Introduction
Women with ovarian cancer usually present at an
advanced stage with FIGO stage III and IV disease in >75% of
cases (1). Therefore, the prognosis
of ovarian cancer is poor with high recurrence rates between 60 and
85% within five years despite aggressive multimodality treatment
with cytoreductive surgery and adjuvant cytotoxic chemotherapy
(2). In order to improve the
prognosis of women with ovarian cancer, alternative therapy
modalities have been explored, among them a variety of
anti-angiogenic drugs such as bevacizumab, pazopanib, and
nintedanib (3). Specifically,
bevacizumab, a monoclonal humanized antibody against the vascular
endothelial growth factor (VEGF), has been shown to significantly
prolong recurrence-free and progression-free survival after
first-line therapy (4,5) and in women with platinum-sensitive
(6) and platinum-resistant recurrence
(7). To date, no effect on overall
survival was ascertained. Based on the results of randomized
clinical trials (4–7), adding bevazicumab to standard systemic
chemotherapy has become the standard of care in the adjuvant and
recurrent situations.
Another anti-angiogenic agent, pazopanib, an oral,
tyrosine kinase inhibitor of the VEGF receptors 1, 2 and 3, the
platelet-derived growth factor receptor (PDGFR), and c-Kit, has
been tested as a maintenance treatment after initial surgery and
adjuvant chemotherapy. In a large randomized trial, maintenance
therapy with pazopanib 800 mg once per day or placebo for up to 24
months, significantly prolonged the duration of progression-free
survival (PFS) from a median of 12.3 to 17.9 months. Overall
survival was not improved (8).
Besides bevacizumab and pazopanib, a third anti-angiogenic drug,
nintedanib, has been demonstrated to improve PFS in ovarian cancer
patients. Nintedanib is an oral triple angiokinase inhibitor of the
VEGF- and PDGF-receptors and the fibroblast growth factor receptor
(FGFR). In a randomized phase III trial, maintenance treatment with
200 mg of nintedanib twice daily on days 2 to 21 of every 3 week
cycle for up to 120 weeks was tested against placebo. Median PFS
was significantly longer in the nintedanib group, although the
absolute difference was small (17.2 vs. 16.6 months) (9). Taken together, these trials demonstrate
that blocking tumor angiogenesis at various levels is a successful
strategy to delay disease progression in ovarian cancer. Therefore,
it is reasonable to investigate additional anti-angiogenic drugs in
this patient population.
Thalidomide and lenalidomide are such drugs, because
they are immunomodulatory agents with strong anti-angiogenic
properties. Thalidomide inhibits the processing of mRNA encoding
different peptide molecules such as tumor necrosis factor-α (TNF-α)
and VEGF (10). In addition,
thalidomide modulates intracellular signaling pathways via the
mediation of VEGF, phosphoinositide-kinase/protein kinase B and
nuclear factor-κB (NF-κB), and the mammalian target of rapamycin
(mTOR), which integrates these signaling systems (11). In pre-clinical studies, thalidomide
has been shown to suppress malignant cell proliferation and
angiogenesis as well as invasion and metastasis (12).
Historically, thalidomide was developed as a drug
for the treatment of morning sickness in pregnant women (13). After its teratogenic effects leading
to limb defects became apparent in the early sixties of the
20th century (14),
thalidomide was retracted worldwide from the market. However, the
drug has made a remarkable comeback in the last decade as a highly
effective agent for leprosy, multiple myeloma, and Crohn's disease
(15).
For example, thalidomide is being used effectively
in the treatment of inflammatory bowel diseases refractory to
first-line and second-line treatments. In a recent systematic
literature review, Bramuzzo et al identified 2 randomized
controlled trials and 29 uncontrolled trials with a total of 489
patients (16). Thalidomide induced a
clinical response in 296/427 (69%) patients. Clinical remission was
achieved in 220/427 (51%) cases. Maintenance of remission was
reported in 128/160 (80%) patients at 6 months and in 96/133 (72%)
at 12 months. Reduction in steroid dosage, clinical improvement of
fistulas, and endoscopic improvement were also reported.
Neurological disturbances in 341/530 (64%) patients accounted for
most adverse events and were the most frequent cause of drug
withdrawal.
Regarding the use of thalidomide as an antitumor
drug, a number of malignant diseases have been investigated, among
them glioblastoma (17),
hepatocellular carcinoma (18), and
multiple myeloma (19). For example,
in a meta-analysis of randomized trials, Gao et al
summarized the results of 22 randomized trials with 9,098 multiple
myeloma patients (19). Induction
therapy with thalidomide significantly improved the overall
response rate, progression-free survival, and overall survival in
patients who were not allowed to receive autologous stem cell
transplantation. Among patients who were allowed to receive
autologous stem cell transplantation, induction treatment with
thalidomide significantly improved the overall response rate and
progression-free survival, but not overall survival. The most
notable side effect of thalidomide in these trials was an increased
rate of venous thromboembolism (VTE).
Lenalidomide has immunomodulatory and
anti-angiogenic effects. For example, it modulates the substrate
specificity of the CRL4 (CRBN) E3 ubiquitin ligase complex
(20). Polyubiquitination and
subsequent proteasomal degradation of IKZF1 and IKZF3 in multiple
myeloma and CK1α in del (5q) myelodysplastic syndrome has been
described as the basis of the therapeutic efficacy of lenalidomide.
Harnessing ubiquitin ligase substrate specificity facilitates the
degradation of other ‘undruggable’ proteins and thus allows for the
separation of detrimental side effects of IMiD compounds from those
associated with therapeutic efficacy (20,21).
Lenalidomide (Revlimid®) has been approved by the FDA
for previously treated multiple myeloma in combination with
dexamethasone.
Based on the well-known fact that anti-angiogenesis
is effectively delaying disease progression in ovarian cancer
patients, the compassionate use of thalidomide has been reported in
heavily pre-treated patients with ovarian cancer recurrence
(22–24). These case reports indicated that
thalidomide may be active in ovarian cancer. In order to further
highlight the safety and efficacy of thalidomide and lenalidomide
in patients with recurrent ovarian, fallopian tube, and primary
peritoneal cancer, we performed a systematic literature review of
retrospective and prospective clinical trials evaluating the
efficacy and safety of these two anti-angiogenic agents.
Materials and methods
A systematic review of the literature was conducted
to identify studies with thalidomide and lenalidomide in women with
recurrent ovarian, fallopian tube, or primary peritoneal cancer.
The databases Medline and the Cochrane Central Register of
Controlled Trials were searched for published English language case
reports, clinical trials and other studies that described the
safety and efficacy of thalidomide or lenalidomide alone or in
combination with other drugs, using the search terms ‘thalidomide’
[MeSH Terms] OR ‘thalidomide’ [All Fields]) AND (‘ovarian
neoplasms’ [MeSH Terms] OR (‘ovarian’ [All Fields] AND ‘neoplasms’
[All Fields]) OR ‘ovarian neoplasms’ [All Fields] OR (‘ovarian’
[All Fields] AND ‘cancer’ [All Fields]) OR ‘ovarian cancer’ [All
Fields]). In a second step, we used the search terms ‘lenalidomide’
[Supplementary Concept] OR ‘lenalidomide’ [All Fields]) AND
(‘ovarian neoplasms’ [MeSH Terms] OR (‘ovarian’ [All Fields] AND
‘neoplasms’ [All Fields]) OR ‘ovarian neoplasms’ [All Fields] OR
(‘ovarian’ [All Fields] AND ‘cancer’ [All Fields]) OR ‘ovarian
cancer’ [All Fields]).
Abstracts were screened for the following
eligibility criteria: Studies describing at least one woman with
recurrent ovarian, fallopian tube or primary peritoneal cancer
treated with thalidomide or lenalidomide with description of the
safety and/or efficacy and/or side effects of this treatment.
Duplicate publications were excluded. No size or design
restrictions were applied, ie case reports, case series,
retrospective and prospective cohort studies, case control studies,
and randomized controlled trials were allowed. Relevant full text
articles were retrieved and analyzed for outcomes related to the
goal of this review-to assess the safety, efficacy, and side
effects of thalidomide and lenalidomide in women with recurrent
ovarian, fallopian tube or primary peritoneal cancer.
Results
In a systematic literature search of the databases
PubMed and Cochrane Central Register of Controlled Trials (search
date 01-11-2016) using the search terms ‘thalidomide’ [MeSH Terms]
OR ‘thalidomide’ [All Fields]) AND (‘ovarian neoplasms’ [MeSH
Terms] OR (‘ovarian’ [All Fields] AND ‘neoplasms’ [All Fields]) OR
‘ovarian neoplasms’ [All Fields] OR (‘ovarian’ [All Fields] AND
‘cancer’ [All Fields]) OR ‘ovarian cancer’ [All Fields]), we
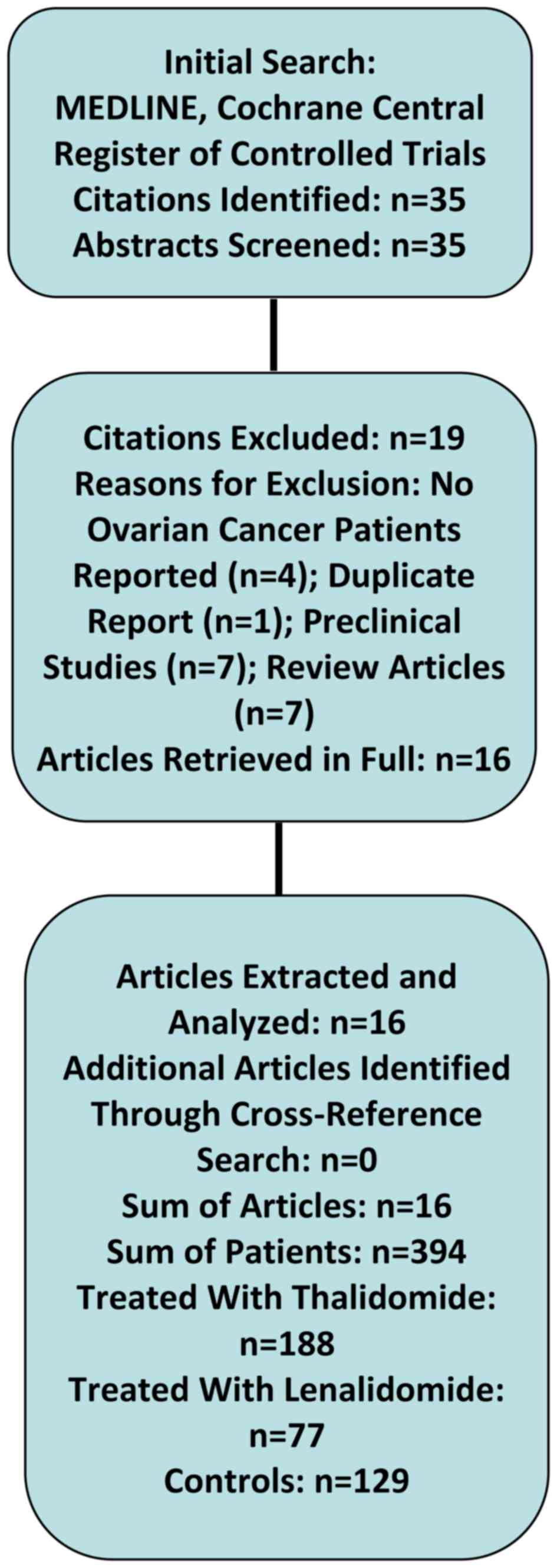
identified 32 studies (15,22–52). In a
second step, we used the search terms ‘lenalidomide’ [Supplementary
Concept] OR ‘lenalidomide’ [All Fields]) AND (‘ovarian neoplasms’
[MeSH Terms] OR (‘ovarian’ [All Fields] AND ‘neoplasms’ [All
Fields]) OR ‘ovarian neoplasms’ [All Fields] OR (‘ovarian’ [All
Fields] AND ‘cancer’ [All Fields]) OR ‘ovarian cancer’ [All
Fields]) and identified 3 additional studies (53–55). After
screening all abstracts of these studies, we excluded 4 studies
(51,53–55)
because they did not report on patients with ovarian cancer and one
study (52) because the patients
reported in this study were already published in another trial by
the same authors (42). Further 7
citations comprised preclinical studies describing in vitro
and in vivo data on the antitumor effects of thalidomide and
lenalidomide (25–31). Seven citations were review articles
not containing individual patients data (15,32–37). Thus,
in summary, we included 16 clinical studies describing 394 patients
treated with thalidomide (n=188), lenalidomide (n=77), and 129
controls. These studies included 5 case reports (n=6), 3 case
series (n=45), 2 phase I trials (n=27), 4 phase II trials (n=109),
and 2 randomized phase III trials (n=207). Table I shows the study characteristics and
results of the 5 case reports and 11 clinical trials in detail.
Figure 1 shows a flow diagram of the
literature search algorithm. In a pooled analysis of trials with
thalidomide tested as a single drug, the overall response rate was
43% (43/99) with a mean time to progression of 5.6 months. Among
these patients, complete response was observed in 11%, partial
response in 13%, and stable disease in 19%. The dosage of
thalidomide used in these studies ranged from 100 to 1,200 mg/day.
In one phase III trial, the combination of thalidomide and
topotecan significantly increased the overall response rate
compared to topotecan alone (14/30 [47%] vs. 8/39 [21%]). In
another phase III trial of women with biochemical ovarian cancer
recurrence, thalidomide was not more effective than tamoxifen.
 | Table I.Clinical studies describing women
with ovarian, fallopian tube or peritoneal cancer treated with
thalidomide or lenalidomide. |
Table I.
Clinical studies describing women
with ovarian, fallopian tube or peritoneal cancer treated with
thalidomide or lenalidomide.
| Author | Study type | Number of cases
(n) | Primary/disease
recurrent | Histology | Previous
therapies | Treatment
regimen | Dosage | Response (n) | Time to progression
(mo) | (Refs.) |
|---|
|
|---|
| A, Thalidomide
trials |
|---|
| Eisen et
al | Phase II | 19 | REC | EOC | CRS, CHXT | T | 100 mg/d | CR (0), PR (0), SD
(1) | Not evaluated | (40) |
| Jeyakumar et
al | Case report | 1 | REC | YST | CRS, CHXT | T+DOC, GEM | 200 mg/d | CR (1) | – | (39) |
| Abramson et
al | CS | 10 | REC | EOC, PPC | CRS, CHXT | T | 200 mg/d escalated
by 100 mg/d/week | PR (3) | Not evaluated | (41) |
| Chan et
al | CS | 17 | REC | EOC | CRS, CHXT | T | 100 to 1,200
mg/d | PR (3), SD (6) | 10 | (44) |
| Gordinier et
al | CS | 18 | REC | EOC, PPC | CRS, CHXT | T | 200 mg/d | PR (1), SD (7) | 3.8 | (52) |
| Kanwar et
al | Case report | 1 | PD | SCC | – | CRS, CHXT, T,
Ima | 60 mg/d | CR (1) | 36 | (22) |
| Buttin and
Moore | Case report | 1 | REC | EOC | CRS, CHXT | T+TOP | 200 md/d | SD (1) | 3 | (24) |
| Downs et
al | RCT | 69 | REC | EOC | CRS, CHXT | T+TOP (30) vs. TOP
(39) | 200 mg/d | CR (9), PR (5) vs.
CR (7), PR (1) | 6 vs. 4 | (45) |
| Phippen and
Leath | Case report | 2 | REC | EOC | CRS, CHXT | T+TOP | – | PR (1), SD (1) | 6 | (23) |
| Hurteau et
al | RCT | 138 | REC
(Bioche-mical) | EOC | CRS, CHXT | T (68) vs. TAM
(70) | 200 to 400
mg/d | Not evaluated | 3.2 | (46) |
| Muthuramalingam
et al | Phase II | 40 | PD | EOC IC-IV | CRS | T+C (20) vs. C
(20) | 100 mg/d | CR+PR (15) | Not evaluated | (48) |
| Benesch et
al | Case report | 1 | REC | GCT | CRS, CHXT (2
lines), RXT | T+PAC, BEV,
IFN | – | CR (1) | 72 | (38) |
| Zhang et
al | Phase I | 20 | REC | EOC, PPC | CRS, CHXT | L | 25 mg/d d1-21,
q28 | SD (9) | 5.8 | (43) |
| Carter et
al | Phase II | 5 | REC | EOC | CRS, CHXT | L+TOP | 5 mg/d | Not evaluated | 1 | (47) |
| Ganesan et
al | Phase I | 7 | REC | EOC | CRS, CHXT | L+S | 10 to 25 mg/d | SD (1) | 3.5 | (49) |
|
| B, Lenalidomide
trials |
|
| Selle et
al | Phase II | 45 | REC | EOC | CRS, CHXT | L | 20 mg/d | PR (4), SD
(21) | 3.4 | (50) |
| Pooled
analysis | – | 394 T (188); L
(77); Controls (129) | REC (353), PD
(41) | – | – | – | T: 100–1,200 mg/d
(range); L: 5–25 mg/d (range) | T: CR (11%), PR
(13%), CBR (43%)a L: SD
(19%); CR (0%), PR (6%), SD (46%); CBR (52%)b | T: 5.6, L:
4.6c |
|
|
Lenalidomide was investigated in 3 phase I trials
and in one phase II trial with an overall response rate of 52%
(34/65) and a mean time to progression of 4.6 months. Among these
patients, complete response was observed in 0%, partial response in
6%, and stable disease in 46%. The dosage of lenalidomide used in
these studies ranged from 5 to 25 mg/day.
Systemic toxicity of both drugs was noted in up to
77% of patients with pneumonitis/pneumonia, fatigue, neutropenia,
neuropathy, and VTE cited as the most common side effects. Table II shows the side effects of
thalidomide observed in 12 clinical trials with 317 patients in
detail. Grade 3 or 4 adverse events were noted in 61/132 (46%) of
women with thalidomide as a single treatment. Specifically, the
most often cited side effects of thalidomide were pulmonary
problems/pneumonia/pneumonitis (4 studies), neuropathy (4 studies),
VTE (3 studies), weakness/fatigue (3 studies), constipation (3
studies), and somnolence (3 studies). Table III shows the side effects of
lenalidomide in 4 clinical trials with 77 patients in detail. 43/65
(66%) patients with lenalidomide as a single treatment showed grade
3 adverse events and 7/65 (11%) patients showed grade 4 adverse
events. The most often cited side effects of lenalidomide were
neutropenia (4 studies), VTE (2 studies), rash (2 studies),
constipation (2 studies), and weakness (2 studies).
 | Table II.Clinical studies describing the
toxicity of thalidomide in women with ovarian, fallopian tube or
peritoneal cancer. |
Table II.
Clinical studies describing the
toxicity of thalidomide in women with ovarian, fallopian tube or
peritoneal cancer.
| Author | Number of cases
(n) | Treatment
regimen | Grade 3 events | Grade 4 events | (Refs.) |
|---|
| Eisen et al | 19 | T | 0 | 0 | (40) |
| Jeyakumar et
al | 1 | T+DOC, GEM | 0 | 1 (Bowel
otbstruction) | (39) |
| Abramson et al | 10 | T | 0 | 0 | (41) |
| Chan et al | 17 | T | 2 (VTE), 1 (CON), 1
(Confusion), 1 (Speech impairment) |
| (44) |
| Gordinier et
al | 18 | T | 8 (Dyspnea), 2
(CON), 2 (Sedation) | 0 | (42) |
| Kanwar et al | 1 | CRS, CHXT, T,
IMA | 3
(Neurological) | 0 | (22) |
| Buttin and
Moore | 1 | T+TOP | 1 (IP) | 0 | (24) |
| Downs et al | 69 | T+TOP (30) vs. TOP
(39) | 28 (Neutropenia), 6
(Thrombocytopenia), 4 (Anemia), 4 (Neurologic), 3 Pulmonary, 2
(Infection), 2 (Constitutional), 1 (VTE), 1 (Dermatology), 1
(Ocular), 1 (Gastrointestinal) Phippen and Leath |
| (45) |
| 2 | T+TOP | 2 (Fatigue) | 0 | (23) |
| Hurteau et al | 138 | T (68) vs. TAM
(70) | 8 (Constitutional),
7 (Somnolence), 7 (Neurologic), 6 (Pulmonary), 5 (Dermatologic), 3
(Pain), 3 (Gastrointestinal), 2 (VTE) | 2 (VTE), 1
(Somnolence) | (46) |
| Muthuramalingam et
al | 40 | T+C (20) vs. C
(20) | – | – | (48) |
| Benesch et al | 1 | T+PAC, BEV,
IFN | 0 | 0 | (38) |
| Pooled
analysis | 317 T (188);
Controls (129) | – |
| 61/132
(46%)a |
|
 | Table III.Clinical studies describing the
toxicity of lenalidomide in women with ovarian, fallopian tube or
peritoneal cancer. |
Table III.
Clinical studies describing the
toxicity of lenalidomide in women with ovarian, fallopian tube or
peritoneal cancer.
| Author | Number of cases
(n) | Treatment
regimen | Grade 3 events | Grade 4 events | (Refs.) |
|---|
| Zhang et al | 20 | L | 7 (NEU), 2
(Anemia), 1 (Fatigue), 1 (Nausea), 1 (Diarrhea), 1 (VTE) | 0 | (43) |
| Carter et al | 5 | L+TOP | 3 (NEU), 2 (THR), 2
(Anemia), 1 (Fatigue), 1 (VTE) | 0 | (47) |
| Ganesan et al | 7 | L+S | 7 (NEU), 2 (THR), 2
(Rash), 1 (VTE) | 1 (PE) | (49) |
| Selle et al | 45 | L | 10 (NEU), 3 (Pain),
3 (Obstruction), 2 (VTE), 2 (Edema), 2 (Vomiting), 1 (Dyspnea), 1
(Diarrhea) | 3 (Neutropenia), 3
(VTE) | (50) |
| Pooled
analysis | 77 L (77); Controls
(0) | – | 43/65
(66%)a | 7/65
(11%)a |
Experimental studies
Several experimental studies assessed the antitumor
effects of thalidomide in ovarian cancer cell lines, ex vivo, and
in vivo models. For example, Kobayashi et al examined
whether thalidomide is able to suppress the expression of
urokinase-type plasminogen activator receptor (uPAR) mRNA and
protein in the human ovarian cancer cell line HRA (28). They found that thalidomide had
multiple synergistic effects. Specifically, it suppressed the
expression of uPAR mRNA and protein as well as the NF-κB activation
system, which is necessary for the transforming growth factor
(TGF)-β1-induced increase in uPAR expression. Furthermore, the
once-daily intraperitoneal administration of thalidomide (400 µg/g
body weight/day) decreased progressive growth of HRA tumors and
ascites formation in an in vivo animal model. The once-daily
intraperitoneal administration of thalidomide in combination with
paclitaxel significantly decreased the growth of HRA cells in a
synergistic fashion. These data suggest that thalidomide
downregulates constitutive and TGF-β1-stimulated uPAR mRNA and
protein expression via the suppression of NF-κB and may have
synergistic effects with paclitaxel. Piura et al assessed
the effect of thalidomide on the ovarian cancer cell secretome of
SKOV-3 cells (31). TNF-α,
interleukin (IL)-6 and matrix metalloproteinase (MMP) secretion in
epithelial ovarian cancer cells. In these experiments, thalidomide
significantly decreased the secretion of TNF-α, MMP-9, and
MMP-2.
Bauer et al evaluated the antiangiogenic
activity of interferon (IFN)-α 2b and thalidomide. They used a
murine dermis model in nude mice. The combination of IFN-α 2b and
thalidomide had synergistic effects in reducing angiogenesis and
tumor activity in this model (27).
In another murine xenograft model, Li et al studied the
effect of thalidomide alone or in combination with cytoxan on the
growth and angiogenesis of human ovarian cancer cells transplanted
subcutaneously into nude mice (29).
Both thalidomide and the combination of thalidomide and cytoxan
reduced the expression of VEGF mRNA and serum levels, tumor volume,
microvessel density, and macroscopic tumor volumes.
Taken together, the experimental evidence strongly
suggests that thalidomide is active in ovarian cancer through the
downregulation of constitutive and TGF-beta1-stimulated uPAR
expression via the suppression of NF-κB. In addition, it affects
the ovarian cancer cell secretome and reduces angiogenesis,
proliferation, and tumor growth.
Thalidomide trials
In the largest trial, sponsored by the Gynecologic
Oncology Group (GOG), Hurteau et al analyzed 138 women with
ovarian, fallopian tube or peritoneal cancer FIGO stages III and IV
who were free of disease following first-line chemotherapy and
subsequently developed biochemical recurrence (46). Biochemical recurrence was defined as a
rising CA-125 exceeding twice the upper limit of normal without
evidence of disease in imaging studies. Women were randomized to
oral thalidomide 200 mg daily with escalation to a maximum of 400
mg or tamoxifen 20 mg orally twice daily for up to 1 year.
Treatment was given until progression or limiting toxicity. In this
trial, thalidomide did not reduce the recurrence rate relative to
tamoxifen. Specifically, thalidomide vs. tamoxifen was associated
with a similar risk of progression [hazard ratio (HR)=1.31, 95%
confidence interval (CI)=0.93–1.85]. Of note, thalidomide even
resulted in a significantly increased risk of death (HR=1.76, 95%
CI=1.16–2.68) and had significantly more grade 3 and 4 toxicities
(55% vs. 3%). The most common grade 3 and 4 toxicities of
thalidomide were constitutional (12%), somnolence (12%), pulmonary
(9%), VTE (6%) and peripheral neurologic toxicity (6%). This trial
demonstrates that thalidomide should not be used in women with
biochemical recurrence in order to prolong time to progression. In
this indication, thalidomide clearly does more harm than good with
significant increases in morbidity and even mortality.
In contrast to the Hurteau et al (46) trial, the second largest trial was
positive. In a randomized phase III trial, Downs et al and
compared the response rates of 69 women with recurrent ovarian
cancer treated with topotecan or topotecan and thalidomide
(45). Eligible patients had
recurrent epithelial ovarian carcinoma with measurable disease or
elevated CA 125 values. Patients received topotecan at a dose of
1.25 mg/m2 on days 1 through 5 of a 21-day cycle or
topotecan and thalidomide with a starting dose of 200 mg per day
and then increasing the dose as tolerated. In this positive trial,
the overall response rate in the topotecan only arm was 21%
[complete responses (CR) in 18% and partial responses (PR) in 3% of
patients] compared with 47% in the topotecan and thalidomide arm
(CR 30%, PR 17%). This difference was clinically and statistically
significant (P=0.03). The median progression-free survival was
significantly longer in the topotecan and thalidomide arm (6 vs. 4
months; P=0.02). Of note, overall survival time was also longer
with topotecan and thalidomide (19 vs. 15 months), but this
difference was not statistically significant. In contrast to the
Hurteau et al (46) trial,
toxicities in this study were similar in both treatment arms.
Specifically, there were not more grade 3 and 4 events in the
combination arm. This trial is notable due to its randomized design
and the positive result indicating a statistically and clinically
significant additive antitumor effect of thalidomide in combination
with topotecan. Moreover, thalidomide did not increase overall
toxicity.
In a prospective phase II trial, Eisen et al
found no objective tumor responses in 19 women with recurrent
ovarian cancer treated with low dose oral thalidomide (100 mg per
day) (40). They reported no toxicity
for thalidomide. To the contrary, thalidomide improved appetite and
sleeping patterns. In three retrospective case series of 10, 17,
and 18 women, Abramson et al (41), Chan et al (44), and Gordinier et al (52) reported response rates of 33% (3/10),
53% (9/17), and 44% (8/18), respectively. In all three case series,
no complete response was noted. Responses consisted of partial
response and stable disease. Toxicity of thalidomide was moderate
described as mild weakness, agitation, somnolence, rash, and
constipation in one study (41).
However, Gordinier et al observed grade 3 dyspnea in 8/18
(44%) and grade 3 constipation in 2/18 (11%) patients, respectively
(52). In Chan's study, two women
experienced grade 3/4 toxicity (constipation and neurologic
toxicity) and stopped treatment (44).
Five case reports describing the effects of
thalidomide in women with recurrent ovarian cancer were identified.
Complete responses under thalidomide were reported in 3 case
reports (22,38,39).
However, in all 3 cases thalidomide was combined with systemic
chemotherapy. In 2 further case reports of 3 patients (23,24),
thalidomide combined with systemic chemotherapy achieved a partial
response in one and stable disease in 2 patients. In these case
reports, thalidomide was combined with topotecan, docetaxel,
gemcitabine, and paclitaxel. As relevant toxicity, one case of
interstitial pneumonitis was reported among these 6 patients.
Lenalidomide trials
In a prospective phase II trial of 45 women with
recurrent ovarian cancer, continuous oral lenalidomide at a dose of
20 mg per day achieved 4 partial responses and 21 cases of stable
disease for an overall response rate of 56% (25/45) (50). The most frequent toxicity was
hematologic, notably grade 3/4 neutropenia in 29% of patients,
along with fatigue (69%), gastrointestinal toxicity (constipation
53%, abdominal pain 49%, diarrhea 38%, nausea/vomiting 36%) and
thrombosis (11%). Eight patients withdrew due to intolerable
toxicity. Thus, this study indicates that lenalidomide as a single
treatment has significant activity in this patient population
despite a considerable toxicity. In another small phase I/II dose
escalation trial, lenalidomide was escalated from 5 to 25 mg per
day in 5 mg increments and combined with intravenous topotecan 1.25
mg/m2 on days 1 to 5 of a 21-day cycle (47). Of note, 4 patients discontinued
because of dose-limiting toxicity, most commonly grade 4
neutropenia and the study was terminated early for reasons of
toxicity.
Lenalidomide was further investigated in 2 phase I
trials with 20 and 7 participants (43,49). Nine
of 20 women (45%) were noted to have stable disease in one trial
(43) and 1/7 women (14%) in the
second trial, in which lenalidomide was combined with sorafenib
(49). The toxicities observed were
considerable. In the Ganesan et al (49) trial with 7 participants, 15 grade 3/4
events were observed, most notably neutropenia (n=7),
thrombocytopenia, rash, VTE, and pulmonary embolism (all n=2). In
the Zhang et al (43) trial of
20 women, lenalidomide led to 14 grade 3/4 events (neutropenia,
n=7; anemia, n=2; fatigue, nausea, diarrhea, and VTE.
Discussion
In a systematic literature search, we identified 16
clinical studies with 394 patients treated with thalidomide or
lenalidomide. Overall, thalidomide achieved an overall response
rate of 43% with a mean time to progression of 5.6 months and
lenalidomide achieved an overall response rate of 52% with a mean
time to progression of 4.6 months. Systemic toxicity of both drugs
was considerable and was documented in up to 77% of patients with
pneumonitis/pneumonia, neutropenia, rash, constipation, somnolence,
fatigue, neuropathy, and VTE cited as the most common side effects.
Based on these data, we conclude that both thalidomide and
lenalidomide are active drugs in recurrent ovarian cancer. The
toxicity of both drugs, however, is high with
pneumonitis/pneumonia, fatigue, neuropathy, neutropenia, and VTE
being the most important side effects. More data are available for
thalidomide than for lenalidomide. The high toxicity of thalidomide
and lenalidomide clearly limits the clinical usefulness of these
drugs in the palliative situation of recurrent ovarian cancer.
Thalidomide has synergistic effects with topotecan.
This is the notable result of a randomized trial comparing
topotecan alone and topotecan combined with thalidomide (45) in women with recurrent ovarian cancer.
The combination treatment arm doubled the response rate from 21 to
47%. Moreover, adding thalidomide to the standard topotecan
chemotherapy regime did not increase tocixity. Unfortunately, there
is no independent confirmation of this result and the number of
patients recruited for this trial was low with only 69 probands.
Adding the antiangiogenic agent thalidomide to cytotoxic
chemotherapy is a reasonable strategy given that other
antiangiogenic agents such as bevacizumab have been clearly
demonstrated to enhance the antitumoral effect of cytotoxic
chemotherapy (3–7). If this additive effect of thalidomide to
systemic chemotherapy is confirmed in more trials, adding
thalidomide to standard palliative chemotherapy could be a
promising strategy to integrate thalidomide into clinical
practice.
Both thalidomide and lenalidomide have considerable
side effects. For example, grade 3 or 4 adverse events were noted
in 61/132 (46%) of women with thalidomide as a single treatment.
The frequency of severe side effects was even higher with
lenalidomide. In 54/65 (77%) of women treated with lenalidomide as
a single treatment, a grade 3 or 4 adverse event was noted.
Proteinuria and hypertension, which are well-known side effects of
the antiangiogenic agent bevacizumab, have not been noted in women
treated with thalidomide or lenalidomide. Thus, the side effect
profile of thalidomide and lenalidomide is specific and clearly
differs from other antiangiogenic drugs. Typical side effects noted
in many studies with thalidomide and lenalidomide are an increased
pulmonary tocixity and a high rate of VTE. Thus, prophylactic
heparinization may be discussed with patients prior to initiating
thalidomide or lenalidomide, especially in those patients with
additional VTE risk factors. In view of the fact that all patients
with recurrent ovarian, fallopian tube, and peritoneal cancer are
in a palliative situation, the pronounced side effect profile of
thalidomide and lenalidomide is a severe limitation of these
agents. When considering to use these agents, a detailed discussion
with the patient about the treatment benefit and the
drug-associated toxicity is necessary.
Based on the overall estimation of a response rate
of 25% (complete responses and partial responses) for thalidomide
and 6% (complete responses and partial responses) for lenalidomide,
both drugs should not be used routinely in women with recurrent
ovarian cancer. In addition, neither thalidomide nor lenalidomide
are approved for this patient population. Thus, the use of both
drugs in ovarian cancer patients is off-label. This fact may also
limit the practical use of thalidomide and lenalidomide in women
with recurrent ovarian, fallopian tube, and peritoneal cancer,
because off-label use has a number of practical consequences for
the treating physician regarding liability, informed consent, and
reimbursement issues (54).
In summary, we found that the antiangiogenic agents
thalidomide and lenalidomide are active drugs in recurrent ovarian
cancer. Thalidomide has synergistic effects with topotecan. The
toxicity of both drugs is considerable with pneumonitis/pneumonia,
fatigue, neuropathy, and VTE. More data are available for
thalidomide than for lenalidomide. Both thalidomide and
lenalidomide are not recommended for women with recurrent ovarian
cancer. However, the available data support further investigations
of the combination of thalidomide and topotecan within the setting
of clinical trials.
References
|
1
|
Malvezzi M, Carioli G, Rodriguez T, Negri
E and La Vecchia C: Global trends and predictions in ovarian cancer
mortality. Ann Oncol. 27:2017–2025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Foley OW, Rauh-Hain JA and del Carmen MG:
Recurrent epithelial ovarian cancer: An update on treatment.
Oncology (Williston Park). 27:288–294, 298. 2003.
|
|
3
|
Wu YS, Shui L, Shen D and Chen X:
Bevacizumab combined with chemotherapy for ovarian cancer: An
updated systematic review and meta-analysis of randomized
controlled trials. Oncotarget. 8:10703–10713. 2017.PubMed/NCBI
|
|
4
|
Oza AM, Cook AD, Pfisterer J, Embleton A,
Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P,
Cervantes A, et al: Standard chemotherapy with or without
bevacizumab for women with newly diagnosed ovarian cancer (ICON7):
Overall survival results of a phase 3 randomised trial. Lancet
Oncol. 16:928–936. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burger RA, Brady MF, Bookman MA, Fleming
GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE,
et al: Incorporation of bevacizumab in the primary treatment of
ovarian cancer. N Engl J Med. 365:2473–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aghajanian C, Blank SV, Goff BA, Judson
PL, Teneriello MG, Husain A, Sovak MA, Yi J and Nycum LR: OCEANS: A
randomized, double-blind, placebo-controlled phase III trial of
chemotherapy with or without bevacizumab in patients with
platinum-sensitive recurrent epithelial ovarian, primary
peritoneal, or fallopian tube cancer. J Clin Oncol. 30:2039–2045.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum resistant recurrent ovarian cancer: The AURELIA open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
du Bois A, Floquet A, Kim JW, Rau J, del
Campo JM, Friedlander M, Pignata S, Fujiwara K, Vergote I, Colombo
N, et al: Incorporation of pazopanib in maintenance therapy of
ovarian cancer. J Clin Oncol. 32:3374–3382. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
du Bois A, Kristensen G, Ray-Coquard I,
Reuss A, Pignata S, Colombo N, Denison U, Vergote I, Del Campo JM,
Ottevanger P, et al: Standard first-line chemotherapy with or
without nintedanib for advanced ovarian cancer (AGO-OVAR 12): A
randomised, double blind, placebo-controlled phase 3 trial. Lancet
Oncol. 17:78–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Shen Y, Li S, Lv M, Zhang X and
Yang J, Wang F and Yang J: Importance of the interaction between
immune cells and tumor vasculature mediated by thalidomide in
cancer treatment (Review). Int J Mol Med. 38:1021–1029.
2016.PubMed/NCBI
|
|
11
|
Sherbet GV: Therapeutic potential of
thalidomide and its analogues in the treatment of cancer.
Anticancer Res. 35:5767–5772. 2015.PubMed/NCBI
|
|
12
|
Bauer JA, Morrison BH, Grane RW, Jacobs
BS, Borden EC and Lindner DJ: IFN-alpha2b and thalidomide
synergistically inhibit tumor-induced angiogenesis. J Interferon
Cytokine Res. 23:3–10. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vargesson N: Thalidomide-induced limb
defects: Resolving a 50-year-old puzzle. Bioessays. 31:1327–1336.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McBride W: Thalidomide and congenital
malformations. Lancet. 1:3581961.PubMed/NCBI
|
|
15
|
Connors T: Anticancer drug development:
The way forward. Oncologist. 1:180–181. 1996.PubMed/NCBI
|
|
16
|
Bramuzzo M, Ventura A, Martelossi S and
Lazzerini M: Thalidomide for inflammatory bowel disease: Systematic
review. Medicine (Baltimore). 95:e42392016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Milanovic D, Sticht C, Röhrich M, Maier P,
Grosu AL and Herskind C: Inhibition of 13-cis retinoic acid-induced
gene expression of reactive-resistance genes by thalidomide in
glioblastoma tumours in vivo. Oncotarget. 6:28938–28948. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang PC, Ch'ang HJ, Hsu C, Chen LT, Shih
TT and Liu TW: Perfusion parameters of dynamic contrast-enhanced
magnetic resonance imaging predict outcomes of hepatocellular
carcinoma receiving radiotherapy with or without thalidomide.
Hepatol Int. 9:258–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao M, Kong Y, Wang H, Xie B, Yang G, Gao
L, Zhang Y, Zhan F, Dai B, Tao Y and Shi J: Thalidomide treatment
for patients with previously untreated multiple myeloma: A
meta-analysis of randomized controlled trials. Tumour Biol.
37:11081–11098. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guirguis AA and Ebert BL: Lenalidomide:
Deciphering mechanisms of action in myeloma, myelodysplastic
syndrome and beyond. Curr Opin Cell Biol. 37:61–67. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galustian C and Dalgleish A: Lenalidomide:
A novel anticancer drug with multiple modalities. Expert Opin
Pharmacother. 10:125–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanwar VS, Heath J, Krasner CN and Pearce
JM: Advanced small cell carcinoma of the ovary in a
seventeen-year-old female, successfully treated with surgery and
multi-agent chemotherapy. Pediatr Blood Cancer. 50:1060–1062. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Phippen NT and Leath CA III: Weekly
topotecan and daily thalidomide in patients with recurrent
epithelial ovarian cancer: A report of 2 cases. J Reprod Med.
54:583–586. 2009.PubMed/NCBI
|
|
24
|
Buttin BM and Moore MJ:
Thalidomide-induced reversible interstitial pneumonitis in a
patient with recurrent ovarian cancer. Gynecol Oncol. 111:546–548.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Braun AG and Weinreb SL: Teratogen
metabolism: Spontaneous decay products of thalidomide and
thalidomide analogues are not bioactivated by liver microsomes.
Teratog Carcinog Mutagen. 5:149–158. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Braun AG and Dailey JP: Thalidomide
metabolite inhibits tumor cell attachment to concanavalin A coated
surfaces. Biochem Biophys Res Commun. 98:1029–1034. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bauer JA, Morrison BH, Grane RW, Jacobs
BS, Borden EC and Lindner DJ: IFN-alpha2b and thalidomide
synergistically inhibit tumor-induced angiogenesis. J Interferon
Cytokine Res. 23:3–10. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kobayashi H, Yagyu T, Kondo T, Kurita N,
Inagaki K, Haruta S, Kawaguchi R, Kitanaka T, Sakamoto Y, Yamada Y,
et al: Suppression of urokinase receptor expression by thalidomide
is associated with inhibition of nuclear factor kappaB activation
and subsequently suppressed ovarian cancer dissemination. Cancer
Res. 65:10464–10471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li W, Peng ZL and Cao ZY: Study of
thalidomide on the growth and angiogenesis of ovary cancer SKOV3
transplanted subcutaneously in nude mice. Zhonghua Fu Chan Ke Za
Zhi. 40:186–189. 2005.(In Chinese). PubMed/NCBI
|
|
30
|
Strese S, Fryknäs M, Larsson R and Gullbo
J: Effects of hypoxia on human cancer cell line chemosensitivity.
BMC Cancer. 13:3312013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Piura B, Medina L, Rabinovich A, Dyomin V
and Huleihel M: Thalidomide distinctly affected TNF-8.5, IL-6 and
MMP secretion by an ovarian cancer cell line (SKOV-3) and primary
ovarian cancer cells. Eur Cytokine Netw. 24:122–129.
2013.PubMed/NCBI
|
|
32
|
Eisen TG: Thalidomide in solid tumors: The
London experience. Oncology (Williston Park). 14:(12 Suppl 13).
S17–S20. 2000.
|
|
33
|
Eleutherakis-Papaiakovou V, Bamias A and
Dimopoulos MA: Thalidomide in cancer medicine. Ann Oncol.
15:1151–1160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma WW and Jimeno A: Strategies for
suppressing angiogenesis in gynecological cancers. Drugs Today
(Barc). 43:259–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Galustian C and Dalgleish A: Lenalidomide:
A novel anticancer drug with multiple modalities. Expert Opin
Pharmacother. 10:125–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Malayev Y, Levene R and Gonzalez F:
Palliative chemotherapy for malignant ascites secondary to ovarian
cancer. Am J Hosp Palliat Care. 29:515–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gadducci A, Sergiampietri C and Guiggi I:
Antiangiogenic agents in advanced, persistent or recurrent
endometrial cancer: A novel treatment option. Gynecol Endocrinol.
29:811–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Benesch M, Lackner H, Pilhatsch A,
Gürtl-Lackner B, Schwinger W and Urban C: Long-term remission in a
female with multiple relapsed juvenile granulosa cell tumor. J
Pediatr Hematol Oncol. 37:e486–e489. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jeyakumar A, Chalas E and Hindenburg A:
Sustained complete remission in a patient with platinum-resistant
ovarian yolk sac tumor. Gynecol Oncol. 82:578–580. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eisen T, Boshoff C, Mak I, Sapunar F,
Vaughan MM, Pyle L, Johnston SR, Ahern R, Smith IE and Gore ME:
Continuous low dose Thalidomide: A phase II study in advanced
melanoma, renal cell, ovarian and breast cancer. Br J Cancer.
82:812–817. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Abramson N, Stokes PK, Luke M, Marks AR
and Harris JM: Ovarian and papillary-serous peritoneal carcinoma:
Pilot study with thalidomide. J Clin Oncol. 20:1147–1149. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gordinier ME, Dizon DS, Weitzen S,
Disilvestro PA, Moore RG and Granai CO: Oral thalidomide as
palliative chemotherapy in women with advanced ovarian cancer. J
Palliat Med. 10:61–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang MM, Chan JK, Husain A, Guo HY and
Teng NN: Safety and efficacy of lenalidomide (Revlimid) in
recurrent ovarian and primary peritoneal carcinoma. Gynecol Oncol.
105:194–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chan JK, Manuel MR, Ciaravino G, Cheung
MK, Husain A and Teng NN: Safety and efficacy of thalidomide in
recurrent epithelial ovarian and peritoneal carcinoma. Gynecol
Oncol. 103:919–923. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Downs LS Jr, Judson PL, Argenta PA, Ghebre
R, Geller MA, Bliss RL, Boente MP, Nahhas WA, Abu-Ghazaleh SZ, Chen
MD and Carson LF: A prospective randomized trial of thalidomide
with topotecan compared with topotecan alone in women with
recurrent epithelial ovarian carcinoma. Cancer. 112:331–339. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hurteau JA, Brady MF, Darcy KM, McGuire
WP, Edmonds P, Pearl ML, Ivanov I, Tewari KS, Mannel RS, Zanotti K
and Benbrook DM: Randomized phase III trial of tamoxifen versus
thalidomide in women with biochemical-recurrent-only epithelial
ovarian, fallopian tube or primary peritoneal carcinoma after a
complete response to first-line platinum/taxane chemotherapy with
an evaluation of serum vascular endothelial growth factor (VEGF): A
gynecologic oncology group study. Gynecol Oncol. 119:444–450. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Carter JS and Downs LS Jr: A prospective
clinical trial of lenalidomide with topotecan in women with
advanced epithelial ovarian carcinoma. Int J Clin Oncol.
16:666–670. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Muthuramalingam SR, Braybrooke JP, Blann
AD, Madhusudan S, Wilner S, Jenkins A, Han C, Kaur K, Perren T and
Ganesan TS: A prospective randomised phase II trial of thalidomide
with carboplatin compared with carboplatin alone as a first-line
therapy in women with ovarian cancer, with evaluation of potential
surrogate markers of angiogenesis. Eur J Gynaecol Oncol.
32:253–258. 2011.PubMed/NCBI
|
|
49
|
Ganesan P, Piha-Paul S, Naing A, Falchook
G, Wheler J, Fu S, Hong DS, Kurzrock R, Janku F, Laday S, et al:
Phase I clinical trial of lenalidomide in combination with
sorafenib in patients with advanced cancer. Invest New Drugs.
32:279–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Selle F, Sevin E, Ray-Coquard I, Mari V,
Berton-Rigaud D, Favier L, Fabbro M, Lesoin A, Lortholary A and
Pujade-Lauraine E: A phase II study of lenalidomide in
platinum-sensitive recurrent ovarian carcinoma. Ann Oncol.
25:2191–2196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tilluckdharry L, Dean R, Farver C and
Ahmad M: Thalidomide-related eosinophilic pneumonia: A case report
and brief literature review. Cases J. 1:1432008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gordinier ME and Dizon DS: Dyspnea during
thalidomide treatment for advanced ovarian cancer. Ann
Pharmacother. 39:962–965. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Santi RM, Ceccarelli M, Catania G,
Monagheddu C, Evangelista A, Bernocco E, Monaco F, Federico M,
Vitolo U, Cortelazzo S, et al: PO-03 - Khorana score and histotype
predict the incidence of early venous thromboembolism (VTE) in non
hodgkin lymphoma (NHL). A pooled data analysis of twelve clinical
trials of fondazione italiana linfomi (FIL). Thromb Res. 140:(Suppl
1). S1772016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Joerger M, Schaer-Thuer C, Koeberle D,
Matter-Walstra K, Gibbons-Marsico J, Diem S, Thuerlimann B and
Cerny T: Off-label use of anticancer drugs in eastern Switzerland:
A population-based prospective cohort study. Eur J Clin Pharmacol.
70:719–725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dasanu CA, Mewawalla P and Grabska J:
Multiple myeloma and its therapies: to what extent do they
contribute to the increased incidence of second malignant
neoplasms? Curr Med Res Opin. 28:1129–1140. 2012. View Article : Google Scholar : PubMed/NCBI
|