Introduction
Colorectal cancer is one of the most common types of
cancer worldwide (1). Despite
advances in diagnostic and therapeutic strategies, clinical
outcomes and prognoses for patients with colorectal cancer remain
unsatisfactory (2). Therefore, the
identification of molecular markers for the more aggressive
colorectal tumor phenotypes is required, to allow patient treatment
to be adjusted accordingly. However, predictive molecular
indicators of regional disease invasion and metastasis are not well
defined.
Nuclear receptors (NRs) are ligand-activated
transcription factors that control the expression of genes involved
in nearly all aspects of development, physiology, and disease
(3). The majority of NRs are
receptors for small lipophilic ligands (metabolites, hormones,
drugs, and environmental compounds) that directly modulate their
transcriptional activities (4). The
NRs liver X receptor α (LXRα) and β are key regulators of lipid,
cholesterol and carbohydrate metabolism and homeostasis (5). They function as transcription factors by
heterodimerizing with retinoid X receptor (RXR) and increasing the
expression of target genes that encode proteins implicated in lipid
metabolism, particularly in cholesterol efflux and fatty acid
synthesis (6). Cholesterol controls
cell proliferation; disruptions in cholesterol metabolism are
associated with the development of colon cancer (7). Previous studies have indicated that LXRs
may couple cholesterol homeostasis to proliferation (8–14).
Synthetic (compounds T0901317 and GW3965) and natural
(22[R]-hydroxycholesterol and 24[S]-hydroxycholesterol) LXR ligands
suppress the proliferation of a number of human cancer cell lines,
including prostate, breast, colon, ovarian and leukemia cancer
cells (8–14). Furthermore, downregulation of the
S-phase-associated kinase protein-2 (Skp2) component of ubiquitin
ligase, which regulates p27Kip1 degradation (15) and the resulting p27Kip1
protein stabilization and retinoblastoma protein dephosphorylation,
may contribute to the inhibition of cell proliferation (16). In addition, LXRs inhibit the
proliferation of human colorectal cancer cells and the growth of
intestinal tumors in mice (7).
RXR is an NR family member that has been implicated
in cancer chemoprevention (17,18). RXRα
expression is decreased in mouse skin tumors (19), whereas RXRβ expression is increased in
non-small cell lung tumors (20)
compared with healthy tissue. However, the clinical significance of
RXR in colorectal cancer remains unclear.
Sterol regulatory element-binding protein-1c
(SREBP-1c) is a transcriptional intermediary for the insulin
stimulation of fatty acid synthase (FAS) gene expression (21). Induction of FAS expression and the
consequential enhanced fatty acid synthesis is required for
neoplastic transformation and tumor progression (22). SREBP-1 may be implicated in
tumorigenesis, as the high expression of SREBP-1 is reported to
predict a poor prognosis in patients with pancreatic cancer
(23).
The orphan NR chicken ovalbumin upstream
promoter-transcription factor II (COUP-TFII) is involved in the
regulation of gene expression (24,25),
development, differentiation, and homeostasis (26); however, its role in cancer is debated
as contradictory tumor-suppressive and oncogenic capacities have
been reported (27,28). Increased expression of COUP-TFII was
shown to enhance the invasiveness of human lung carcinoma cells
(27). By contrast, a previous report
demonstrated that overexpression of COUP-TFII in MDA-MB-435 breast
cancer cells led to reduced growth and plating efficiency (28). The prognostic significance of high or
low expression of COUP-TFII appears to vary; its expression may be
a favorable (e.g., ovarian and colon cancer) or an unfavorable
(e.g., breast and prostate cancer) prognostic factor in patients
with different types of cancer, and its expression is
tumor-specific (29). The underlying
mechanisms that trigger altered expression of this gene in
individual tumors remains poorly understood (30–32).
According to a previous study, patients with COUP-TFII-positive
tumors had a significantly higher 3-year overall survival (OS) rate
compared with the COUP-TFII-negative group (33). However, the follow-up period was short
and few patients with colorectal cancer were included in the study.
Therefore, in the present study, the aim was to investigate the
association between COUP-TFII expression and clinicopathological
factors further and to confirm its prognostic significance in a
larger number of patients with colorectal cancer. The association
between LXR, RXRα, and SREBP-1c expression and clinicopathological
factors was also assessed in the study participants.
Materials and methods
Patients and tissue samples
Consecutive patients with colorectal cancer who were
eligible and underwent surgery at Dong-A University Hospital
between March 2002 and July 2011 (n=707) were enrolled in the
study, including 403 males (age range, 29.0–87.0 years; mean age,
61.8 years) and 304 females (age range, 22.0–84.0 years; mean age,
62.1 years). Tissue samples from the patients were formalin-fixed
and paraffin-embedded. Patients with familial adenomatous polyposis
or inflammatory bowel disease or synchronous colorectal or
extracolorectal cancer, and those lost to follow-up, were excluded.
None of the patients had a family history of colorectal cancer, and
none had received preoperative chemotherapy or radiotherapy.
Information concerning age, sex, histological grade and
Tumor-Node-Metastasis (TNM) stage (34) was retrieved by reviewing pathological
and surgical reports. The present study was approved by the
institutional review board of Dong-A University (Busan, Korea;
approval no., 2-104709-AB-N-01-201504-BR-004-02).
Tissue microarrays and
immunohistochemistry
Cores (1 mm) were removed from colorectal cancer
samples that had previously been formalin-fixed and
paraffin-embedded. For all arrays, three cores from different areas
of the tumor were collected and placed in a new blank recipient
paraffin block, according to a previously described method
(35). Sections were deparaffinized
using a series of xylene baths; rehydration was performed using a
series of graded alcohol solutions. Sections (4-µm thick) were used
for immunohistochemical staining. To enhance immunoreactivity,
microwave antigen retrieval was performed at 750 W for 30 min in
Tris EDTA (pH 9.0). Subsequent to blocking endogenous peroxidase
activity with 5% hydrogen peroxidase for 10 min, incubation with
the primary antibody was performed for 1 h at room temperature. The
primary antibodies used in immunostaining included a mouse
monoclonal antibody directed against COUP-TFII (clone H7147;
catalog no., PP-H7147-00; 1:100; Perseus Proteomics Inc., Tokyo,
Japan), a rabbit polyclonal antibody directed against LXRα/β (clone
S-20; catalog no., sc-1000; 1:400; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), a mouse monoclonal antibody directed against RXRα
(clone F-1; catalog no., sc-46659; 1:50; Santa Cruz Biotechnology,
Inc.), and a rabbit polyclonal antibody directed against SREBP-1
(clone H-160; catalog no., sc-8984; 1:100; Santa Cruz
Biotechnology, Inc.). An Envision™Chem™ Detection kit
(DakoCytomation, Carpinteria, CA, USA) was used for the secondary
antibody at room temperature for 30 min. After washing the tissue
samples in TBS for 10 min, 3,3′-diaminobenzidine was used as a
chromogen, and then Mayer's hematoxylin counterstain was applied
for 1 min at room temperature. Archival, 10% formalin-fixed (for
18–48 h at room temperature), paraffin-embedded human normal
kidney, thyroid, skin and testis tissues (obtained from tissue
archives at Dong-A University Hospital) were used as positive
controls for COUP-TFII, LXRα/β, RXRα, and SREBP-1c, according to
the antibody manufacturer's protocol. A negative control was
obtained by substituting the primary antibody with buffer.
Immunohistochemical assessment
The percentage and intensity of immunoreactive tumor
cells in each core were recorded, and the final value of the
positive tumor cells was determined as the mean of the
immunoreactivity of the three cores. The presence of tumor tissue
in ≥2 interpretable cores was required for the inclusion of a case
in statistical analyses. All slides were independently evaluated by
two independent experienced pathologists (MSR and MGP) who were
blinded to clinicopathological data. There were only minor
discrepancies in the evaluation; slides with discrepancies between
evaluations were reevaluated under a multi-head microscope until a
consensus evaluation was obtained. The percentage of positive tumor
cells and the staining intensity (weak or strong) were assessed.
Staining intensity was scored visually and stratified as follows:
Negative, weak (if the staining appeared as a blush), or strong (if
it was markedly positive at 20x magnification).
For COUP-TFII, immunoreactivity was defined as cells
showing nuclear staining in the tumor tissue with minimal
background staining. Tumors with strong staining intensity in
>10% of tumor cells were recorded as having positive
immunoreactivity for COUP-TFII. For LXRα/β, immunoreactivity was
defined as cells exhibiting nuclear staining with/without
cytoplasmic staining patterns in the tumor tissue with minimal
background staining; tumors with strong staining intensity in
>10% of the tumor cells were recorded as having positive
immunoreactivity for LXRα/β. For RXRα, immunoreactivity was defined
as cells exhibiting nuclear staining in the tumor tissue with
minimal background staining. Cases were divided into those with
weak or strong RXRα expression according to staining intensity,
since immunoreactivity was typically evenly distributed within a
tumor sample, but varied in intensity. For SREBP-1c,
immunoreactivity was defined as cells exhibiting nuclear staining
with/without cytoplasmic staining patterns in the tumor tissue with
minimal background staining; tumors with a strong staining
intensity in >10% of tumor cells were recorded as having
positive immunoreactivity for SREBP-1c.
Statistical analysis
The χ2 test was used to analyze differences in
clinical characteristics and immunohistochemically-assessed
expression levels. Survival curves were calculated by the
Kaplan-Meier method, and comparisons of survival curves were made
with the log-rank test. Multiple analyses were performed with the
Cox proportional hazards model to assess the association of
COUP-TFII, LXR, RXRα, and SREBP-1c expression with the OS rate.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed with SAS 9.4
software (SAS Institute, Cary, NC, USA).
Results
Expression of COUP-TFII, LXR, RXRα,
and SREBP-1c in human colorectal cancer tissues
Our previous study revealed expression of COUP-TFII
in 55/95 (57.89%) colorectal carcinoma tissue specimens (33). To confirm the expression pattern of
COUP-TFII in a larger number of patients with human colorectal
carcinoma, immunohistochemistry was performed with an antibody
against COUP-TFII. Positive COUP-TFII expression was observed in
231/707 (32.7%) colorectal carcinoma tissue specimens.
Immunostaining occurred predominantly in the nuclei of tumor cells
(Fig. 1). The expression levels of
LXR, RXRα, and SREBP-1c were also assessed in human colorectal
tumors by immunohistochemistry. Positive expression of LXR and
SREBP-1c was observed in 360 (50.9%) and 295 (41.7%) of the 707
colorectal carcinoma tissue specimens, respectively (Fig. 1; Table
I). Positive expression of RXRα was observed in 399/704 (56.4%)
colorectal carcinoma tissue specimens (Fig. 1 and Table
I). Core tissue was lost during the preparation of three
colorectal carcinoma tissue specimens.
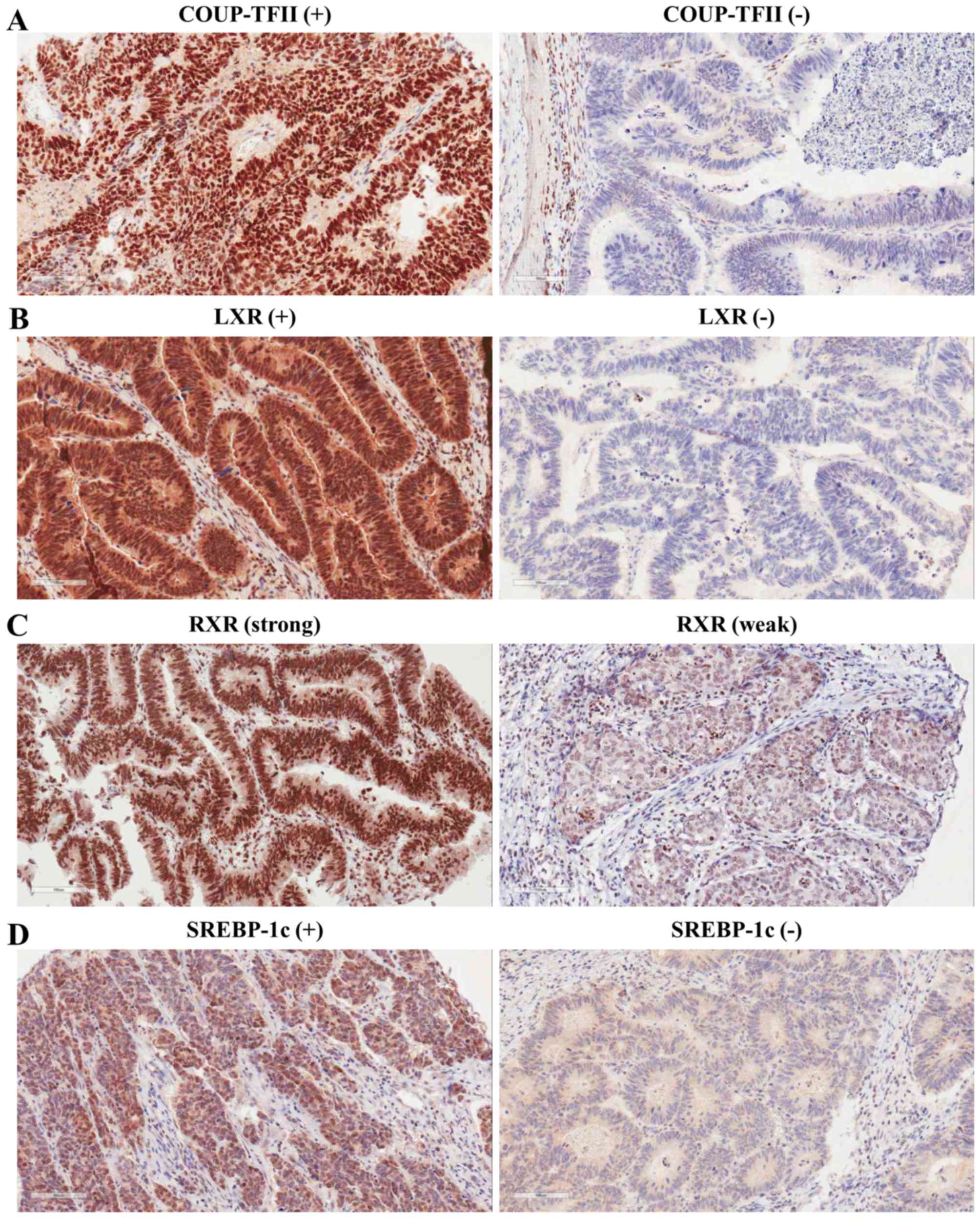 | Figure 1.Representative images of
immunohistochemical staining for COUP-TFII, LXR, RXRα, and SREBP-1c
in colorectal cancer tissue. (A) Left, COUP-TFII-positive
colorectal cancer tissue. Right, COUP-TFII-negative colorectal
cancer tissue. (B) Left, LXR-positive colorectal cancer tissue.
Right, LXR-negative colorectal cancer tissue. (C) Left, strong RXRα
immunoreactivity detected in well-differentiated colorectal cancer
tissues. Right, weak RXRα immunoreactivity detected in poorly
differentiated colorectal cancer tissues. (D) Left,
SREBP-1c-positive colorectal cancer tissue. Right,
SREBP-1c-negative colorectal cancer tissue. Magnification, ×200.
COUP-TFII, chicken ovalbumin upstream promoter-transcription factor
II; LXR, liver X receptor; RXRα, retinoid X receptor α; SREBP-1c,
sterol regulatory element binding protein-1c. |
 | Table I.Clinical characteristics and
immunohistochemistry expressions of the study participants
(n=707). |
Table I.
Clinical characteristics and
immunohistochemistry expressions of the study participants
(n=707).
| Variable | Patients, n (%) |
|---|
| Sex |
|
| Male | 403 (57.0) |
|
Female | 304 (43.0) |
| Age, years |
|
|
<65 | 392 (55.5) |
|
≥65 | 315 (44.6) |
| Grade |
|
| 1 | 398 (56.3) |
| 2 | 262 (37.1) |
|
3+4 | 47 (6.7) |
| Tumor size, cm |
|
|
<5 | 247 (34.9) |
| ≥5 | 460 (65.1) |
| Vascular
invasion |
|
|
Negative | 605 (85.6) |
|
Positive | 102 (14.4) |
| TNM stage |
|
|
0+I | 95 (13.4) |
| II | 295 (41.7) |
|
III+IV | 317 (44.8) |
| COUP-TEII
expression |
|
|
Negative | 476 (67.3) |
|
Positive | 231 (32.7) |
| LXR expression |
|
|
Negative | 347 (49.1) |
|
Positive | 360 (50.9) |
| RXRα
expression |
|
| No
dataa | 3 (0.4) |
|
Negative | 305 (43.1) |
|
Positive | 399 (56.4) |
| SREBP-1c
expression |
|
|
Negative | 412 (58.3) |
|
Positive | 295 (41.7) |
To evaluate the associations between COUP-TFII
expression and LXR, RXRα, and SREBP-1c expression in colorectal
cancer, the χ2 test was used. In 70.7% (163/231) of
patient samples in which COUP-TFII was expressed, LXR was also
expressed (P<0.0001). In 64.1% (148/229) of patient samples in
which COUP-TFII was expressed, RXRα was also expressed (P=0.0035).
In 49.8% (115/231) of patient samples in which COUP-TFII was
expressed, SREBP-1c was also expressed (P=0.0027; Table II). These data suggest that COUP-TFII
expression is positively associated with LXR, RXRα and SREBP-1c
expression.
 | Table II.Differential distribution of LXR,
RXRα, and SREBP-1c according to COUP-TFII expression. |
Table II.
Differential distribution of LXR,
RXRα, and SREBP-1c according to COUP-TFII expression.
|
| LXR expression, n
(%) | RXRα
expressionb, n (%) | SREBP-1c
expression, n (%) |
|---|
|
|
|
|
|
|---|
| COUP-TFII
expression | Negative | Positive | Negative | Positive | Negative | Positive |
|---|
| Negative | 279 (58.6) | 197 (41.4) | 224 (47.1) | 251 (52.7) | 296 (62.2) | 180 (37.8) |
| Positive | 68 (29.4) | 163 (70.7) | 81 (35.1) | 148 (64.1) | 116 (50.2) | 115 (49.8) |
|
P-valuea | <0.0001 | 0.0035 | 0.0027 |
Associations between the expression of
COUP-TFII, LXR, RXRα and SREBP-1c, and clinicopathological
features
Following the analysis of COUP-TFII, LXR, RXRα and
SREBP-1c staining in tumors, the χ2 test was used to evaluate the
association between COUP-TFII, LXR, RXRα and SREBP-1c expression,
and the clinicopathological features of the study population. As
shown in Table III, there was a
significant association of vascular invasion (P=0.0184) and the TNM
stage (P=0.0215) with COUP-TFII expression, and samples that
exhibited vascular invasion and higher TNM stages tended to be
COUP-TFII-negative. No significant association was identified
between COUP-TFII expression and the patient's age or sex, or the
tumor size or grade (Table III).
There was a significant negative association between LXR expression
and vascular invasion (P=0.0334). No significant association was
found between LXR expression and the patient's age or sex, the
tumor size or grade, or the TNM stage (Table III). There was a significant
association between tumor grade (P=0.0160) and RXRα expression,
with high grades tending to be RXRα-negative. No significant
association was identified between RXRα expression and age, sex,
TNM stage, tumor size or vascular invasion (Table III). No significant association was
identified between SREBP-1c expression and age at the time of
surgery, sex, size, grade, TNM stage or vascular invasion (Table III).
 | Table III.Univariate analysis of the
associations between clinical characteristics and COUP-TFII, LXR,
RXRα, and SREBP-1c expression. |
Table III.
Univariate analysis of the
associations between clinical characteristics and COUP-TFII, LXR,
RXRα, and SREBP-1c expression.
|
| COUP-TFII | LXR | RXRα | SREBP-1c |
|---|
|
|
|
|
|
|
|---|
| Variable | Positive, n
(%) | P-value | Positive, n
(%) | P-value | Positive, n
(%) | P-value | Positive, n
(%) | P-value |
|---|
| Sex |
| 0.3883 |
| 0.7376 |
| 0.7935 |
| 0.4272 |
|
Male | 137 (34.0) |
| 203 (50.4) |
| 225 (56.3) |
| 163 (40.5) |
|
|
Female | 94 (30.9) |
| 157 (51.6) |
| 174 (57.2) |
| 132 (43.4) |
|
| Age, years |
| 0.8618 |
| 0.0790 |
| 0.8739 |
| 0.4837 |
|
<65 | 127 (32.4) |
| 188 (48.0) |
| 220 (56.4) |
| 159 (40.6) |
|
|
≥65 | 104 (33.0) |
| 172 (54.6) |
| 179 (57.0) |
| 136 (43.2) |
|
| Grade |
| 0.2914 |
| 0.2749 |
| 0.0160 |
| 0.7015 |
| 1 | 134 (33.7) |
| 196 (49.3) |
| 240 (60.5) |
| 171 (43.0) |
|
| 2 | 78 (29.8) |
| 143 (54.6) |
| 141 (53.8) |
| 104 (39.7) |
|
|
3+4 | 19 (40.4) |
| 21 (44.7) |
| 18 (40.0) |
| 20 (42.6) |
|
| Tumor size, cm |
| 0.6494 |
| 0.6620 |
| 0.2653 |
| 0.0537 |
|
<5 | 153 (33.3) |
| 237 (51.5) |
| 266 (58.2) |
| 204 (44.4) |
|
| ≥5 | 78 (31.6) |
| 123 (49.8) |
| 133 (53.9) |
| 91 (36.8) |
|
| Vascular
invasion |
| 0.0184 |
| 0.0334 |
| 0.6958 |
| 0.7546 |
|
Negative | 208 (34.4%) |
| 42 (41.2) |
| 343 (57.0) |
| 44 (43.1) |
|
|
Positive | 23 (22.6%) |
| 318 (52.6) |
| 56 (54.9) |
| 251 (41.5) |
|
| TNM stage |
| 0.0215 |
| 0.3616 |
| 0.5857 |
| 0.6859 |
|
0+I | 38 (40.0) |
| 51 (53.7) |
| 51 (53.7) |
| 36 (37.9) |
|
| II | 106 (35.9) |
| 157 (53.2) |
| 173 (58.8) |
| 123 (41.7) |
|
|
III+IV | 87 (27.4) |
| 152 (48.0) |
| 175 (55.6) |
| 136 (42.9) |
|
Association of COUP-TFII and LXR
expression with good prognosis in colorectal cancer patients
To assess whether COUP-TFII expression is a
significant prognostic factor for the survival of patients with
surgically resected colorectal carcinoma, a log-rank test was used
with Kaplan-Meier survival curves. The median follow-up duration
was 63.39 months. Of the 707 patients analyzed, the patients
positive for COUP-TFII expression (231 patients) had a
significantly higher OS rate than those negative for COUP-TFII
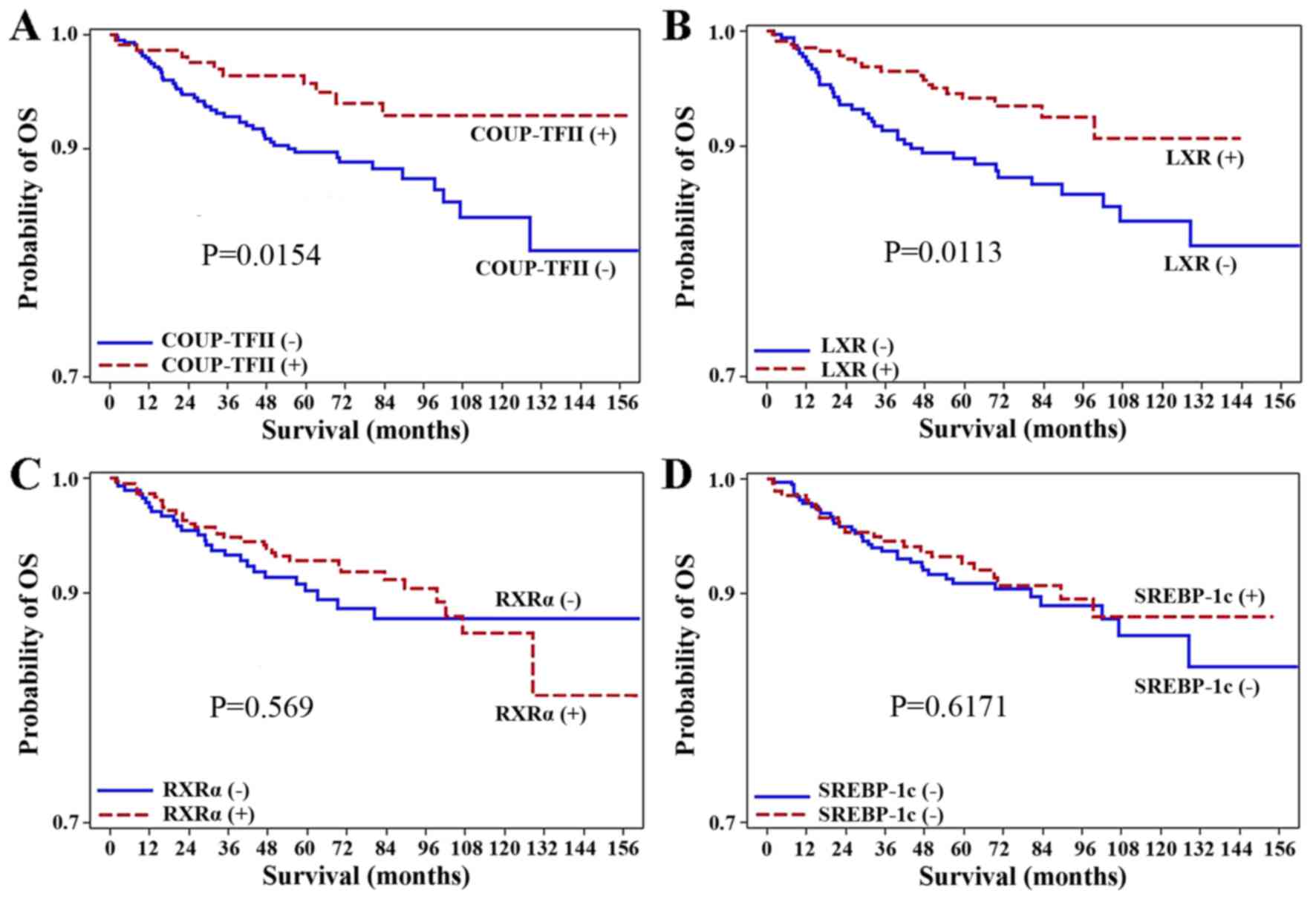
expression (P=0.0154; Fig. 2).
Similarly, the positive expression of LXR was associated with
better OS rate (P=0.0113; Fig. 2).
However, the positive expression of RXRα and SREBP-1c were not
associated with the OS rate (P=0.569, P=0.6171, respectively).
Additionally, Cox proportional hazards regression analysis revealed
that the negative expression of LXR [hazard ratio (HR), 1.99; 95%
confidence interval (CI), 1.16–3.42; P=0.0130] or COUP-TFII (HR,
2.20; 95% CI, 1.14–4.24; P=0.0182) were associated with
significantly worse prognoses than the positive expression of LXR
or COUP-TFII (Tables IV and V).
 | Table IV.Crude HRs for COUP-TFII, LXR, RXRα,
and SREBP-1c expression. |
Table IV.
Crude HRs for COUP-TFII, LXR, RXRα,
and SREBP-1c expression.
| Expression
status | HR | 95% CI | P-value |
|---|
| COUP-TFII |
|
|
|
|
Negative | 2.20 | 1.14, 4.24 | 0.0182 |
|
Positive | ref. |
|
|
| LXR |
|
|
|
|
Negative | 1.99 | 1.16, 3.42 | 0.0130 |
|
Positive | ref. |
|
|
| RXRα |
|
|
|
|
Negative | 1.16 | 0.69, 1.95 | 0.5693 |
|
Positive | ref. |
|
|
| SREBP-1c |
|
|
|
|
Negative | 1.14 | 0.68, 1.93 | 0.6174 |
|
Positive | ref. |
|
|
| COUP-TFII +
LXR |
|
|
|
|
C(−)L(−) | 2.43 | 1.17, 5.04 | 0.0171 |
|
C(−)L(+) | 1.05 | 0.43, 2.53 | 0.9201 |
|
C(+)L(−) | 0.50 | 0.11, 2.33 | 0.3800 |
|
C(+)L(+) | ref. |
|
|
|
 | Table V.HRs for COUP-TFII, LXR, or RXRα
expression and clinical characteristics. |
Table V.
HRs for COUP-TFII, LXR, or RXRα
expression and clinical characteristics.
|
|
|
|
|
|
|
|
|
|
| Multiple
models |
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
|
| Crude model | COUP-TFII | LXR | RXRα | SREBP-1c |
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Expression |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Negative |
|
|
| 2.16 | 1.11–4.23 | 0.0243 | 1.83 | 1.06–3.17 | 0.0306 | 1.16 | 0.69–1.95 | 0.5822 | 1.20 | 0.71–2.03 | 0.5076 |
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Male | 1.80 | 1.03–3.13 | 0.0381 | 1.88 | 1.08–3.29 | 0.0266 | 1.80 | 1.03–3.13 | 0.0399 | 1.76 | 1.00–3.07 | 0.0490 | 1.81 | 1.03–3.15 | 0.0378 |
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<65 | 1.48 | 0.85–2.58 | 0.1641 | 1.39 | 0.80–2.43 | 0.2425 | 1.33 | 0.76–2.33 | 0.3130 | 1.37 | 0.78–2.40 | 0.2678 | 1.39 | 0.80–2.43 | 0.2441 |
| Grade |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3+4 | 1.69 | 0.66–4.44 | 0.2746 | 1.89 | 0.72–4.95 | 0.1954 | 1.57 | 0.61–4.07 | 0.3521 | 1.30 | 0.46–3.73 | 0.6213 | 1.56 | 0.60–4.03 | 0.3601 |
| 2 | 1.19 | 0.69–2.04 | 0.5370 | 0.95 | 0.55–1.65 | 0.8519 | 0.98 | 0.56–1.71 | 0.9370 | 0.92 | 0.53–1.62 | 0.7826 | 0.93 | 0.54–1.62 | 0.8014 |
| Tumor size |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ≥5
cm | 1.30 | 0.75–2.26 | 0.3591 | 1.19 | 0.67–2.12 | 0.5459 | 1.19 | 0.67–2.11 | 0.5454 | 1.16 | 0.66–2.07 | 0.6057 | 1.19 | 0.67–2.12 | 0.5480 |
| Vascular
invasion |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Positive | 2.11 | 1.16–3.84 | 0.0151 | 1.53 | 0.82–2.86 | 0.1839 | 1.56 | 0.84–2.90 | 0.1636 | 1.73 | 0.93–3.21 | 0.0844 | 1.68 | 0.91–3.11 | 0.0983 |
| TNM stage |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
III+IV | 4.29 | 1.32–13.95 | 0.0154 | 3.41 | 1.02–11.46 | 0.0473 | 3.55 | 1.06–11.91 | 0.0406 | 3.52 | 1.04–11.88 | 0.0425 | 3.56 | 1.06–11.96 | 0.0405 |
| II | 2.25 | 0.67–7.58 | 0.1900 | 1.93 | 0.55–6.71 | 0.3017 | 1.96 | 0.57–6.81 | 0.2872 | 1.99 | 0.57–6.96 | 0.2792 | 1.93 | 0.55–6.74 | 0.3014 |
Prognostic significance of
combinations of COUP-TFII and LXR expression
The aforementioned results indicated that COUP-TFII
or LXR expression may be positive prognostic factors for patients
with colorectal cancer. Therefore, the OS rate for patients with
LXR- and COUP-TFII-positive immunostaining was compared with that
of patients with LXR- and COUP-TFII-negative immunostaining.
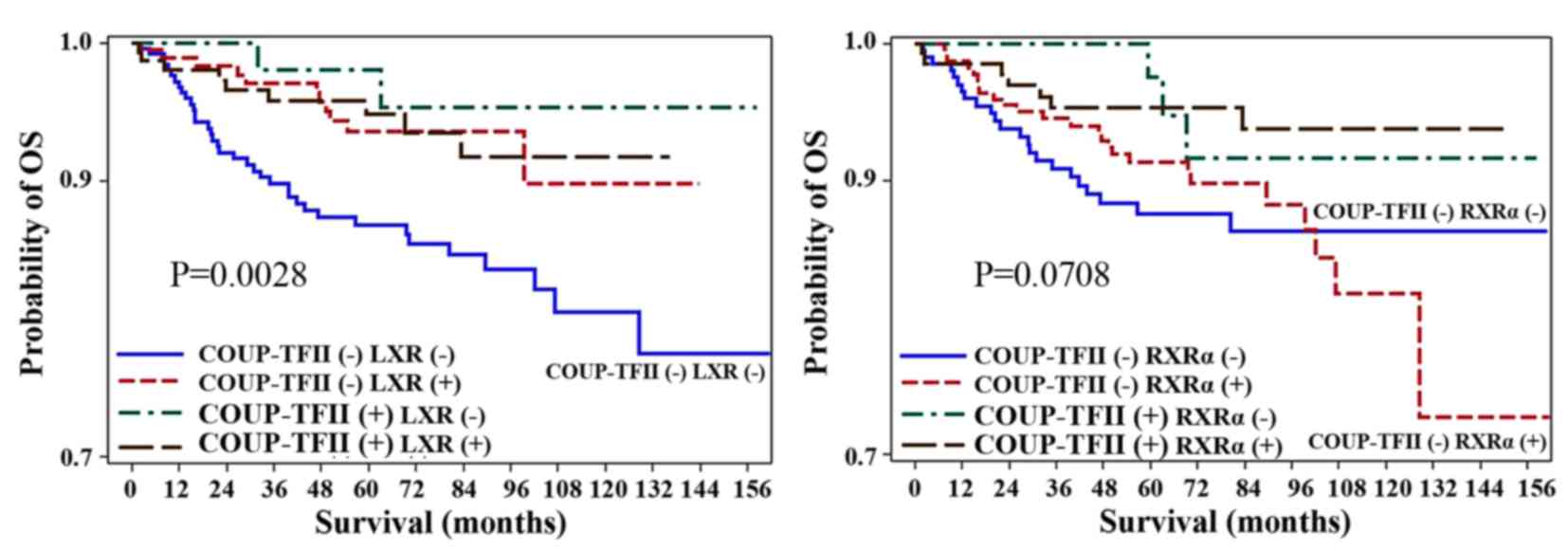
Patients with LXR- and COUP-TFII-positive immunostaining had a
significantly higher OS rate than those with LXR- and
COUP-TFII-negative immunostaining (P=0.0028; Fig. 3). Additionally, Cox proportional
hazards regression analysis revealed that the negative expression
of LXR and COUP-TFII was associated with a significantly worse
prognosis (HR, 2.43; 95% CI, 1.17–5.04; P=0.0171) compared with the
positive expression of LXR and COUP-TFII (Tables IV and VI).
 | Table VI.Adjusted HRs for combinations of
COUP-TFII and LXR expression. |
Table VI.
Adjusted HRs for combinations of
COUP-TFII and LXR expression.
|
| COUP-TFII +
LXR |
|---|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value |
|---|
| C(−)L(−) | 2.33 | 1.11–4.89 | 0.0256 |
| C(−)L(+) | 1.15 | 0.47–2.80 | 0.7597 |
| C(+)L(−) | 0.54 | 0.12–2.50 | 0.4287 |
| C(+)L(+) | ref. |
|
|
| Sex |
|
Male | 1.86 | 1.06–3.26 | 0.0299 |
| Age, years |
|
|
|
|
<65 | 1.36 | 0.78–2.37 | 0.2828 |
| Grade |
|
|
|
3+4 | 1.84 | 0.70–4.82 | 0.2128 |
| 2 | 0.97 | 0.56–1.69 | 0.9240 |
| Tumor size, cm |
|
|
| ≥5 | 1.20 | 0.68–2.13 | 0.5227 |
| Vascular
invasion |
|
|
|
Positive | 1.38 | 0.74–2.60 | 0.3144 |
| TNM stage |
|
|
|
III+IV | 3.28 | 0.98–11.02 | 0.0546 |
| II | 1.85 | 0.53–6.41 | 0.3338 |
Discussion
The identification of biomarkers for predicting the
prognosis of colorectal cancer will aid the adjustment of
therapeutic strategies to individual patients. Cholesterol is a
known risk factor for patients with colorectal cancer. There are
several transcription factors involved in cholesterol homeostasis,
including LXR, RXRα and SREBP-1c. The role served by LXR in
carcinogenesis was investigated in several tumor types in previous
studies (8–16). The tumor-protective actions of LXR
were revealed in a previous study, which revealed that the
ligand-induced activation of LXR or transfection with LXRα blocked
entry into G1 phase, increased caspase-dependent apoptosis and
slowed the growth of xenograft tumors in mice (7). Gene expression analysis revealed that
the activation of LXRα affected lipid metabolic networks and
increased cholesterol efflux in the intestine (7). However, to the best of our knowledge,
the clinical significance of LXR expression in colorectal cancer
has not been previously investigated.
In the present study, LXR expression was observed in
50.9% of colorectal cancer patients and was associated with
favorable clinical outcomes, such as improved OS rates and lack of
vascular invasion. However, it was not possible to discriminate
between the expression of LXRα or β in the present study, as an
anti-LXRα/β antibody was used. A future study will determine which
type of LXR is more predictive of the prognosis in colorectal
cancer. To the best of our knowledge, the present study is the
first to demonstrate that LXR can be a positive prognostic factor
for colorectal cancer, although there are several reports
demonstrating that ligands of LXR inhibit cell proliferation in a
number of cancer cell lines (8–14).
RXR has been implicated in cancer chemoprevention
(17,18). However, the clinical significance of
RXRα in colorectal cancer remains unknown. RXRα expression was
observed in 56.4% of colorectal cancer patients in the present
study and it was inversely associated with tumor grade. Further
studies using RNA interference, or transfection with RXRα and LXR,
are required to reveal the association between OS rates and LXR
expression in patients with colorectal cancer.
No associations were found between expression of
SREBP-1c and clinicopathological characteristics; this result is
different from another study, in which the high expression of
SREBP-1 predicted a poor prognosis for patients with pancreatic
cancer (23). The different roles of
SREBP-1c in cancer may depend on the tumor type.
In the present study, COUP-TFII expression was
associated with an improved OS rate in a large cohort of patients
with colorectal cancer with a long follow-up period. Our previous
study revealed that COUP-TFII expression was not associated with
lymph node metastasis or vascular invasion; this result may have
been due to the relatively small number of patients with colorectal
cancer who were included in the study (33). COUP-TFII expression was significantly
negatively associated with vascular invasion and TNM stage; these
results are similar to those of another study, which identified
that high COUP-TFII transcript levels were associated with
increased survival time, and that its expression inhibits the
transforming growth factor-β (TGF-β)-dependent
epithelial-mesenchymal transition (EMT) in breast cancer (36). In this study, the expression of TGF-β
in patients with colorectal cancer was not examined. However, we
hypothesize that there will be the downregulation of TGFβ in
COUP-TFII-positive tumors. Our future study may investigate the
expression of TGF-β and genes involved in EMT in patients with
colorectal cancer.
Several studies have demonstrated that COUP-TFII is
involved in cancer progression and metastasis (27,37,38). A
recent study described the positive regulation of Snail1 by
COUP-TFII, with the consequent downregulation of E-cadherin in
colon cancer cell lines (37). The
discrepancies between the results of the present study and those of
Bao et al (37) may be due to
other proteins associated with COUP-TFII in different cell lines,
the sample size, and the genetic background of patients with
colorectal cancer who were included in the studies. Although
extensive studies have been performed recently (36–38),
uncertainties remain concerning the role of COUP-TFII in cancer.
Further studies using COUP-TFII-knockdown or overexpression are
required to identify why the expression of COUP-TFII is negatively
associated with TNM stage or vascular invasion in colorectal
cancer.
Patients with LXR- and COUP-TFII-positive
immunostaining had significantly better OS rates than those with
LXR- and COUP-TFII-negative immunostaining. These data suggest that
immunostaining for LXR and COUP-TFII in colorectal cancer samples
at diagnosis may aid the prediction of the prognosis of patients
with colorectal cancer.
The present study has limitations that should be
noted. First, mortality during the study may have been too low to
yield statistically significant data about whether TNM stage and
vascular invasion were prognostic factors for patients in the
study. Second, it was not demonstrated which type of LXR is a more
important prognostic factor for colorectal cancer. Third, the
molecular mechanisms responsible for the observed improvement in OS
in patients with LXR-, or COUP-TFII-positive tumors have not been
identified.
In summary, the present study demonstrated that LXR
and COUP-TFII expression may be positive prognostic markers for
patients with colorectal cancer. The results of the current study
also suggest that the combined immunohistochemical examination of
LXR and COUP-TFII expression in diagnostic samples of colorectal
cancer may aid prognostic prediction. Future prospective and
mechanistic studies evaluating the molecular interactions of LXR
and COUP-TFII are required to confirm the findings of the present
study.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea, funded by the Korean Government
(grant no. 2016R1A5A2007009), and by the Basic Science Research
Program through the National Research Foundation of Korea, funded
by the Ministry of Science, ICT & Future Planning (grant no.
2016R1C1B2007429).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mangeksdorf DJ, Thummel C, Beato M,
Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M,
Chambon P and Evans RM: The nuclear receptor superfamily: The
second decade. Cell. 83:835–839. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McKenna NJ, Cooney AJ, DeMayo FJ, Downes
M, Glass CK, Lanz RB, Lazar MA, Mangelsdorf DJ, Moore DD, Qin J, et
al: Minireview: Evolution of NURSA, the nuclear receptor signaling
atlas. Mol Endocrinol. 23:740–746. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chuu CP, Kokontis JM, Hilpakka RA and Liao
S: Modulation of liver X receptor signaling as novel therapy for
prostate cancer. J Biomed Sci. 14:543–553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wójcicka G, Jamroz-Wisniewska A,
Horoszewicz K and Beltowski J: Liver X receptors (LXRs). Part I:
Structure, function, regulation of activity, and role in lipid
metabolism. Postepy Hig Med Dows (Online). 61:736–759. 2007.
|
|
7
|
Lo Sasso G, Bovenga F, Murzili S,
Salvatore L, Di Tullio G, Martelli N, D'Orazio A, Rainaldi S, Vacca
M, Mangia A, et al: Liver X receptors inhibit proliferation of
human colorectal cancer cells and growth of intestinal tumors in
mice. Gastroenterology. 144(1497–1507): e1–e13. 2013.
|
|
8
|
Fukuchi J, Kokontis JM, Hiipakka RA, Chuu
CP and Liao S: Antiproliferative effect of liver X receptor
agonists on LNCaP human prostate cancer cells. Cancer Res.
64:7686–7689. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chuu CP, Hiipakka RA, Kokontis JM, Fukuchi
J, Chen RY and Liao S: Inhibition of tumor growth and progression
of LNCaP prostate cancer cells in athymic mice by androgen and
liver X receptor agonist. Cancer Res. 66:6482–6486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pommier AJ, Alves G, Viennois E, Bernard
S, Communal Y, Sion B, Marceau G, Damon C, Mouzat K, Caira F, et
al: Liver X receptor activation downregulates AKT survival
signaling in lipid rafts and induces apoptosis of prostate cancer
cells. Oncogene. 29:2712–2723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vedin LL, Lewandowski SA, Parini P,
Gustafsson JA and Steffensen KR: The oxysterol receptor LXR
inhibits proliferation of human breast cancer cells.
Carcinogenesis. 30:575–579. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uno S, Endo K, Jeong Y, Kawana K, Miyachi
H, Hashimoto Y and Makishima M: Suppression of beta-catenin
signaling by liver X receptor ligands. Biochem Pharmacol.
77:186–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scoles DR, Xu X, Wang H, Tran H,
Taylor-Harding B, Li A and Karlan BY: Liver X receptor agonist
inhibits proliferation of ovarian carcinoma cells stimulated by
oxidized low density lipoprotein. Gynecol Oncol. 116:109–116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Geyeregger R, Shehata M, Zeyda M, Kiefer
FW, Stuhlmeier KM, Porpaczy E, Zlabinger GJ, Jäger U and Stulnig
TM: Liver X receptors interfere with cytokine-induced proliferation
and cell survival in normal and leukemic lymphocytes. J Leukoc
Biol. 86:1039–1048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakayama K, Nagahama H, Minamishima YA,
Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R,
Tsukiyama T, Ishida N, et al: Targeted disruption of Skp2 results
in accumulation of cyclin E and (p27)Kip, polyploidy and centrosome
overduplication. EMBO J. 19:2069–2081. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blaschke F, Leppanen O, Takata Y, Caglayan
E, Liu J, Fishbein MC, Kappert K, Nakayama KI, Collins AR, Fleck E,
et al: Liver X receptor agonists suppress vascular smooth muscle
cell proliferation and inhibit neointima formation in
balloon-injured rat carotid arteries. Cir Res. 95:e110–e123. 2004.
View Article : Google Scholar
|
|
17
|
Fan YY, Spencer TE, Wang N, Moyer MP and
Chapkin RS: Chemopreventive n-3 fatty acids activate RXRalpha in
colonocytes. Carcinogenesis. 24:1541–1548. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang SY, Shen SR, Shyu RY, Yu JC, Harn
HJ, Yeh MY, Lee MM and Chang YC: Expression of nuclear retinoid
receptors in normal, premalignant and malignant gastric tissues
determined by in situ hybridization. Br J Cancer. 80:206–214. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar R, Shoemaker AR and Verma AK:
Retinoic acid nuclear receptors and tumor promotion: Decreased
expression of retinoic acid nuclear receptors by the tumor promoter
12-O-tetradecanoylphorbol-13-acetate. Carcinogenesis. 15:701–705.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu XC, Sozzi G, Lee JS, Lee JJ, Pastorino
U, Pilotti S, Kurie JM, Hong WK and Lotan R: Suppression of
retinoic acid receptor beta in non-small-cell lung cancer in vivo:
Implications for lung cancer development. J Natl Cancer Inst.
89:624–629. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang YA, Han WF, Morin PJ, Chrest FJ and
Pizer ES: Activation of fatty acid synthesis during neoplastic
transformation: Role of mitogen-activated protein kinase and
phosphatidylinositol 3-kinase. Exp Cell Res. 279:80–90. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Yu, Morin PJ, Han WF, Chen T, Bomman
DM, Gabrielson EW and Pizer ES: Regulation of fatty acid synthase
expression in breast cancer by sterol regulatory element binding
protein-1c. Exp Cell Res. 282:132–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun Y, He W, Luo M, Zhou Y, Chang G, Ren
W, Wu K, Li X, Shen J, Zhao X and Hu Y: SREBP1 regulates
tumorigenesis and prognosis of pancreatic cancer through targeting
lipid metabolism. Tumour Biol. 36:4133–4141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smirnov DA, Hou S, Liu X, Claudio E,
Siebenlist UK and Ricciardi RP: COUP-TFII is up-regulated in
adenovirus type 12 tumorigenic cells and is a repressor of MHC
class I transcription. Virology. 284:13–19. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Navab R, Wang Y, Chow YH, Wang A, Jankov
RP, Takamoto N, Tsai SY, Tsai MJ, Tanswell AK and Hu J: Regulation
of human Clara cell 10 kD protein expression by chicken ovalbumin
upstream promoter transcription factors (COUP-TFs). Am J Respir
Cell Mol Biol. 27:273–285. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai MJ and O'Malley BW: Molecular
mechanisms of action of steroid/thyroid receptor superfamily
members. Annu Rev Biochem. 63:451–486. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Navab R, Gonzalez-Santos JM, Johnston MR,
Liu J, Brodt P, Tsao MS and Hu J: Expression of chicken ovalbumin
upstream promoter-transcription factor II enhances invasiveness of
human lung carcinoma cells. Cancer Res. 64:5097–5105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakshatri H, Mendonca MS, Bhat-Nakshatri
P, Patel NM, Goulet RJ Jr and Cornetta K: The orphan receptor
COUP-TFII regulates G2/M progression of breast cancer cells by
modulating the expression/activity of p21(WAF1/CIP1), cyclin D1,
and cdk2. Biochem Biophys Res Commun. 270:1144–1153. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Safe S, Jin UH, Hedrick E, Reeder A and
Lee SO: Minireview: Role of orphan nuclear receptors in cancer and
potential as drug targets. Mol Endocrinol. 28:157–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Litchfield LM and Klinge CM: Multiple
roles of COUP-TFII in cancer initiation and progression. J Mol
Endocrinol. 49:R135–R148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qin J, Tsai SY and Tsai MJ: The critical
roles of COUP-TFII in tumor progression and metastasis. Cell
Biosci. 4:582014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boudot A, Le Dily F and Pakdel F:
Involvement of COUP-TFs in cancer progression. Cancers (Basel).
3:700–715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shin SW, Kwon HC, Rho MS, Choi HJ, Kwak JY
and Park JI: Clinical significance of chicken ovalbumin upstream
promoter-transcription factor II expression in human colorectal
cancer. Oncol Rep. 21:101–106. 2009.PubMed/NCBI
|
|
34
|
Lan YT, Yang SH, Chang SC, Liang WY, Li
AF, Wang HS, Jiang JK, Chen WS, Lin TC and Lin JK: Analysis of the
seventh edition of American Joint Committee on colon cancer
staging. Int J Colorectal Dis. 27:657–663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hsu FD, Nielsen TO, Alkushi A, Dupuis B,
Huntsman D, Liu CL, van de Rijn M and Gilks CB: Tissue microarrays
are an effective quality assurance tool for diagnostic
immunohistochemistry. Mod Pathol. 15:1374–1380. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang C, Han Y, Huang H, Qu L and Shou C:
High NR2F2 transcript is associated with increased survival and its
expression inhibits TGF-β-dependent epithelial-mesenchymal
transition in breast cancer. Breast Cancer Res Treat. 147:265–281.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bao Y, Gu D, Feng W, Sun X, Wang X, Zhang
X, Shi Q, Cui G, Yu H, Tang C and Deng A: COUP-TFII regulates
metastasis of colorectal adenocarcinoma cells by modulating Snail1.
Br J Cancer. 111:933–943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bringuier PP, Schalken JA, Hervieu V and
Giroldi LA: Involvement of orphan nuclear receptor COUP-TFII in
cadherin-6 and cadherin-11 regulation: implications in development
and cancer. Mech Dev. 136:64–72. 2015. View Article : Google Scholar : PubMed/NCBI
|