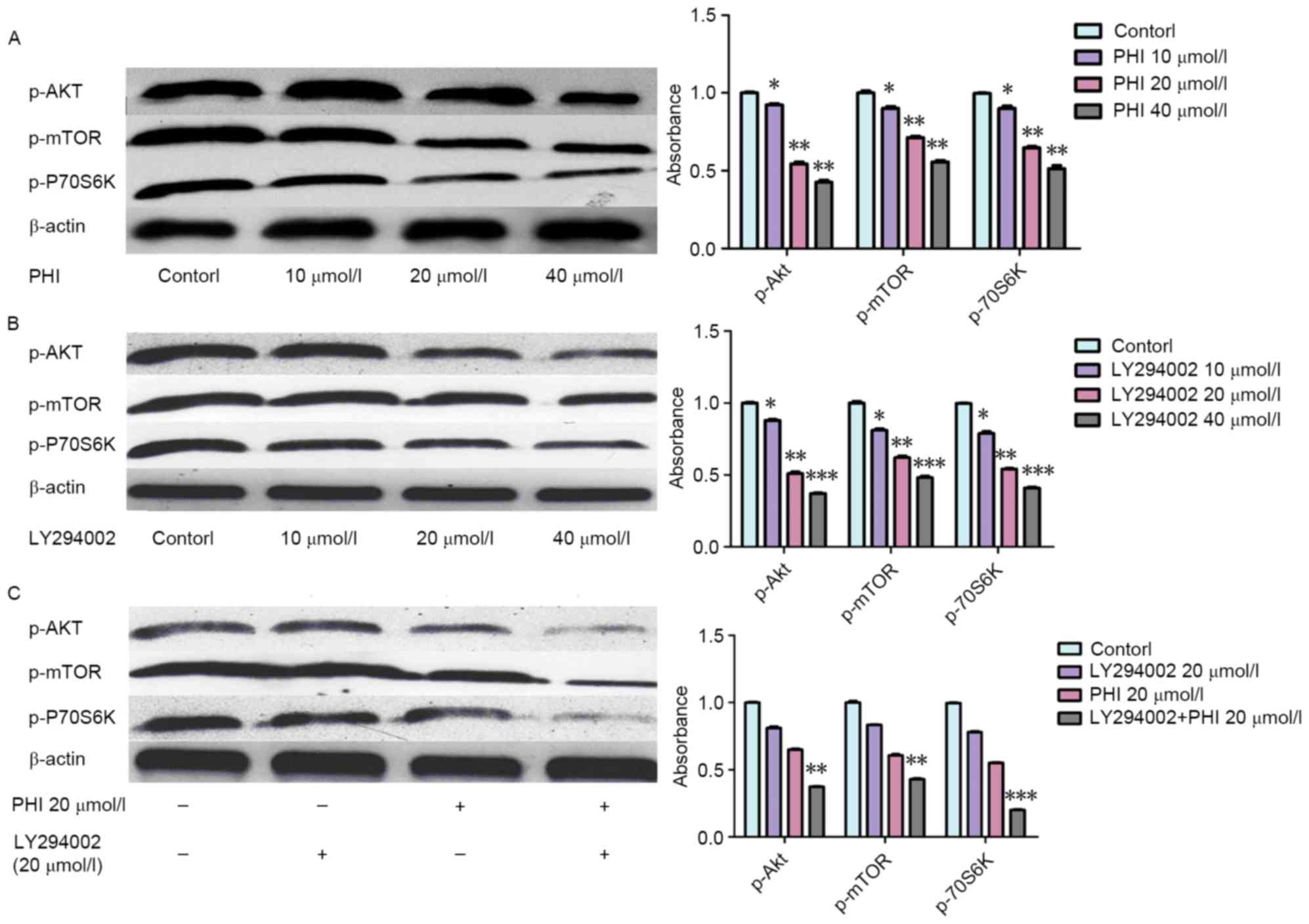
Levels of p-Akt, p-mTOR and p-p70S6K were decreased
following exposure to PHI or LY294002. When PHI was combined with
LY294002, the effect was more marked. p-Akt, p-mTOR and p-p70S6K
levels were decreased following exposure to PHI or LY294002. Levels
of p-Akt, p-mTOR and p-p70S6K were increased 0.92±0.06-, 0.90±0.05-
and 0.89±0.05-fold, respectively, by PHI at 10 µmol/l, 0.55±0.03-,
0.71±0.04- and 0.65±0.03-fold, respectively, by PHI at 20 µmol/l,
and 0.42±0.02-, 0.56±0.03- and 0.51±0.03-fold, respectively, by PHI
at 40 µmol/l for 72 h, compared with the control (Fig. 4A). Similarly, the levels of p-Akt,
p-mTOR and p-p70S6K were increased 0.87±0.03-, 0.81±0.04- and
0.79±0.04-fold, respectively, by LY294002 at 10 µmol/l, 0.51±0.03-,
0.62±0.04- and 0.54±0.03-fold, respectively, by LY294002 at 20
µmol/l, and 0.37±0.02-, 0.48±0.03- and 0.41±0.02-fold,
respectively, by LY294002 at 40 µmol/l for 72 h, compared with the
control (Fig. 4B). The levels of
p-Akt, p-mTOR and p-p70S6K were increased 0.81±0.03-, 0.83±0.05-
and 0.78±0.06-fold, respectively, by LY294002, respectively, at 20
µmol/l for 72 h, and 0.65±0.03-, 0.61±0.04- and 0.55±0.03-fold,
respectively, by PHI at 20 µmol/l for 72 h compared with the
control. However, levels of p-Akt, p-mTOR and p-p70S6K were
increased 0.37±0.04-, 0.43±0.03- and 0.21±0.02-fold, respectively,
by PHI and LY294002 in combination at 20 µmol/l each for 72 h
compared with the control (Fig. 4C).
The combination of PHI and LY294002 enhanced the effect on the
protein levels of the PI3K/Akt signaling pathway.
Discussion
The results of the present study indicate that
LY294002 and PHI induced cell apoptosis, decreased cell viability
and dephosphorylated p-Akt, p-mTOR and p-p70S6K.
Treatment with PHI led to an upregulation of histone acetylation.
Our previous studies indicated that PHI induced cell apoptosis and
inhibited cell viability in various tumor cell lines, including
MOLT-4, PC3 and SMMC7721 cells (23,31,32). These
results of these studies provide evidence of the underlying
molecular mechanisms for the apoptotic effects of PHI, which
modulates histone acetylation, histone methylation and DNA
demethylation. The results are also consistent with the evidence
that PHI dephosphorylated proteins in the PI3K/Akt signaling
pathway, inactivated Akt and modulated histone acetylation in PC3
cells.
Activation of the PI3K/Akt signaling pathway results
in disturbance of cell proliferation and apoptosis, providing a
competitive proliferative advantage for tumor cells (4,33,34). It is clear that upregulation of the
PI3K/Akt axis may be one of the major factors undermining
successful antineoplastic treatment, thus leading to a poor
prognosis in many types of cancer (35). Therefore, the PI3K/Akt pathway is an
attractive target for the development of novel therapeutic
strategies in patients with various types of tumor.
LY294002, a classical PI3K inhibitor, has been used
in in vitro and in vivo studies on cancer cell lines
in which it induces apoptosis and increases sensitivity to
chemotherapeutic drugs (36–38). The results of the present study
indicated that LY294002 may induce apoptosis and inhibit viability
of HL-60 cells. It inhibits the PI3K/Akt signaling pathway by
dephosphorylating p-Akt, p-mTOR and p-p70S6K.
In the present study, it was confirmed for the first
time, to the best of our knowledge, that a combination of LY294002
and PHI exerts synergistic effects on inducing apoptosis and
inhibiting viability of HL-60 cells. The results indicated that PHI
or LY294002 resulted in an increase in the apoptotic rate and
inhibition of cell viability in a dose-dependent manner. PHI and
LY294002 in combination at 20 µmol/l each exhibited a synergistic
effect with a significantly increased apoptosis rate. Treatment
with PHI or LY294002 separately at 20 µmol/l for 72 h led to cell
viability of 57.3±2.5 and 64.2±2.3%, respectively, compared with
the control. PHI and LY294002 in combination at 20 µmol/l each
exhibited a synergistic effect with a significantly increased
inhibition of cell viability. Investigation into the underlying
molecular mechanism indicated that PHI induced an accumulation of
acetylated histone H3 and histone H4 in HL-60 cells; however,
LY294002 exhibited no effect on histone acetylation. However,
upregulation of acetylated histone H3 and histone H4 was increased
by PHI in combination with LY294002 compared with PHI alone. These
results indicated that LY294002 may enhance the effect of PHI on
histone acetylation. PHI and LY294002 each dephosphorylated p-Akt;
in combination, they resulted in a synergistic effect,
dephosphorylating p-Akt further.
Akt synergistically enhanced the activity of histone
acetyltransferases (HATs), inducing p300, and the increasing the
binding capacity with the HAT p300/cyclic adenosine
5′-monophosphate-response element-binding protein-binding protein
(CBP)-associated factor (39).
Rapamycin inhibition of the mTOR signaling pathway may lead to the
release of the oncogene Esa1 from the ribosomal protein gene
promoter and lead to histone H4 deacetylation, thereby affecting
gene expression (40). Resistance to
histone deacetylase inhibitors in non-small cell lung cancer is
mediated in part through the activation of nuclear factor-κβ
through the phosphatidylinositol 3-kinase/Akt-dependent pathway
(41). A number of studies (42–44) have
indicated that the dynamic changes of histone modification may
regulate mTOR signaling pathway protein kinase activity. However,
the underlying molecular mechanism remains unknown. A study by Gan
and Zhang (45) identified that the
activation of phosphatase and tensin homolog deleted on chromosome
10 (PTEN), and Akt phosphorylation was downregulated by
trichostatin A (TSA) treatment. This study demonstrated that
downregulation of Akt phosphorylation induced by TSA may be
mediated by PTEN small interfering RNA. It has been identified that
the phosphorylation of Akt at Ser1834 enhances the
transcriptional activity of p300 by increasing promoter recruitment
and histone acetylation (39).
Chromatin immunoprecipitation assays revealed that CBPs and nuclear
factor erythroid 2-related factor (Nrf2) recruitment to the
antioxidant-response element (ARE) and broad
complex-tramtrack-bric-a-brac and cap‘n’collar homology 1 release
were inhibited by LY294002, along with the partial inhibition of
Nrf2 nuclear accumulation (46).
Furthermore, acetylation of histone H3 at Lys9 and
Lys18, and deacetylation histone H3 at Lys14
were associated with PI3K-dependent ARE activation. Apoptosis
induced by doxorubicin, an inhibitor of the PTEN signaling pathway,
was enhanced by TSA. Furthermore, TSA promoted early growth
response protein 1 (Egr-1) expression, which is the major
transcription factor of PTEN, and this resulted in upregulation of
PTEN expression, which consequently potentiated apoptosis (47). HAT p300 was able to synergistically
activate PTEN transcription with Egr-1, implicating the role of
histone acetylation in the regulation of PTEN expression.
The results of the present study suggest that a
combination of PHI and LY294002 may be a novel treatment for acute
leukemia.
Acknowledgements
The present study was partly supported by a
grant-in-aid from the Foundation of Science and Technology of
Zhangzhou, Fujian, China (grant no. Z07014), the Foundation of
Science and Technology of Fujian Medical University, Fujian, China
(grant no. FZS08018), the Science Research Foundation of the
Ministry of Health, and United Fujian Provincial Health, and
Education Project for Tackling Key Research, China (grant no.
WKJ2008-2-55).
Glossary
Abbreviations
Abbreviations:
|
PHI
|
phenylhexyl isothiocyanate
|
|
AML
|
acute myeloid leukemia
|
|
DMSO
|
dimethyl sulfoxide
|
|
p-Akt
|
phosphorylated protein kinase B
|
|
p-mTOR
|
phosphorylated mammalian target of
rapamycin
|
|
p-p70S6K
|
phosphorylated ribosomal protein S6
kinase
|
|
HAT
|
histone acetyltransferase
|
|
PTEN
|
phosphatase and tensin homolog deleted
on chromosome 10
|
|
TSA
|
trichostatin A; V-fluorescein
isothiocyanate FITC
|
|
CI
|
combination index
|
|
Fa
|
affected fraction
|
References
|
1
|
Löwenberg B, Downing JR and Burnett A:
Acute myeloid leukemia. N Engl J Med. 341:1051–1062. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wolff SN, Herzig RH, Fay JW, Phillips GL,
Lazarus HM, Flexner JM, Stein RS, Greer JP, Cooper B and Herzig GP:
High-dose cytarabine and daunorubicin as consolidation therapy for
acute myeloid leukemia in first remission: Long-term follow-up and
results. J Clin Oncol. 7:1260–1267. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wells RJ, Woods WG, Buckley JD, Odom LF,
Benjamin D, Bernstein I, Betcher D, Feig S, Kim T, Ruymann F, et
al: Treatment of newly diagnosed children and adolescents with
acute myeloid leukemia: A Childrens cancer group study. J Clin
Oncol. 12:2367–2377. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bellacosa A, Kumar CC, Di Cristofano A and
Testa JR: Activation of AKT kinases in cancer: Implications for
therapeutic targeting. Adv Cancer Res. 94:29–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kubota Y, Ohnishi H, Kitanaka A, Ishida T
and Tanaka T: Constitutive activation of PI3K is involved in the
spontaneous proliferation of primary acute myeloid leukemia cells:
Direct evidence of PI3K activation. Leukemia. 18:1438–1440. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Min YH, Eom JI, Cheong JW, Maeng HO, Kim
JY, Jeung HK, Lee ST, Lee MH, Hahn JS and Ko YW: Constitutive
phosphorylation of Akt/PKB protein in acute myeloid leukemia: Its
significance as a prognostic variable. Leukemia. 17:995–997. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu Q, Simpson SE, Scialla TJ, Bagg A and
Carroll M: Survival of acute myeloid leukemia cells requires PI3
Kinase activation. Blood. 102:972–980. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao S, Konopleva M, Cabreira-Hansen M,
Xie Z, Hu W, Milella M, Estrov Z, Mills GB and Andreeff M:
Inhibition of phosphatidylinositol 3-kinase dephosphorylates BAD
and promotes apoptosis in myeloid leukemias. Leukemia. 18:267–275.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stauffer F, Holzer P and García-Echeverria
C: Blocking the PI3K/PKB pathway in tumor cells. Curr Med Chem
Anticancer Agents. 5:449–462. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brazil DP, Yang ZZ and Hemmings BA:
Advances in protein kinase B signalling: AKTion on multiple fronts.
Trends Biochem Sci. 29:233–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanada M, Feng J and Hemmings BA:
Structure, regulation and function of PKB/AKT-a major therapeutic
target. Biochim Biophys Acta. 1697:3–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edlind MP and Hsieh AC: PI3K-AKT-mTOR
signaling in prostate cancer progression and androgen deprivation
therapy resistance. Asian J Androl. 16:378–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang P, Liu N, Pang Q, Qu C, Wang B and
Guo H: PI3K/AKT signaling pathway in the regulation of non-small
cell lung cancer radiosensitivity after hypofractionated radiation
therapy. Int J Radia Oncol Biol Physics. 84:(Suppl). S6702012.
View Article : Google Scholar
|
|
14
|
Roche S, Koegl M and Courtneidge SA: The
phosphatidylinositol 3-kinase alpha is required for DNA synthesis
induced by some, but not all, growth factors. Proc Natl Acad Sci
USA. 91:9185–9189. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shivakrupa R, Bernstein A, Watring N and
Linnekin D: Phosphatidylinositol 3′-kinase is required for growth
of mast cells expressing the kit catalytic domain mutant. Cancer
Res. 63:4412–4419. 2003.PubMed/NCBI
|
|
16
|
Bondar VM, Sweeney-Gotsch B, Andreeff M,
Mills GB and McConkey DJ: Inhibition of the phosphatidylinositol
3′-kinase-AKT pathway induces apoptosis in pancreatic carcinoma
cells in vitro and in vivo. Mol Cancer Ther. 1:989–997.
2002.PubMed/NCBI
|
|
17
|
Hu H, Jiang C, Li G and Lü J: PKB/AKT and
ERK regulation of caspase-mediated apoptosis by methylseleninic
acid in LNCaP prostate cancer cells. Carcinogenesis. 26:1374–1381.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schultz RM, Merriman RL, Andis SL,
Bonjouklian R, Grindey GB, Rutherford PG, Gallegos A, Massey K and
Powis G: In vitro and in vivo antitumor activity of the
phosphatidylinositol-3-kinase inhibitor, wortmannin. Anticancer
Res. 15:1135–1139. 1995.PubMed/NCBI
|
|
19
|
Hu L, Zaloudek C, Mills GB, Gray J and
Jaffe RB: In vivo and in vitro ovarian carcinoma growth inhibition
by a phosphatidylinositol 3-kinase inhibitor (LY294002). Clin
Cancer Res. 6:880–886. 2000.PubMed/NCBI
|
|
20
|
Semba S, Itoh N, Ito M, Harada M and
Yamakawa M: The in vitro and in vivo effects of
2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific
inhibitor of phosphatidylinositol 3′-kinase, in human colon cancer
cells. Clin Cancer Res. 8:1957–1963. 2002.PubMed/NCBI
|
|
21
|
Minarini A, Milelli A, Fimognari C, Simoni
E, Turrini E and Tumiatti V: Exploring the effects of
isothiocyanates on chemotherapeutic drugs. Expert Opin Drug Metab
Toxicol. 10:25–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cavell BE, Syed Alwi SS, Donlevy A and
Packham G: Anti-angiogenic effects of dietary isothiocyanates:
Mechanisms of action and implications for human health. Biochem
Pharmacol. 81:327–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma X, Fang Y, Beklemisheva A, Dai W, Feng
J, Ahmed T, Liu D and Chiao JW: Phenylhexyl isothiocyanate inhibits
histone deacetylases and remodels chromatins to induce growth
arrest in human leukemia cells. Int J Oncol. 28:1287–1293.
2006.PubMed/NCBI
|
|
24
|
Xiao L, Huang Y, Zhen R, Chiao JW, Liu D
and Ma X: Deficient histone acetylation in acute leukemia and the
correction by an isothiocyanate. Acta Haematol. 123:71–76. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang YQ, Ma XD, Zhen RJ, Chiao JW and Liu
DL: Experiment study of PHI on histone methylation and acetylation
in Molt-4 cells. Zhonghua Xue Ye Xue Za Zhi. 28:612–615. 2007.(In
Chinese). PubMed/NCBI
|
|
26
|
Jiang S, Ma X, Huang Y, Xu Y, Zheng R and
Chiao JW: Reactivating aberrantly hypermethylated p15 gene in
leukemic T cells by a phenylhexyl isothiocyanate mediated
inter-active mechanism on DNA and chromatin. J Hematol Oncol.
3:482010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zou Y, Ma X, Huang Y, Hong L and Chiao JW:
Effect of phenylhexyl isothiocyanate on aberrant histone H3
methylation in primary human acute leukemia. J Hematol Oncol.
5:362012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiarini F, Del Sole M, Mongiorgi S,
Gaboardi GC, Cappellini A, Mantovani I, Follo MY, McCubrey JA and
Martelli AM: The novel Akt inhibitor, perifosine, induces
caspase-dependent apoptosis and downregulates P-glycoprotein
expression in multidrug-resistant human T-acute leukemia cells by a
JNK-dependent mechanism. Leukemia. 22:1106–1116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Liu D, Ahmed T, Chung FL, Conaway
C and Chiao JW: Targeting cell cycle machinery as a molecular
mechanism of sulforaphane in prostate cancer prevention. Int J
Oncol. 24:187–192. 2004.PubMed/NCBI
|
|
30
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beklemisheva AA, Fang Y, Feng J, Ma X, Dai
W and Chiao JW: Epigenetic mechanism of growth inhibition induced
by phenylhexyl isothiocyanate in prostate cancer cells. Anticancer
Res. 26:1225–1230. 2006.PubMed/NCBI
|
|
32
|
Lai YD, Ma XD, Huang YQ, Xu XN, Wang XZ,
Chiao DJ and Liu D: Modulation of histone acetylation and induction
of apoptosis in SMMC-7721 cells by phenylhexyl isothiocyanate.
Zhonghua Zhong Liu Za Zhi. 32:804–807. 2010.(In Chinese).
PubMed/NCBI
|
|
33
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhuang Z, Ma X, Huang Y, Zheng Z, Zheng Y
and Jiang S: Study on histone acetylation modulation and Akt
signaling pathway inhibition by phenyhexyle isothiocyanate in
prostate cancer PC3 cell line. Chin J Urol. 31:707–709. 2010.
|
|
35
|
Kim D, Dan HC, Park S, Yang L, Liu Q,
Kaneko S, Ning J, He L, Yang H, Sun M, et al: AKT/PKB signaling
mechanisms in cancer and chemoresistance. Front Biosci. 10:975–987.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
West KA, Castillo SS and Dennis PA:
Activation of the PI3K/Akt pathway and chemotherapeutic resistance.
Drug Resist Updat. 5:234–248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martelli AM, Tabellini G, Bortul R,
Tazzari PL, Cappellini A, Billi AM and Cocco L: Involvement of the
phosphoinositide 3-kinase/Akt signaling pathway in the resistance
to therapeutic treatments of human leukemias. Histol Histopathol.
20:239–252. 2005.PubMed/NCBI
|
|
38
|
Luo J, Manning BD and Cantley LC:
Targeting the PI3K-Akt pathway in human cancer: Rationale and
promise. Cancer Cell. 4:257–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang WC and Chen CC: Akt phosphorylation
of p300 at Ser-1834 is essential for its histone acetyltransferase
and transcriptional activity. Mol Cell Biol. 25:6592–6602. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rohde JR and Cardenas ME: The tor pathway
regulates gene expression by linking nutrient sensing to histone
acetylation. Mol Cell Biol. 23:629–635. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Denlinger CE, Rundall BK and Jones DR:
Inhibition of phosphatidylinositol 3-kinase/Akt and histone
deacetylase activity induces apoptosis in non-small cell lung
cancer in vitro and in vivo. J Thorac Cardiovasc Surg.
130:1422–1429. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang X, Sun DF and Fang JY: Research
advances on the relationship of PI3-kinase/Akt/mTOR pathway and
epigenetic modification. Yi Chuan. 28:1585–1590. 2006.(In Chinese).
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wei FZ, Cao Z, Wang X, Wang H, Cai MY, Li
T, Hattori N, Wang D, Du Y, Song B, et al: Epigenetic regulation of
autophagy by the methyltransferase EZH2 through an MTOR-dependent
pathway. Autophagy. 11:2309–2322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nishioka C, Ikezoe T, Yang J, Koeffler HP
and Yokoyama A: Blockade of mTOR signaling potentiates the ability
of histone deacetylase inhibitor to induce growth arrest and
differentiation of acute myelogenous leukemia cells. Leukemia.
22:2159–2568. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gan YH and Zhang S: PTEN/AKT pathway
involved in histone deacetylases inhibitor induced cell growth
inhibition and apoptosis of oral squamous cell carcinoma cells.
Oral Oncol. 45:e150–e154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sakamoto K, Iwasaki K, Sugiyama H and
Tsuji Y: Role of the tumor suppressor PTEN in antioxidant
responsive element-mediated transcription and associated histone
modifications. Mol Biol Cell. 20:1606–1617. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pan L, Lu J, Wang X, Han L, Zhang Y, Han S
and Huang B: Histone deacetylase inhibitor trichostatin a
potentiates doxorubicin-induced apoptosis by up-regulating PTEN
expression. Cancer. 109:1676–1688. 2007. View Article : Google Scholar : PubMed/NCBI
|