Background
The repurposing of drugs is becoming increasingly
attractive as it avoids the expense and time required to develop
novel therapeutics (1–3). Itraconazole is a Food and Drug
Administration-approved agent that has passed toxicity testing, has
recognised anti-neoplastic properties and has already been assessed
in cancer therapy (4,5).
The original function of itraconazole was as a
broad-spectrum anti-fungal agent that inhibits lanosterol
14-α-demethylase (14LDM), an enzyme that produces ergosterol in
fungi and cholesterol in mammals (4–8). It is
used to treat fungal infections, including aspergillosis,
candidiasis and histoplasmosis, and for prophylaxis in
immunosuppressive disorders (9,10).
Itraconazole is a relatively safe drug, with rare side effects,
including neutropenia, liver failure and heart failure (9).
An emerging body of in vivo, in vitro and
clinical evidence has confirmed that itraconazole possesses
antineoplastic activity and has a synergistic action when combined
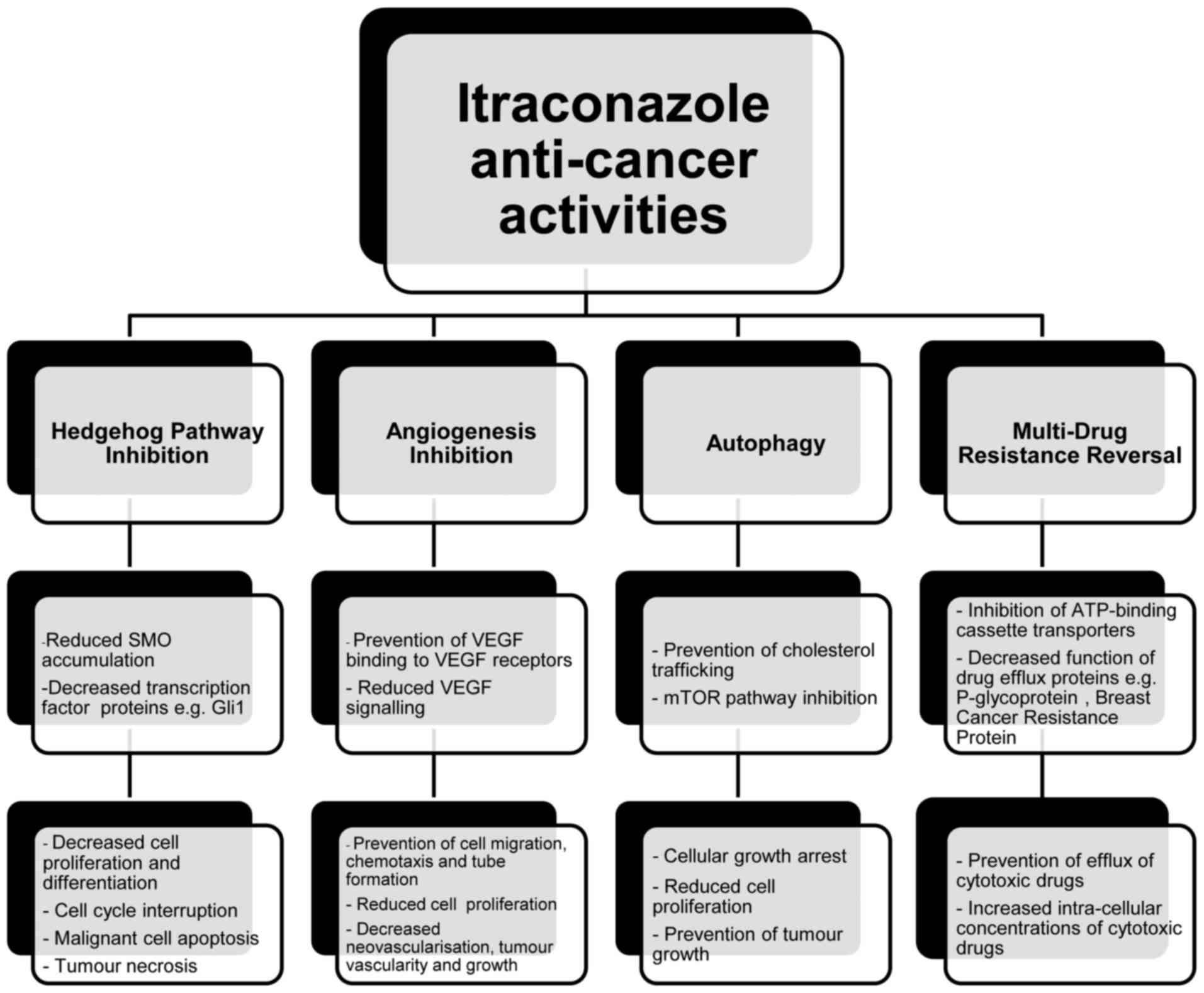
with other chemotherapeutic agents (4–33). It acts
via several underlying mechanisms to prevent tumour growth
(Fig. 1), including inhibition of the
Hedgehog pathway, prevention of angiogenesis, decreased endothelial
cell proliferation, cell cycle arrest, reversal of drug resistance
and induction of auto-phagocytosis (9–11).
Itraconazole's ability to prevent angiogenesis appears to be
associated with its anti-fungal properties, yet all other
mechanisms are not associated with the inhibition of 14LDM
(4–8,12).
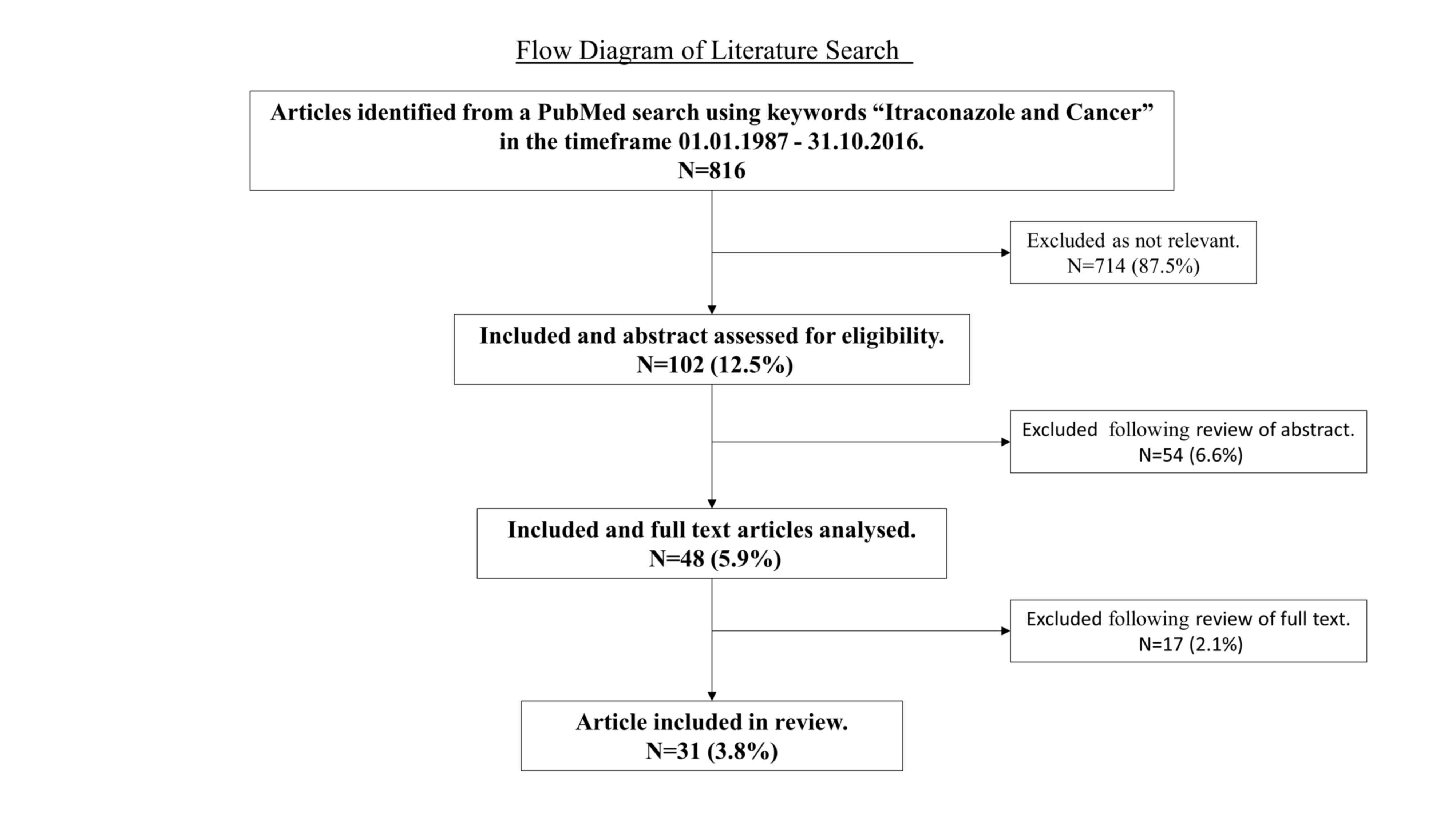
This paper reviews the currently available
literature regarding the use of itraconazole in a variety of
malignancies. A literature search was performed using PubMed with
the keywords ‘Itraconazole and Cancer’ from January 1987 to October
2016. Those articles with titles relevant to our review topic were
assessed for eligibility; abstracts that either described the
clinical use of itraconazole as a cancer treatment in patients or
illustrated evidence of itraconazole's antineoplastic activity from
in vivo or in vitro studies were included. These
selected articles were obtained and analysed in full, with 31
included in our review. Fig. 2
demonstrates the articles initially identified and those included
for the review of the literature.
Itraconazole and the Hedgehog pathway
The Hedgehog pathway controls necessary
developmental and embryogenic processes that are involved in tissue
patterning and morphogenesis (4,11,13). While essentially quiescent in adult
tissues, the Hedgehog pathway is involved in the maintenance of
certain epithelial progenitor cell populations and is activated
during tissue regeneration and wound healing (4,13). In the
absence of Sonic Hedgehog ligand (Shh), patched 1 (PTCH1) represses
the activity of smoothened (SMO), and the pathway is turned off.
Binding of Shh ligand to PTCH1 relieves its suppression of SMO,
resulting in protein stabilisation and nuclear translocation of the
GLI transcription factors (34–36). The
GLI proteins, of which there are three (GLI1-3), activate a
plethora of downstream targets that effect cell growth, survival
and differentiation (37). In the
majority of situations, expression of GLI1 mRNA is used as a
surrogate marker for Hedgehog pathway activity (4). Fig. 3
depicts the Hedgehog signalling pathway, when activated and
supressed.
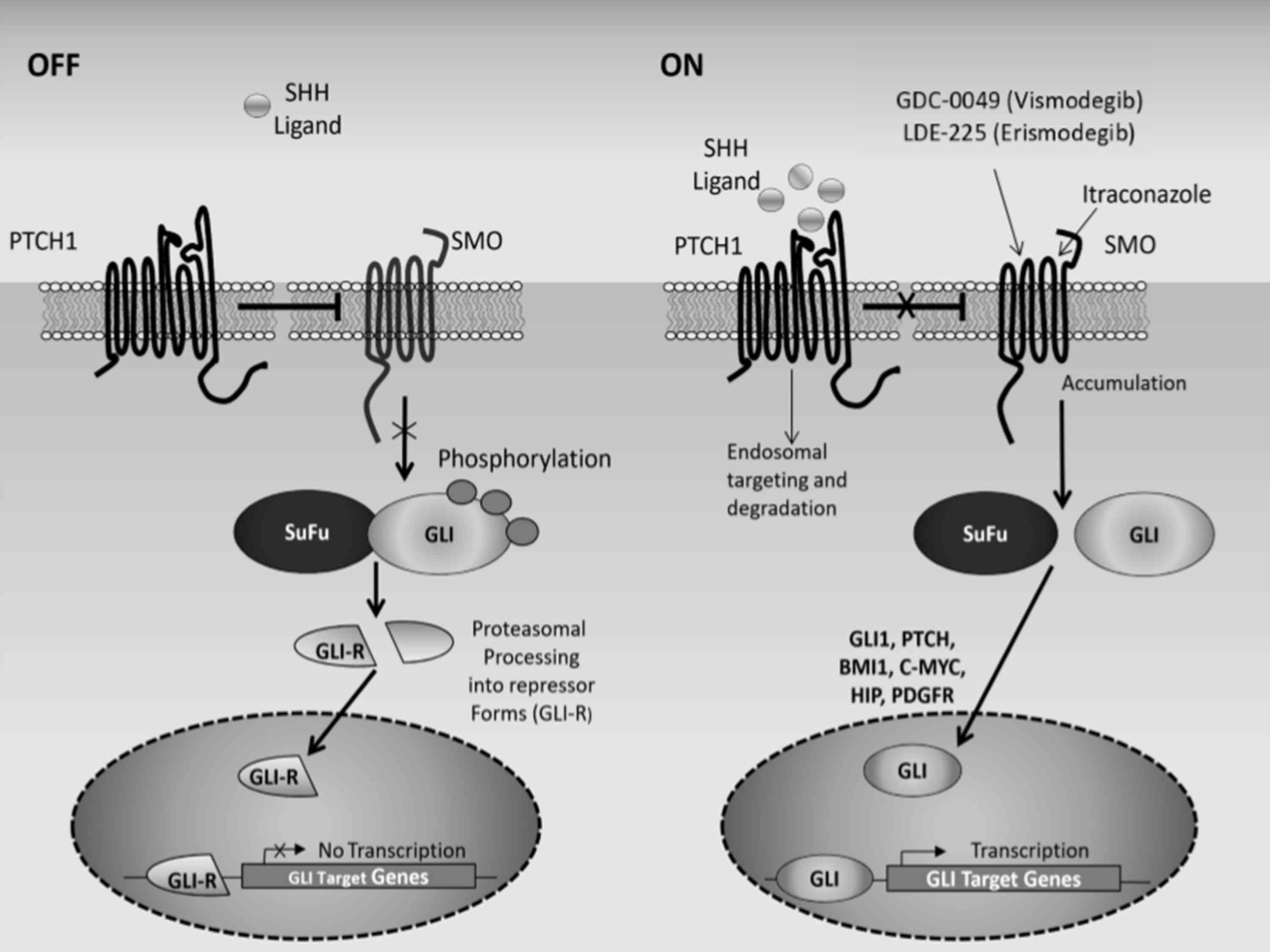 | Figure 3.Schematic representation of the
Hedgehog signaling pathway. In the absence of Shh, PTCH1 suppresses
the activity of SMO. SuFu induces proteasomal degradation of the
GLI (glioma-associated oncogene) transcription factors. The cleaved
GLI factors, GLI-R (GLI repressor form), translocate to the nucleus
where they suppress the expression of Hedgehog target genes. In
this setting, the pathway is turned off. In the presence of Shh,
PTCH1 suppression of SMO is relieved, which modulates SuFu
activity. SuFu no longer associates with the GLI transcription
factors, which translocate to the nucleus, activating Hedgehog
target genes. These include pathway effectors (GLI1) and regulators
(PTCH1 and HIP), and proteins involved in cell proliferation
(PDGRF, cyclin D2, BMI1 (B-cell-specific Moloney murine leukemia
virus integration site 1) and c-MYC). Hedgehog pathway inhibitors
referred to include GDC-0449 (vismodegib), LDE225 (erismodegib) and
itraconazole, which directly inhibit SMO. Sonic hedgehog ligand,
Shh; patched 1, PTCH1; SMO, smoothened; SuFu, suppressor of fused;
HIP, Hedgehog interacting protein; PDGFR, platelet-derived growth
factor receptor. |
Abnormalities in Hedgehog signalling can result in
congenital malformations, and inappropriate activation of the
pathway may lead to the development of cancer (4,11). In
addition to the overexpression of Shh, Hedgehog pathway activation
may follow loss-of-function of PTCH1, gain-of-function mutations in
SMO and epigenetic modulation of key pathway components, such as
suppressor of fused, which is a negative regulator of the Hedgehog
pathway (11).
Thus far, drugs designed to treat Hedgehog-driven
malignancies have been developed to target SMO, although other
compounds have been identified to inhibit or modulate the activity
of the GLI proteins (38). Drugs
demonstrated to block the Hedgehog pathway include vismodegib
(GDC-0449), sonidegib (LDE-225) and cyclopamine (39). These drugs are known to act by binding
to and antagonising the function of SMO (4,11,14). Itraconazole similarly inhibits the
Hedgehog pathway by acting directly on SMO but, unlike other drugs,
it binds to a different site on the SMO protein (4,11). This is
the likely explanation for its synergistic activity with other
anti-neoplastic agents, such as vismodegib. Itraconazole can
therefore be used in combination with or, in cases of drug
resistance, as an alternative to other Hedgehog pathway inhibitors
(4). When other signalling pathways
facilitate neoplastic growth, tumours may survive even in the
presence of Hedgehog pathway inhibition, and therefore, a
combination of drugs acting on multiple pathways may be required
(14).
Evidence from pre-clinical studies has confirmed the
capacity of itraconazole to inhibit the Hedgehog pathway (4,12,13,15,16).
Treatment of allografted medulloblastomas in a murine model
resulted in a reduction in GLI1 mRNA and growth suppression
(4). When combined with cyclopamine
the effect was greater than with itraconazole treatment alone
(4,11). Shh and GLI1 expression was revealed in
low grade, stage 1A human endometrial cancer tissue samples
(15). In vitro cell
proliferation was then significantly inhibited by itraconazole,
even when cells were treated with an oral anti-fungal dose
(15). In cultured pleural
mesothelioma cells, activated Hedgehog signalling was inhibited
with itraconazole and arsenic trioxide, an anti-leukaemia drug,
which is known to target the GLI1 protein (13). The two drugs reduced the expression of
GLI1, decreased cell viability, perturbed cell cycle progression
and induced apoptosis (13,16). The Hedgehog pathway is aberrantly
activated in basal cell carcinoma (BCC), due primarily to the
presence of a mutated or inherited defective PTCH1 gene (6). Itraconazole administration reduced the
growth of BCC in mouse models, decreased the expression of GLI1
mRNA and induced tumour necrosis (4).
Itraconazole and angiogenesis
The growth of solid tumours is
angiogenesis-dependent (8).
Anti-angiogenic agents are used in cancer therapy, and itraconazole
has been shown to act on numerous pathways necessary for
angiogenesis (8). It inhibits
vascular endothelial growth factor (VEGF) signalling by preventing
VEGF binding to the VEGF receptor 2, thereby reducing endothelial
cell proliferation (5,17). Itraconazole can also prevent cell
migration, chemotaxis and tube formation, all of which are
essential for neovascularisation and angiogenesis (8).
The suppression of tumour growth with itraconazole
treatment has been demonstrated in non-small cell lung cancer
xenografts (8,18). In one such study, growth was reduced
by 72 and 79% (P<0.001) in two primary xenograft models
(8). Greater tumour growth
suppression was observed with combination therapy involving
itraconazole and cisplatin (8). As
well as inhibiting cell proliferation in response to angiogenesis
factors (VEGF and fibroblast growth factor), the migration and
formation of tube networks were also prevented. These are necessary
for capillary bed production; therefore, the area of tumour
vascularity significantly decreased (8,18). In
addition, itraconazole has been demonstrated to reduce pleural
effusion volumes, the number of pleural tumour foci and VEGF-C
levels in xenograft models with Lewis lung carcinoma (7).
Itraconazole and drug resistance
Drug resistance is a major obstacle in the desire to
cure malignancies (33). Combination
therapy is believed to reduce the development of drug resistance
when compared with treatment with one drug alone (40,41). It is
therefore possible that itraconazole can prevent the resistance
associated with monotherapy when combined with other medications.
Furthermore, all opportunities in reversing resistance should be
explored, particularly with therapies that have minimal sequela,
such as itraconazole. It has been revealed in vitro to
reverse multi-drug resistance in numerous types of malignancies
(9). In ovarian and breast cancer,
drug resistance to chemotherapy is associated with permeability
glycoprotein (also termed p-glycoprotein), multidrug resistance
protein 1 and ATP-binding cassette sub-family B member 1 (14,18). This
is an efflux pump present on cell membranes that reduces
intracellular drug concentrations, conferring cellular resistance
to genotoxic therapies (42). Ovarian
clear cell carcinoma, one of the more aggressive disease subtypes,
has been identified to have increased expression of these efflux
pumps, thereby preventing the accumulation of cytotoxic agents
within the malignant cells (43).
In vitro studies confirm that itraconazole inhibits the
efflux pump, thus reversing resistance (10,14,19,20).
This has also been observed in resistant leukaemia and human
embryonic kidney cells (21,22).
Clinical papers
The use of itraconazole as a therapy has received
extensive attention, primarily in phase I/II studies. Details of
recent clinical studies are presented in Table I.
 | Table I.Clinical papers included in the
literature review. |
Table I.
Clinical papers included in the
literature review.
| Authors | Study type | Cancer type | No. of
patients | Itraconazole dose
and treatment schedule | Response rates | Progression-free
survival | Overall
survival | Reported
pathway | Adverse
outcomes | (Refs.) |
|---|
| Kim DJ, et
al 2014 | Phase II | Basal cell
carcinoma | 29: 19 treated, 10
controls | High-dose: 200 mg
twice daily for 4 weeks (n=15) Low-dose: 100 mg twice daily for
average of 2.3 months (n=4) | Cell proliferation
reduced by 45% (P=0.04), GLI1 mRNA reduced by 65% (P=0.028) in
high-dose. Tumour area reduced by 24% (95% CI, 18.2–30.0%) with
both doses. | – | – | Hedgehog pathway
inhibition | Fatigue (grade 2),
heart failure (grade 4) | (6) |
| Lockhart NR, et
al 2016 | Case report | Stage III
un-resectable pancreatic adeno-carcinoma | 1 | 200 mg daily for 9
months | Tumour size.
reduced and resected | No recurrence of
disease following surgery. | – | Hedgehog pathway
inhibition |
| (10) |
| Rudin CM, et
al 2013 | Phase II | Metastatic
non-squamous non-small cell lung cancer | 23 | 200 mg daily for 21
days combined with IV pemetrexed (n=15,) single agent IV pemetrexed
on day 1 (n=8) | Disease
stabilisation at 3 months: 67% with itraconazole, 29% without.
P=0.11 | 5.5 months with
itraconazole, 2.8 months without. P=0.089 | 32 months with
itraconazole, 8 months without P=0.012 | – | Nil | (18) |
| Tsubamoto H, et
al 2014 | Retrospective
analysis | Recurrent clear
cell ovarian carcinoma | 9 | 400 mg daily on
days. 2 to +2 with docetaxel and carboplatin-based chemotherapy on
day 1. Regime repeated every 2 weeks. | Response rate of
44% (95%, CI 12.77%). | 544 (median; 95%
CI, 82.544) days. | 1,047 (median; 95%
CI, 462.1,332) days. | – | Deranged liver
function (grade 1), anorexia (grade 2). | (19) |
| Tsubamoto H, et
al 2014 | Retrospective
analysis | Resistant ovarian
cancer | 55 (19 treated, 36
controls) | Treated group:
400.600 mg daily on days. 2 to +2 alongside 2nd line chemotherapy
(n=19). Controls: chemotherapy only (n=36). | Overall response
rate of 18% (95% CI, 8.28%). 32% in treated group, 11% in control
group. P=0.06 | 103 days (median)
in treated group, 53 days in controls. P=0.014. | 642 (median for
treated; 95% CI 238.1, 166) days, 139 (median in controls; 95%
CI, | – | – | (23) |
| Antonarakis ES
et al 2013 | Phase II | Chemotherapy naive,
metastatic castration-resistant prostate cancer | 46 (17 in low-dose
group, 29 in high-dose group) | Low-dose: 200
mg/day. High-dose: 600 mg/day, until disease progression or
toxicity. | Serum PSA response
in low-dose: 0% (0.19.5%). In high-dose: 14.3% (4.32.7%). Disease
progression in 15 (low-dose) and 22 (high-dose). | Low-dose: 11.9
weeks (median), 18.8% at 24 weeks. High-dose: 35.9 weeks,
61.6%. |
| Hedgehog pathway
inhibition | Grade 3 toxicities:
Fatigue, anorexia, rash, hyper-tension and hypo-kalaemia. | (24) |
| Suzman DL, et
al 2014 | Case report | Biochemically
recurrent non-metastatic prostate cancer | 1 | 300 mg twice daily
for 5 months. | PSA fell by <50%
after 3 months, testosterone levels unchanged, DHEA increased. | – | – | – | Increased ACTH,
hypoaldost-eronism, hyperbili-rubinaemia. | (25) |
| Ademuyiwa FO, et
al 2011 | Pilot trial | Metastatic breast
cancer | 13 | 200 mg daily for 28
day cycles (median 2 cycles) | Partial response in
1, stable disease in 3, progressive disease in 9 | 1.8 months | 19.3 months | Angio-genesis
inhibition | Increased ACTH,
hypoaldost-eronism, hyperbili-rubinaemia. | (26) |
| Tsubamoto H, et
al 2014 | Retrospective
analysis | Recurrent
triple-negative breast cancer | 13 | 400 mg daily on
days. 2 to +2, given with chemotherapy on day 1. Regime repeated
every 2 weeks. 5 patients also received bevacizumab. | 62% (95% CI,
33.88%): Complete response in 23%, partial in 38%. Progressive
disease in 15%. | 10.8 months
(median; 95% CI 7.6.15.3 months) | 20.4 months
(median; 95% CI 13.1.41.4 months) | – | Fatigue, insomnia,
nausea, vomiting, dyspnoea, haemorrhage, pain (grade 3.4) | (27) |
| Ally MS, et
al 2016 | Phase II | Metastatic basal
cell carcinoma | 5 | 400 mg daily on
days 6.28, intravenous arsenic trioxide on days 1.5. | GLI1 mRNA reduced
by 75% (P<0.001). Stable disease in 3 patients. | – | 4 survived with
disease and 1 died after mean follow-up of 12 months | Hedgehog pathway
inhibition | Fatigue (grade 2),
heart failure (grade 4) | (29) |
| Tsubamoto H, et
al 2015 | Retrospective
analysis | Metastatic
pancreatic cancer | 38 | 400 mg daily on
days. 2 to +2 in combination with chemotherapy (docetaxel,
gemcitabine and carboplatin) every 2 weeks for 3.11 cycles. | 37%, complete
response in 1 and partial response 13 patients. | – | 11.4 months
(median) | Hedgehog pathway,
angiogenesis and P-glyco-protein inhibition | – | (30) |
| Tsubamoto H, et
al 2015 | Retrospective
analysis | Metastatic biliary
tract cancer | 28 | 400 mg daily on
days. 2 to +2, every 2 weeks for 2.17 cycles. 26 received
docetaxel, gemcitabine and carboplatin. 2 received docetaxel and
irinotecan. | 57%, complete
response in 2 and partial response in 14. | – | 12 months
(median) | Hedgehog pathway
and P-glycoprotein inhibition. | Trans-aminitis,
leukopenia, infection, atrial flutter | (31) |
| Cooper SM, et
al 2003 | Case report | Mycosis
fungoides | 1 | 200 mg daily for 7
days | Complete response
on 4 occasions | – | – | – | Grade 4
neutropeniagrade 4 thrombo-cytopenia. | (32) |
| Vreugdenhil G,
et al 1993 | Double-blind
placebo-controlled trial | Acute
leukaemia | 65 Treated: 11 ALL,
17 AML-Control: 12 ALL, 25 AML | 100 mg twice
daily | Increased disease
remission with itraconazole in AML, no difference in ALL | Increased disease
free survival with itraconazole in ALL (P<0.06), no difference
in AML | – | Cytochrome P.450
and P-glyco-protein | Grade 3/4
neutropenia anaemia, thrombocytopenia. | (33) |
Ovarian cancer
At presentation, ovarian cancer is at an advanced
stage in 70–75% of patients, and has a 5-year survival rate of ~40%
(44,45). Although the initial response rates to
first line chemotherapy are high, resistance is common, as
reflected by poor survival (46).
Itraconazole has been utilised in refractory disease to try and
reverse such chemo-resistance (19,23). In a
retrospective review, 55 patients were treated either with
chemotherapy alone (regimes of pegylated liposomal doxorubicin,
gemcitabine, docetaxel, irinotecan or paclitaxel) or chemotherapy
(docetaxel based in 79%) combined with itraconazole. The
combination therapy was given biweekly to 19 female patients, with
400–600 mg itraconazole administered daily for 4 or 5 days. The
median progression-free survival time was significantly longer for
those receiving itraconazole (103 days, compared with 53 days in
those who did not receive itraconazole; P=0.014), as was the
overall survival time (642 days, compared with 139 days in those
who did not receive itraconazole; P=0.0006). The overall response
rate following treatment was 18%, with a greater proportion of the
itraconazole group exhibiting a response (32% in the itraconazole
group, 11% in the control group). The continued use of itraconazole
is the likely explanation for the improved survival rates (23).
In another study (19), 9 patients with recurrent clear cell
ovarian cancer had itraconazole added to their treatment regime
with the objective of improving chemotherapeutic efficacy.
Itraconazole 400 mg daily was administered over 4 days every 2
weeks. A response rate of 44% was achieved, with a higher median
overall survival time (1,047 days) compared with that previously
reported in other studies, which ranged between 7 and 10 months
(47,48).
As chemotherapy is typically discontinued following
resistance, few patients with refractory disease are eligible for
such studies, and small numbers of female patients included
(49). Another limitation is that
cytotoxic regimens differ between patients, doses are frequently
altered and patients are not randomised.
Prostate cancer
In advanced prostate cancer, although androgen
deprivation therapy can be successful, inevitably resistance will
develop (50). When this occurs,
therapeutic options are limited. A subsequent randomised phase II
trial explored the use of either 200 or 600 mg itraconazole daily
for the treatment of metastatic castration-resistant prostate
cancer in 46 patients (24). The
higher dose increased progression-free survival times and
prostate-specific antigen (PSA) progression-free survival rates. In
addition, skin biopsies exhibited a down-modulation of GLI1
(reflecting inhibition of the Hedgehog pathway) in the two
treatment arms, which was associated with a significantly longer
median PSA progression-free survival time (24).
A further case report describes the use of high dose
itraconazole (300 mg twice daily) to treat a biochemical recurrence
following radical prostatectomy in non-metastatic disease (25). After the patient declined castration
treatment, itraconazole was administered and the PSA level reduced
by >50% in 3 months. Although the PSA continued to decline
during an additional 2 months of treatment, levels began to rise
upon termination of the therapy (25). As such, itraconazole may be an
alternative therapy for those wishing to avoid castrating or
cytotoxic therapy, although additional trials are required to
confirm this (24,25).
Breast cancer
A pilot trial evaluated the pharmacokinetics of
itraconazole when administered to 13 patients with metastatic
breast cancer (26). As the plasma
levels of itraconazole increased, higher levels of
thrombospondin-1, which inhibits angiogenesis, were detected. In
addition, the levels of other angiogenic factors, basic fibroblast
growth factor and placenta-derived growth factor decreased, albeit
lacking a direct association between the fall in angiogenic factors
and itraconazole levels.
In another study, 13 patients with progressive
triple-negative breast cancer, despite extensive chemotherapy, were
administered itraconazole (27).
Patients commenced itraconazole treatment (400 mg daily for 4 days,
repeated every 2 weeks) alongside cytotoxic agents, with 5 patients
also receiving bevacizumab. Response rates were high (62%), with
23% complete responses. Overall survival rates were advantageous
compared with previous findings of itraconazole use. Earlier
studies of triple-negative disease failed to demonstrate such
improvements: A phase III trial using bevacizumab and a
retrospective analysis of platinum-based chemotherapy did not
reveal overall survival benefits, while a meta-analysis identified
only short-term improvements with platinum-based chemotherapy in
non-metastatic disease (51–53).
Lung cancer
Itraconazole has been analysed as a second line
treatment in metastatic non-squamous non-small cell lung cancer
(18). A phase II study on 23
patients randomised to either single agent pemetrexed or combined
pemetrexed and itraconazole (200 mg daily for 21 day cycles)
reported the anticipated response rates in the pemetrexed only arm,
with improved outcomes in those exposed to itraconazole (18). The proportion with disease
stabilisation at 3 months was higher, median progression-free
survival increased and overall survival was greater compared to
those treated with pemetrexed alone. Future trials will explore its
use as a first line treatment alongside other agents.
BCC
BCC, the most common form of skin cancer, has been a
focus for Hedgehog pathway inhibitors (6,28,29,54,55).
Studies using vismodegib, sonidegib and itraconazole to treat BCC
have all demonstrated efficacious results; however, resistance is
frequently problematic (6,28,29,55–58).
One phase II trial compared high dose itraconazole (200 mg twice
daily for 4 weeks) with a control group, demonstrating a reduction
in cell proliferation (Ki-67) and Hedgehog pathway activity (GLI1
mRNA levels) with itraconazole (6).
The tumour area decreased when treated with either the high dose or
with a lower dose over a longer time period (100 mg twice daily for
1–4 months). The findings were not replicated in those with prior
vismodegib exposure, questioning the value of itraconazole
following resistance to this drug (6).
Another review also determined that clinical
responses were limited following vismodegib resistance (29). A total of 5 patients with metastatic
BCC were treated with combined itraconazole (400 mg daily on days
6–28) and intravenous arsenic trioxide (on days 1–5). Despite a 75%
decrease in GLI1 mRNA levels, a reduction in tumour size was not
evident. While vismodegib and sonidegib appear to provide higher
response rates and greater Hedgehog pathway inhibition, it may be
beneficial to use itraconazole following resistance or as a
combined therapy. It remains unclear whether continuous high dose
itraconazole administered over a longer period could give similar
results to those observed with vismodegib and sonidegib (6,28,29).
Pancreatic cancer
In a previous study (30), 38 patients with progressive pancreatic
cancer received itraconazole (400 mg daily for 4 days) in
combination with chemotherapy (docetaxel, gemcitabine and
carboplatin) over 2 week cycles. A response rate of 37% was
achieved, with complete and partial responses in 1 and 13 patients,
respectively. In total, 35 patients who either had stable disease
or had a complete or partial response continued itraconazole
treatment with irinotecan-based chemotherapy. The response rate
increased to 47%, with a median overall survival time of 11.4
months. This was greater than the median overall survival time of 6
months found in an earlier analysis of clinical trials that
investigated second-line treatment in advanced pancreatic disease
(59). The advantageous results in
this study are possibly due to the administration of triple
chemotherapeutic agents.
A serendipitous case of pancreatic cancer treated by
itraconazole has previously been reported (10). Histoplasmosis infection was detected
in a patient with stage III locally advanced unresectable
pancreatic adenocarcinoma. Palliative chemotherapy was paused, a
9-month course of itraconazole 200 mg daily commenced and, upon
completion, the tumour was revealed to have decreased in size. It
was deemed resectable and following surgery the patient remained
disease free, with no evidence of recurrence. As chemotherapy had
been withheld, the reduction was thought to have been caused by
itraconazole and Hedgehog pathway inhibition.
Biliary tract cancer
Biliary tract cancer is a rare condition and has a
poor prognosis (60). Favourable
response rates and acceptable toxicity effects have been
demonstrated in a study of patients with refractory metastatic
biliary tract carcinoma treated with itraconazole (31). A total of 28 patients received
itraconazole (400 mg daily for 4 days) in addition to chemotherapy
regimens (docetaxel, gemcitabine and carboplatin in 26 patients,
docetaxel and irinotecan in 2 patients). A complete response was
observed in 2 patients, while 14 had a partial response. The
overall response rate was 57% and the median overall survival time
was 12 months. This compares to 7.2 months in a systematic review
of second-line treatment for advanced biliary tract carcinoma
(61). Despite the small number of
patients in this study, itraconazole appears to be a promising
therapeutic alternative after first-line treatment in recurrent
disease.
Mycosis fungoides
Another study on successful itraconazole treatment
is that of a patient with Mycosis fungoides (32), the most common type of cutaneous
T-cell lymphoma. The patient developed erythematous plaques on four
separate occasions, yet no cause was identified. Following no
improvement with miconazole or topical steroids, itraconazole 200
mg daily was administered for 7 days. The lesions completely
resolved and additional episodes again only responded following
itraconazole treatment. Eventually biopsy and histology results
supported a diagnosis of Mycosis fungoides. The mechanism of action
in this condition is unclear.
Acute leukaemia
As previously stated, itraconazole is used for
fungal infection prophylaxis in immunosuppressive conditions
(9). In patients with acute leukaemia
it is often administered for prophylactic purposes in those
receiving chemotherapy (33,62). Resistance to the cytotoxic agent
daunorubicin has been reversed by itraconazole (63). It has been demonstrated that the
addition of itraconazole (100 mg twice daily) improves remission
rates in acute myelogenous leukaemia and disease-free survival in
acute lymphoblastic leukaemia (33).
This supports itraconazole's action of reversing drug resistance
and is considered to be associated with its involvement with
cytochrome P-450 and P-glycoprotein.
Conclusion
There is understandable reticence regarding the
repurposing of drugs. Although the initial focus of these therapies
is to treat non-malignant disease, the principle of cell
destruction and elimination is the same as in agents created to
target malignancy. To have a drug acting singularly on a recognised
essential pathway in the malignant process is ideal, but, in
reality, few drugs act in such a manner. Thus, the use of therapies
with multiple targets would be reasonable to explore.
Treatments that cause fewer adverse effects, give
greater survival benefits and are more cost-effective are greatly
required (8). Itraconazole has been
shown to be safe in humans and is cheap to purchase, thus making it
a viable option for future studies (9,23). By
avoiding the lengthy process and cost-implications associated with
bringing a novel drug to market, further study into its actions and
potential benefits make it an attractive prospect.
Evidence from in vivo, in vitro and clinical
studies have demonstrated the antineoplastic effects of
itraconazole and have revealed at a number of the critical pathways
that it targets (4–33). These results allow itraconazole, alone
or in combination with other chemotherapeutic agents, to increase
drug efficacy and overcome drug resistance. Exploration in
aggressive and refractory disease, including ovarian cancer, with
greater participant numbers and consistent treatment regimens is
required. While trials are currently underway and additional
studies are planned, studies need to use itraconazole in
combination with other drugs affecting cell survival. They need to
use itraconazole over longer time periods, at various stages of
disease, in tumours associated with drug resistance and in other
malignancies known to be affected by the Hedgehog pathway and
angiogenesis (9,10).
Acknowledgements
This review was supported by Ovacome, The Ovarian
Cancer Support Charity.
References
|
1
|
Chong CR and Sullivan DJ Jr: New uses for
old drugs. Nature. 448:645–646. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pantziarka P, Bouche G, Meheus L, Sukhatme
V, Sukhatme VP and Vikas P: The repurposing drugs in oncology
(ReDO) project. Ecancermedicalscience. 8:4422014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DiMasi JA, Hansen RW and Grabowski HG: The
price of innovation: New estimates of drug development costs. J
Health Econ. 22:151–185. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim J, Tang JY, Gong R, Kim J, Lee JJ,
Clemons KV, Chong CR, Chang KS, Fereshteh M, Gardner D, et al:
Itraconazole, a commonly used antifungal that inhibits Hedgehog
pathway activity and cancer growth. Cancer Cell. 17:388–399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Navec BA, Grassi P, Dell A, Haslam SM and
Liu JO: The antifungal drug itraconazole inhibits vascular
endothelial growth factor receptor 2 (VEGFR2) glycosylation,
trafficking, and signalling in endothelial cells. J Biol Chem.
286:44045–44056. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim DJ, Kim J, Spaunhurst K, Montoya J,
Khodosh R, Chandra K, Fu T, Gilliam A, Molgo M, Beachy PA and Tang
JY: Open-label, exploratory phase II trial of oral itraconazole for
the treatment of basal cell carcinoma. J Clin Oncol. 32:745–751.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Yao Y, Liu H, Ma X, Lv T, Yuan D,
Xiao X, Yin J and Song Y: Itraconazole can inhibit malignant
pleural effusion by suppressing lymphangiogenesis in mice. Transl
Lung Cancer Res. 4:27–35. 2015.PubMed/NCBI
|
|
8
|
Aftab BT, Dobromilskaya I, Liu JO and
Rudin CM: Itraconazole inhibits angiogenesis and tumor growth in
non-small cell lung cancer. Cancer Res. 71:6764–6772. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pantziarka P, Sukhatme V, Bouche G, Meheus
L and Sukhatme VP: Repurposing drugs in oncology
(ReDO)-itraconazole as an anti-cancer agent. Ecancermedicalscience.
9:5212015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lockhart NR, Waddell JA and Schrock NE:
Itraconazole therapy in a pancreatic adenocarcinoma patient: A case
report. J Oncol Pharm Pract. 22:528–532. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dirix L: Discovery and exploitation of
novel targets by approved drugs. J Clin Oncol. 32:720–721. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pace JR, DeBerardinis AM, Sail V,
Tacheva-Grigorova SK, Chan KA, Tran R, Raccuia DS, Wechsler-Reya RJ
and Hadden MK: Repurposing the clinically efficacious antifungal
agent itraconazole as an anticancer chemotherapeutic. J Med Chem.
59:3635–3649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
You M, Varona-Santos J, Singh S, Robbins
DJ, Savaraj N and Nguyen DM: Targeting the hedgehog signal
transduction pathway suppresses survival of malignant pleural
mesothelioma cells in vitro. J Thorac Cardiovasc Surg. 147:508–516.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kast RE, Karpel-Massler G and Halatsch ME:
CUSP9* treatment protocol for recurrent glioblastoma: Aprepitant,
artesunate, auranofin, captopril, celecoxib, disulfiram,
itraconazole, ritonavir, sertraline augmenting continuous low dose
temozolomide. Oncotarget. 5:8052–8082. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inoue K, Tsubamoto H, Sakata K, Sakane R,
Hao H, Hirota S, Sonoda T and Shibahara H: Expression of hedgehog
signals and growth inhibition by itraconazole in endometrial
cancer. Anticancer Res. 36:149–153. 2016.PubMed/NCBI
|
|
16
|
Kim J, Aftab BT, Tang JY, Kim D, Lee AH,
Rezaee M, Kim J, Chen B, King EM, Borodovsky A, et al: Itraconazole
and arsenic trioxide inhibit Hedgehog pathway activation and tumor
growth associated with acquired resistance to smoothened
antagonists. Cancer Cell. 23:23–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chong CR, Xu J, Lu J, Bhat S, Sullivan DJ
Jr and Liu JO: Inhibition of angiogenesis by the antifungal drug
itraconazole. ACS Chem Biol. 2:263–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rudin CM, Brahmer JR, Juergens RA, Hann
CL, Ettinger DS, Sebree R, Smith R, Aftab BT, Huang P and Liu JO:
Phase 2 study of pemetrexed and itraconazole as second-line therapy
for metastatic nonsquamous non-small cell lung cancer. J Thorac
Oncol. 8:619–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsubamoto H, Sonoda T, Yamasaki M and
Inoue K: Impact of combination chemotherapy with itraconazole on
survival for patients with recurrent or persistent ovarian clear
cell carcinoma. Anticancer Res. 34:2007–2014. 2014.PubMed/NCBI
|
|
20
|
Takara K, Tanigawara Y, Komada F,
Nishiguchi K, Sakaeda T and Okumura K: Cellular pharmacokinetic
aspects of reversal effect of itraconazole on
P-glycoprotein-mediated resistance of anticancer drugs. Biol Pharm
Bull. 22:1355–1359. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gupta A, Unadkat JD and Mao Q:
Interactions of azole antifungal agents with the human breast
cancer resistance protein (BCRP). J Pharm Sci. 96:3226–3235. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kurosawa M, Okabe M, Hara N, Kawamura K,
Suzuki S, Sakurada K and Asaka M: Reversal effect of itraconazole
on adriamycin and etoposide resistance in human leukemia cells. Ann
Hematol. 72:17–21. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsubamoto H, Sonoda T, Yamasaki M and
Inoue K: Impact of combination chemotherapy with itraconazole on
survival of patients with refractory ovarian cancer. Anticancer
Res. 34:2481–2487. 2014.PubMed/NCBI
|
|
24
|
Antonarakis ES, Heath EI, Smith DC,
Rathkopf D, Blackford AL, Danila DC, King S, Frost A, Ajiboye AS,
Zhao M, et al: Repurposing itraconazole as a treatment for advanced
prostate cancer: A noncomparative randomised phase II trial in men
with metastatic castration-resistant prostate cancer. Oncologist.
18:163–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzman DL and Antonarakis ES: High-dose
itraconazole as a noncastrating therapy for a patient with
biochemically recurrent prostate cancer. Clin Genitourin Cancer.
12:e51–e53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ademuyiwa FO, Zhao Q, Perkins SM,
Gebregziabher N, Jones DR, Vaughn LG, Sledge GW and Miller K: A
pilot trial of itraconazole pharmacokinetics in patients with
metastatic breast cancer. J Clin Oncol. 29:(15 Suppl). e135652011.
View Article : Google Scholar
|
|
27
|
Tsubamoto H, Sonoda T and Inoue K: Impact
of itraconazole on the survival of heavily pre-treated patients
with triple-negative breast cancer. Anticancer Res. 34:3839–3844.
2014.PubMed/NCBI
|
|
28
|
Wahid M, Jawed A, Mandal RK, Dar SA, Khan
S, Akhter N and Haque S: Vismodegib, itraconazole and sonidegib as
hedgehog pathway inhibitors and their relative competencies in the
treatment of basal cell carcinomas. Crit Rev Oncol Hematol.
98:235–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ally MS, Ransohoff K, Sarin K, Atwood SX,
Rezaee M, Bailey-Healy I, Kim J, Beachy PA, Chang AL, Oro A, et al:
Effects of combined treatment with arsenic trioxide and
itraconazole in patients with refractory metastatic basal cell
carcinoma. JAMA Dermatol. 152:452–456. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsubamoto H, Sonoda T, Ikuta S, Tani S,
Inoue K and Yamanaka N: Combination chemotherapy with itraconazole
for treating metastatic pancreatic cancer in the second-line or
additional setting. Anticancer Res. 35:4191–4196. 2015.PubMed/NCBI
|
|
31
|
Tsubamoto H, Sonoda T, Ikuta S, Tani S,
Inoue K and Yamanaka N: Impact of itraconazole after first-line
chemotherapy on survival of patients with metastatic biliary tract
cancer. Anticancer Res. 35:4923–4927. 2015.PubMed/NCBI
|
|
32
|
Cooper SM, Sheridan A and Burge S: Mycosis
fungoides responding to systemic itraconazole. J Eur Acad Dermatol
Venereol. 17:588–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vreugdenhil G, Raemaekers JM, van Dijke BJ
and de Pauw BE: Itraconazole and multidrug resistance: Possible
effects on remission rate and disease-free survival in acute
leukaemia. Ann Haematol. 67:107–109. 1993. View Article : Google Scholar
|
|
34
|
Kalderon D: Transducing the hedgehog
signal. Cell. 103:371–374. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
di Pasca Magliano M and Hebrok M: Hedgehog
signalling in cancer formation and maintenance. Nat Rev Cancer.
3:903–911. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ruiz i Altaba A, Sánchez P and Dahmane N:
Gli and hedgehog in cancer: Tumours, embryos and stem cells. Nat
Rev Cancer. 2:361–372. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stecca B, Ruiz I and Altaba A:
Context-dependent regulation of the GLI code in cancer by HEDGEHOG
and non-HEDGEHOG signals. J Mol Cell Biol. 2:84–95. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rimkus TK, Carpenter RL, Qasem S, Chan M
and Lo HW: Targeting the sonic hedgehog signaling pathway: Review
of smoothened and GLI inhibitors. Cancers (Basel). 8:pii: E22.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sekulic A and Von Hoff D: Hedgehog pathway
inhibition. Cell. 164:8312016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sarkar S, Goldgar S, Byler S, Rosenthal S
and Heerboth S: Demethylation and re-expression of epigenetically
silenced tumor suppressor genes: Sensitization of cancer cells by
combination therapy. Epigenomics. 5:87–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Callaghan R, Luk F and Bebawy M:
Inhibition of the multidrug resistance P-glycoprotein: Time for a
change of strategy? Drug Metab Dispos. 42:623–631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Undevia SD, Gomez-Abuin G and Ratain MJ:
Pharmacokinetic variability of anticancer agents. Nat Rev Cancer.
5:447–458. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Itamochi H, Kigawa J, Sultana H, Iba T,
Akeshima R, Kamazawa S, Kanamori Y and Terakawa N: Sensitivity to
anticancer agents and resistance mechanisms in clear cell carcinoma
of the ovary. Jpn J Cancer Res. 93:723–728. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maringe C, Walters S, Butler J, Coleman
MP, Hacker N, Hanna L, Mosgaard BJ, Nordin A, Rosen B, Engholm G,
et al: Stage at diagnosis and ovarian cancer survival: Evidence
from the international cancer benchmarking partnership. Gynecol
Oncol. 127:75–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cancer research UK cancer statistics,
ovarian cancer survival statistics. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer#heading-TwoAccessed.
April;2017.
|
|
46
|
Perez RP, Hamilton TC, Ozols RF and Young
RC: Mechanisms and modulation of resistance to chemotherapy in
ovarian cancer. Cancer. 71:(4 Suppl). S1571–S1580. 1993. View Article : Google Scholar
|
|
47
|
Yoshino K, Enomoto T, Fujita M, Ueda Y and
Kimura T, Kobayashi E, Tsutsui T and Kimura T: Salvage chemotherapy
for recurrent or persistent clear cell carcinoma of the ovary: A
single-institution experience for a series of 20 patients. Int J
Clin Oncol. 18:148–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kajiyama H, Shibata K, Mizuno M, Yamamoto
E, Fujiwara S, Umezu T, Suzuki S, Nakanishi T, Nagasaka T and
Kikkawa F: Postrecurrent oncologic outcome of patients with ovarian
clear cell carcinoma. Int J Gynecol Cancer. 22:801–806. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tsubaoto H, Ito Y, Kanazawa R, Wada R,
Hosoda Y, Honda O, Takeyama R, Sakane R, Wakimoto Y and Shibahara
H: Benefit of palliative chemotherapy and hospice enrolment in
late-stage ovarian cancer patients. J Obstet Gynaecol Res.
40:1399–1406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et
al: Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Brufsky A, Valero V, Tiangco B, Dakhil S,
Brize A, Rugo HS, Rivera R, Duenne A, Bousfoul N and Yardley DA:
Second-line bevacizumab-containing therapy in patients with
triple-negative breast cancer: Subgroup analysis of the RIBBON-2
trial. Breast Cancer Res Treat. 133:1067–1075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Staudacher L, Cottu PH, Diéras V,
Vincent-Salomon A, Guilhaume MN, Escalup L, Dorval T, Beuzeboc P,
Mignot L and Pierga JY: Platinum-based chemotherapy in metastatic
triple-negative breast cancer: The institut curie experience. Ann
Oncol. 22:848–856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu M, Mo QG, Wei CY, Qin QH, Huang Z and
He J: Platinum-based chemotherapy in triple-negative breast cancer:
A meta-analysis. Oncol Lett. 5:983–991. 2013.PubMed/NCBI
|
|
54
|
American cancer society, . cancer facts
and figures 201s. 2016 https://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdfAccessed.
April;2017.
|
|
55
|
Miller DL and Weinstock MA: Nonmelanoma
skin cancer in the United States: Incidence. J Am Acad Dermatol.
30:774–778. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sekulic A, Migden MR, Oro AE, Dirix L,
Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander
PA, et al: Efficacy and safety of vismodegib in advanced basal-cell
carcinoma. N Engl J Med. 366:2171–2179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pivotal data for Novartis' investigational
compound LDE225 show marked tumor responses in advanced basal cell
carcinoma. 2014 http://www.oncotrends.de/pivotal-data-for-novartis-investigational-compound-lde225-sonidegib-show-marked-tumor-responses-in-advanced-basal-cell-carcinoma-422202Accessed.
April;2017.
|
|
58
|
Migden MR, Guminski AD, Gutzmer R, Dirix
LY, Lewis KD, Combemale P, Herd R, Gogov S, Yi T, Mone M, et al:
Randomized, double-blind study of sonidegib (LDE225) in patients
(pts) with locally advanced (La) or metastatic basal-cell carcinoma
(BCC). J Clin Oncol. 32:5s2014.
|
|
59
|
Rahma OE, Duffy A, Liewehr DJ, Steinberg
SM and Greten TF: Second-line treatment in advanced pancreatic
cancer: A comprehensive analysis of published clinical trials. Ann
Oncol. 24:1972–1979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Patel T: Increasing incidence and
mortality of primary intrahepatic cholangiocarcinoma in the United
States. Hepatology. 33:1353–1357. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lamarca A, Hubner RA, David Ryder W and
Valle JW: Second-line chemotherapy in advanced biliary cancer: A
systematic review. Ann Oncol. 25:2328–2338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Vreugdenhil G, Van Dijke BJ, Donnelly JP,
Novakova IR, Raemaekers JM, Hoogkamp-Korstanje MA, Koster M and de
Pauw BE: Efficacy of itraconazole in the prevention of fungal
infections among neutropenic patients with hematologic malignancies
and intensive chemotherapy. A double blind, placebo controlled
study. Leuk Lymphoma. 11:353–358. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gupta S, Kim J and Gollapudi S: Reversal
of daunorubicin resistance in P388/ADR cells by itraconazole. J
Clin Invest. 87:1467–1469. 1991. View Article : Google Scholar : PubMed/NCBI
|