Colorectal cancer (CRC) is the fourth most frequent
type of malignancy in the world (1).
According to data collected by several authorities, including the
National Cancer Institute and the National Center for Health
Statistics, it was estimated that there would be 136,830 new cases
of CRC and 50,310 moralities from CRC in 2014. In the United
States, CRC represents 8.2% of all new cases of cancer and 8.6% of
all cancer-associated mortalities.
Metastasis is the most lethal characteristic of CRC,
accounting for 90% of the moralities of patients with colon cancer.
The 5-year survival rates of patients with early-stage disease are
up to 90%, but the 5-year survival rates of patients with distant
metastasis drop to 10%. In addition, the metastatic dissemination
of primary tumors is a pivotal cause for the failure of treatment
(2,3).
However, there is currently no molecular marker to predict the
possibility of CRC metastasis in an early and precise manner
(4,5).
Through the examination of MACC1 mRNA expression
levels in colon mucosa, normal liver, adenoma, primary tumors and
distant metastasis, a previous study illustrated that more MACC1
mRNA was expressed in malignant tissue compared with normal tissue
and adenoma (P<0.0001). Comparatively, tumors with metachronous
metastasis expressed significantly higher levels of MACC1 mRNA
compared with those that did not metastasize (P<0.0001). More
pivotally, the 5-year survival rates of patients with high and low
MACC1 mRNA expression were 15 and 80%, respectively, indicating
that MACC1 expression was an independent prognostic marker for
colon cancer metastasis (6).
Subsequently, the relevance of MACC1 expression to disease
prognosis was also corroborated (58). In a clinical study with 52 CRC tumor
samples available, it was revealed that MACC1 expression was
significantly correlated with peritoneal dissemination (P=0.042)
and the stage of tumor node metastasis classification (P=0.007).
Recently, Koelzer et al further verified MACC1 expression as
a predictive biomarker in a retrospective cohort study (67).
In addition, by examining MACC1 copy numbers and
mRNA expression levels of 103 metastatic CRC tissues, it was
confirmed that MACC1 expression was significantly correlated with
colon cancer metastasis (71).
Furthermore, the results of another study suggested that MACC1
expression was more than a prognostic marker for colon cancer
metastasis, as it was also revealed to be associated with the
recurrence of CRC (72), as confirmed
by Nitsche et al (73) in
2012. According to individualized risk assessment of fresh frozen
colon cancer tissue from 232 complete tumor resection patients with
Union for International Cancer Control stage II disease, it was
verified that the risk of cancer recurrence was markedly associated
with an increased expression of MACC1 (P<0.001), independent of
other biomarkers such as the mutation of KRAS proto-oncogene
(73). Notably, MACC1 was the only
independent parameter for recurrence prediction (hazard ratio, 6.2;
P<0.001) in CRC liver metastases (62). In addition, MACC1 was revealed to be
an independent biomarker for post-operative liver metastasis in
patients with colon cancer (70).
To overcome the inherent limitation of obtaining
tumor tissue via an invasive method, Stein et al (61) described a non-invasive assay for the
quantification of MACC1 transcripts in the plasma of 312 patients
with CRC. The results of the aforementioned study demonstrated that
MACC1 transcript levels in plasma increased in patients with all
stages of cancer in comparison with tumor-free volunteers. Similar
to findings in the tumor tissues, high MACC1 levels in the plasma
were also correlated with unfavorable survival (P<0.0001).
Qualitative studies have demonstrated that the alterations in DNA
and RNA extracted from the plasma of patients are similar to the
alterations of primary tumor nucleic acids, meaning that tumor
cells may be the origin of plasma or serum nucleic acids (74,75). In
addition, numerous studies verified the clinical value of
extracellular RNA in plasma from patients with cancer (76–82), and
the extracellular RNA in plasma was also revealed to be protected
in a multiparticle complex and was actively released by tumor cells
(83). Thus, it was hypothesized that
MACC1 transcripts in plasma were released from tumor cells in a
protected manner, and circulating MACC1 transcripts in plasma may
be a prognostic indicator for the survival and metastasis of
patients with CRC. The association between MACC1 status in the
blood and patient prognosis requires additional investigation in a
larger clinical study.
However, the focus of the majority of the
aforementioned studies was CRC tissue. It is difficult to reflect
disease progression and response to therapy using a single type of
tissue sample or a single time point (85). Circulating tumor DNA (ctDNA) was
revealed to be positively correlated with tumor progression
(86). In addition, ctDNA was
suggested to be an applicable, sensitive and specific biomarker in
CRC (87). Therefore, the present
review suggests that studies focusing on whether MACC1 SNPs in
plasma are associated with overall survival of patients with CRC
patients are required. Additional studies with large sample sizes
are required to reveal the potential association between MACC1 SNPs
and stage classification, recurrence or prognosis.
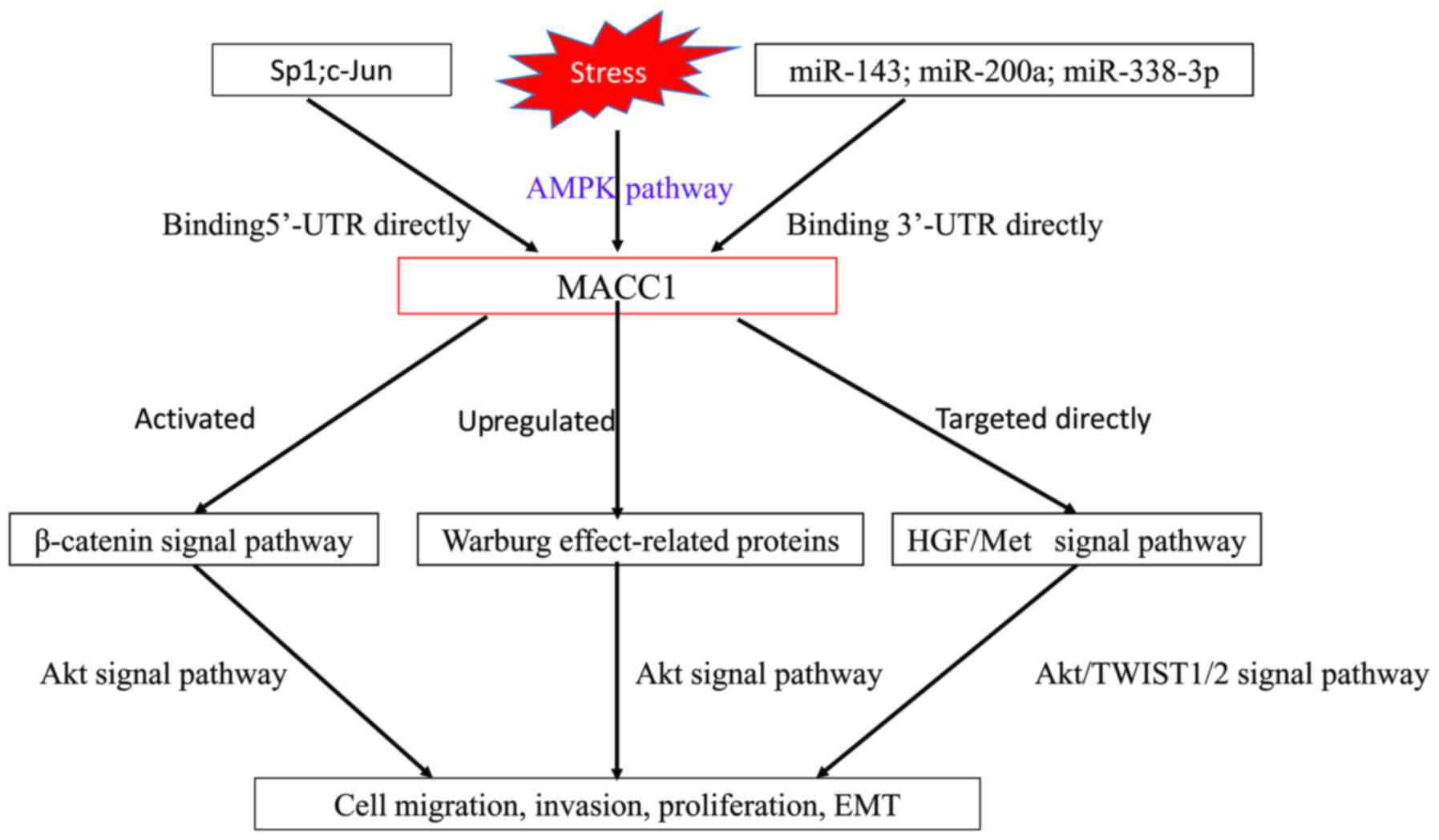
MicroRNAs (miRNAs/miRs) have been revealed to serve
an important role in promoting or suppressing tumor invasion and
metastasis via regulating metastasis-associated genes (88,89). Using
in silico prediction and western blot assays, a negative
correlation was identified between miR-143 and MACC1 in CRC.
Through 3′ untranslated region luciferase reporter gene analysis,
it was revealed that miR-143 directly targeted MACC1 (90). In addition, miR-338-3p and miR-200a
were also revealed to transcriptionally regulate MACC1 in gastric
cancer and hepatocellular carcinoma, respectively (91,92).
According to several studies (93–96),
miR-338-3p and miR-200a are associated with invasion, migration,
EMT and prognosis in patients with CRC. We hypothesize that they
may negatively regulate MACC1 in CRC.
Recently, it was revealed that the cell-free DNA of
tumors acts as a prometastatic factor through the induction of
MACC1 via the Toll-like receptor 9 (TLR9) signaling pathway
(106). TRL families serve a
fundamental role in the activation of innate immunity. In
particular, TLR9 signaling affects colorectal carcinogenesis and
colonic inflammation (107).
Notably, MACC1 is significantly associated with conventional
colitis-associated colorectal cancer (CAC) tumorigenesis (64). Thus, it is hypothesized that the
activation of the TRL9 signaling pathway by the cell-free DNA of
tumors may respond to the stepwise upregulation of MACC1 expression
from inflammatory bowel disease-associated colitis to dysplasia to
adenocarcinoma.
The MACC1 promoter region from −426 to −18 was
identified to be the essential domain, containing the functional
binding sites for the transcription factors activator protein 1
(AP-1), specificity protein 1 (Sp1) and CCAAT-enhancer-binding
protein (C/EBP). Using an electrophoretic mobility shift assay
(EMSA) and a chromatin immunoprecipitation (ChIP) assay, it was
additionally demonstrated that these transcription factors bound to
the minimal essential MACC1 core promoter regions and regulated the
transcription of the gene. In CRC tumors, the expression levels of
c-Jun and Sp1 were significantly correlated with MACC1 expression
levels (P=0.0007 and P=0.02, respectively) and the development of
metachronous metastases (P=0.01 and P=0.001, respectively). At
present, the upstream regulation of MACC1 in CRC remains unclear
and requires additional investigation.
MACC1 was revealed to be was significantly
associated with cisplatin resistance. The downregulation of MACC1
reduced the level of cisplatin resistance and induced apoptosis in
tongue squamous cell carcinoma and human glioblastoma U251 cells
(48,112). Stein et al (109) revealed that MACC1 functioned via
binding to a special consensus sequence of Met promoter, described
as a Sp1 binding site. The ATP-binding cassette sub-family G member
2 (ABCG2) promoter region exhibited the same consensus sequence and
the inhibition of Sp1-dependent ABCG2 expression caused
chemosensitization to cisplatin (113). Therefore, it is possible that ABCG2
may be a transcriptional target of MACC1, and cisplatin resistance
may be caused by the increase of ABCG2 induced by the
overexpression of MACC1. Recently, multiple studies showed that the
activation of the Wnt/β-catenin pathway enhanced cisplatin
resistance, whereas Wnt/β-catenin pathway inhibition sensitized
cancer cells to cisplatin (114–118).
Overall, the Wnt/β-catenin pathway serves a critical role in
cisplatin resistance and MACC1 overexpression may enhance cisplatin
resistance via activing the Wnt/β-catenin pathway.
MACC1 has been demonstrated to promote vasculogenic
mimicry (VM) by upregulating Twist family BHLH transcription factor
(TWIST)1/2 through the HGF/Met signal pathway in gastric cancer
(110). TWIST1/2 were revealed to be
associated with EMT and were also valuable biomarkers in CRC
(119,120). Thus, it was hypothesized that MACC1
induced EMT via the HGF/MET/TWIST1/2 signal pathway in CRC.
Despite advances with regard to our understanding of
the association between MACC1 expression and the survival of
patients with CRC, little is known about the mechanisms behind the
induction of cell proliferation, invasion and migration by MACC1 in
CRC cell cultures. It is therefore necessary to identify these
complex internal mechanisms.
In CRC, metastasis is the most frequent cause of
treatment failure and it is responsible for 90% of patient
mortality. However, there is no molecular biomarker sufficient for
predicting the risk of tumor progression and metastasis. Numerous
studies have revealed that MACC1 expression and SNPs are correlated
with metastasis-free survival. Therefore, MACC1 status may be
regarded as a tumor stage-independent predictor for CRC
metastasis.
Met, identified as a transcriptional target of
MACC1, is associated with CRC metastasis (121–129).
However, MACC1 induces colon cancer cell growth, invasion and
migration not only by the HGF/Met signal pathway, but also by the
β-catenin signal pathway. Additionally, miRNA (miR-143) and several
transcription factors (AP-1, Sp1, and C/EBP) have been revealed to
be involved in the negative or positive regulation of MACC1.
However, the specific mechanism behind the upstream regulation of
MACC1 remains unclear.
Based on the clinical and experimental evidence of
MACC1 in CRC, it may be considered as a promising biomarker for the
prediction of CRC metastasis and disease prognosis. Several studies
have reported that the downregulation of MACC1 inhibits colorectal
tumor progression and metastasis in CRC cells and xenografted mice
(6,90,130).
MACC1 may also act as a therapeutic target in the treatment of CRC.
Due to the significantly higher expression of MACC1 in CRC tissues
compared with other organs, enteric-coated products targeting MACC1
may be a good treatment strategy. However, little was previously
known with regard to how MACC1 functions in CRC. The specific
mechanisms of MACC1 should be additionally investigated, and
MACC1-based retrospective studies or interventional strategies
should be developed in larger clinical trials of CRC.
The authors would like to thank Mr. Chao Fang, Mr.
Xiang-Guang Meng and Mr. Wei-Hua Huang for their valuable
suggestions for the present study. The present study was supported
by the National Natural Scientific Foundation of China (grant nos.
81273595, 81202594 and 81001445) and the ‘863’ Project (grant no.
2012AA02A518).
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stein U and Schlag PM: Clinical,
biological, and molecular aspects of metastasis in colorectal
cancer. Recent Results Cancer Res. 176:61–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deliu IC, Georgescu EF and Bezna MC:
Analysis of prognostic factors in colorectal carcinoma. Rev Med
Chir Soc Med Nat Iasi. 118:808–816. 2014.PubMed/NCBI
|
|
4
|
Sethi N and Kang Y: Unravelling the
complexity of metastasis-molecular understanding and targeted
therapies. Nat Rev Cancer. 11:735–748. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wanebo HJ, LeGolvan M, Paty PB, Saha S,
Zuber M, D'Angelica MI and Kemeny NE: Meeting the biologic
challenge of colorectal metastases. Clin Exp Metastasis.
29:821–839. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stein U, Walther W, Arlt F, Schwabe H,
Smith J, Fichtner I, Birchmeier W and Schlag PM: MACC1, a newly
identified key regulator of HGF-MET signaling, predicts colon
cancer metastasis. Nat Med. 15:59–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stein U, Dahlmann M and Walther W:
MACC1-more than metastasis? Facts and predictions about a novel
gene. J Mol Med (Berl). 88:11–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kokoszyńska K, Kryński J, Rychlewski L and
Wyrwicz LS: Unexpected domain composition of MACC1 links MET
signaling and apoptosis. Acta Biochim Pol. 56:317–323.
2009.PubMed/NCBI
|
|
9
|
Wang R, Wei Z, Jin H, Wu H, Yu C, Wen W,
Chan LN, Wen Z and Zhang M: Autoinhibition of UNC5b revealed by the
cytoplasmic domain structure of the receptor. Mol Cell. 33:692–703.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ipsaro JJ, Huang L and Mondragon A:
Structures of the spectrin-ankyrin interaction binding domains.
Blood. 113:5385–5393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reed JC, Doctor KS and Godzik A: The
domains of apoptosis: A genomics perspective. Sci STKE.
2004:re92004.PubMed/NCBI
|
|
12
|
Shirahata A, Sakata M, Kitamura Y,
Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K,
Kigawa G, et al: MACC 1 as a marker for peritoneal-disseminated
gastric carcinoma. Anticancer Res. 30:3441–3444. 2010.PubMed/NCBI
|
|
13
|
Ge SH, Wu XJ, Wang XH, Xing XF, Zhang LH,
Zhu YB, Du H, Dong B, Hu Y and Ji JF: Over-expression of
metastasis-associated in colon cancer-1 (MACC1) associates with
better prognosis of gastric cancer patients. Chin J Cancer Res.
23:153–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Wu Y, Lin L, Liu P, Huang H, Liao
W, Zheng D, Zuo Q, Sun L, Huang N, et al: Metastasis-associated in
colon cancer-1 upregulation predicts a poor prognosis of gastric
cancer, and promotes tumor cell proliferation and invasion. Int J
Cancer. 133:1419–1430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma J, Ma J, Meng Q, Zhao ZS and Xu WJ:
Prognostic value and clinical pathology of MACC-1 and c-MET
expression in gastric carcinoma. Pathol Oncol Res. 19:821–832.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo T, Yang J, Yao J, Zhang Y, Da M and
Duan Y: Expression of MACC1 and c-Met in human gastric cancer and
its clinical significance. Cancer Cell Int. 13:1212013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun L, Duan J, Jiang Y, Wang L, Huang N,
Lin L, Liao Y and Liao W: Metastasis-associated in colon cancer-1
upregulates vascular endothelial growth factor-C/D to promote
lymphangiogenesis in human gastric cancer. Cancer Lett.
357:242–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burock S, Herrmann P, Wendler I,
Niederstrasser M, Wernecke KD and Stein U: Circulating metastasis
associated in colon cancer 1 transcripts in gastric cancer patient
plasma as diagnostic and prognostic biomarker. World J
Gastroenterol. 21:333–341. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimokawa H, Uramoto H, Onitsuka T, et al:
Macc1 amplification predicts postoperative recurrence in lung
adenocarcinoma. Ann Oncol. 21:24–25. 2010.
|
|
20
|
Shimokawa H, Uramoto H, Onitsuka T,
Chundong G, Hanagiri T, Oyama T and Yasumoto K: Overexpression of
MACC1 mRNA in lung adenocarcinoma is associated with postoperative
recurrence. J Thorac Cardiovasc Surg. 141:895–898. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gu CD, Uramoto H, Onitsuka T, Shimokawa H,
Iwanami T, Nakagawa M, Oyama T and Tanaka F: Molecular Diagnosis of
MACC1 status in lung adenocarcinoma by immunohistochemical
analysis. Anticancer Res. 31:1141–1145. 2011.PubMed/NCBI
|
|
22
|
Hu X, Fu X, Wen S, Zou X and Liu Y:
Prognostic value of MACC1 and c-met expressions in non-small cell
lung cancer. Zhongguo Fei Ai Za Zhi. 15:399–403. 2012.(In Chinese).
PubMed/NCBI
|
|
23
|
Wang Z, Li Z, Wu C, Wang Y, Xia Y, Chen L,
Zhu Q and Chen Y: MACC1 overexpression predicts a poor prognosis
for non-small cell lung cancer. Med Oncol. 31:7902014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Cai M, Weng Y, Zhang F, Meng D,
Song J, Zhou H and Xie Z: Circulating MACC1 as a novel diagnostic
and prognostic biomarker for nonsmall cell lung cancer. J Cancer
Res Clin Oncol. 141:1353–1361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang Y, Zhang H, Cai J, Fang L, Wu J, Ye
C, Zhu X and Li M: Overexpression of MACC1 and its significance in
human breast cancer progression. Cell Biosci. 3:162013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muendlein A, Hubalek M, Geller-Rhomberg S,
Gasser K, Winder T, Drexel H, Decker T, Mueller-Holzner E, Chamson
M, Marth C and Lang AH: Significant survival impact of MACC1
polymorphisms in HER2 positive breast cancer patients. Eur J
Cancer. 50:2134–2141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim GE, Lee JS, Park MH and Yoon JH:
Metastasis associated in colon cancer 1 predicts poor outcomes in
patients with breast cancer. Anal Quant Cytopathol Histpathol.
37:96–104. 2015.PubMed/NCBI
|
|
28
|
Qiu J, Huang P, Liu Q, Hong J, Li B, Lu C,
Wang L, Wang J and Yuan Y: Identification of MACC1 as a novel
prognostic marker in hepatocellular carcinoma. J Transl Med.
9:1662011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qu JH, Chang XJ, Lu YY, Bai WL, Chen Y,
Zhou L, Zeng Z, Wang CP, An LJ, Hao LY, et al: Overexpression of
metastasis-associated in colon cancer 1 predicts a poor outcome of
hepatitis B virus-related hepatocellular carcinoma. World J
Gastroenterol. 18:2995–3003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang XJ, Wang CP, Qu JH, Lu YY, Chen Y,
Bai WL, Gao XD, Hao LY, Xu GL, Wang H and Yang YP: Expression and
clinical significance of metastasis-associated in colon cancer 1
(MACC1) in HBV-related hepatocellular carcinoma. Zhonghua Zhong Liu
Za Zhi. 34:748–752. 2012.PubMed/NCBI
|
|
31
|
Yang YP, Qu JH, Chang XJ, Lu YY, Bai WL,
Dong Z, Wang H, An LJ, Xu ZX, Wang CP, et al: High intratumoral
metastasis-associated in colon cancer-1 expression predicts poor
outcomes of cryoablation therapy for advanced hepatocellular
carcinoma. J Transl Med. 11:412013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie C, Wu J, Yun J, Lai J, Yuan Y, Gao Z,
Li M, Li J and Song L: MACC1 as a prognostic biomarker for
early-stage and AFP-normal hepatocellular carcinoma. PLoS One.
8:e642352013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie C, Li J, Xie DY, Wu JH, Li MF and Gao
ZL: 761 Macc1 protein: A promising immunomarker for the detection
of early stage and afp normal hepatocellular carcinomas. J Hepatol.
56:S2992012. View Article : Google Scholar
|
|
34
|
Gao S, Lin BY, Yang Z, Zheng ZY, Liu ZK,
Wu LM, Xie HY, Zhou L and Zheng SS: Role of overexpression of MACC1
and/or FAK in predicting prognosis of hepatocellular carcinoma
after liver transplantation. Int J Med Sci. 11:268–275. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ji D, Lu ZT, Li YQ, Liang ZY, Zhang PF, Li
C, Zhang JL, Zheng X and Yao YM: MACC1 Expression correlates with
PFKFB2 and survival in hepatocellular carcinoma. Asian Pac J Cancer
P. 15:999–1003. 2014. View Article : Google Scholar
|
|
36
|
Li Y, Lu Z, Liang Z, Ji D, Zhang P, Liu Q,
Zheng X and Yao Y: Metastasis-associated in colon cancer-1 is
associated with poor prognosis in hepatocellular carcinoma, partly
by promoting proliferation through enhanced glucose metabolism. Mol
Med Rep. 12:426–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun DW, Zhang YY, Qi Y, Liu GQ, Chen YG,
Ma J and Lv GY: Prognostic and clinicopathological significance of
MACC1 expression in hepatocellular carcinoma patients: A
meta-analysis. Int J Clin Exp Med. 8:4769–4777. 2015.PubMed/NCBI
|
|
38
|
Yao Y, Dou C, Lu Z, Zheng X and Liu Q:
MACC1 suppresses cell apoptosis in hepatocellular carcinoma by
targeting the HGF/c-MET/AKT pathway. Cell Physiol Biochem.
35:983–996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang RT, Shi HR, Huang HL, Chen ZM, Liu
HN and Yuan ZF: Expressions of MACC1, HGF and C-met protein in
epithelial ovarian cancer and their significance. Nan Fang Yi Ke Da
Xue Xue Bao. 31:1551–1555. 2011.(In Chinese). PubMed/NCBI
|
|
40
|
Zhang RT, Ren F and Shi HR: Expression of
metastasis-associated in colon cancer-1 in different stages of
epithelial ovarian cancer. Zhongguo Yi Xue Ke Xue Yuan Xue Bao.
36:47–51. 2014.(In Chinese). PubMed/NCBI
|
|
41
|
Li H, Zhang H, Zhao S, Shi Y, Yao J, Zhang
Y, Guo H and Liu X: Overexpression of MACC1 and the association
with hepatocyte growth factor/c-Met in epithelial ovarian cancer.
Oncol Lett. 9:1989–1996. 2015.PubMed/NCBI
|
|
42
|
Hu H, Tian D, Chen T, Han R, Sun Y and Wu
C: Metastasis-associated in colon cancer 1 is a novel
survival-related biomarker for human patients with renal pelvis
carcinoma. PLoS One. 9:e1001612014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jin Z, Xu N, Guo K, Xu P, Li P, Zhang Y,
Li X, Zheng S, Liu C, Xu A and Huang P: Increased expression of
metastasis-associated in colon cancer-1 in renal cell carcinoma is
associated with poor prognosis. Int J Clin Exp Pathol. 8:3857–3863.
2015.PubMed/NCBI
|
|
44
|
Yang T, Kong B, Kuang YQ, Cheng L, Gu JW,
Zhang JH, Shu HF, Yu SX, He WQ, Xing XM and Huang HD:
Overexpression of MACC1 protein and its clinical implications in
patients with glioma. Tumor Biol. 35:815–819. 2014. View Article : Google Scholar
|
|
45
|
Hagemann C, Fuchs S, Monoranu CM, Herrmann
P, Smith J, Hohmann T, Grabiec U, Kessler AF, Dehghani F, Löhr M,
et al: Impact of MACC1 on human malignant glioma progression and
patients unfavorable prognosis. Neuro Oncol. 15:1696–1709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Hong Q, Wang J, Fang Y and Hu C:
Downregulated expression of metastasis associated in colon cancer 1
(MACC1) reduces gallbladder cancer cell proliferation and invasion.
Tumor Biol. 35:3771–3778. 2014. View Article : Google Scholar
|
|
47
|
Chen L, Wang J, Fu L, Zhang B, Zhang H and
Ye B: Prognostic significance of metastasis associated in colon
cancer 1 (MACC1) expression in patients with gallbladder cancer. J
Cancer Res Ther. 10:1052–1056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li HF, Liu YQ, Shen ZJ, Gan XF, Han JJ,
Liu YY, Li HG1 and Huang ZQ: Downregulation of MACC1 inhibits
invasion, migration and proliferation, attenuates cisplatin
resistance and induces apoptosis in tongue squamous cell carcinoma.
Oncol Rep. 33:651–660. 2015.PubMed/NCBI
|
|
49
|
Zhang K, Zhang Y, Zhu H, Xue N, Liu J,
Shan C and Zhu Q: High expression of MACC1 predicts poor prognosis
in patients with osteosarcoma. Tumor Biol. 35:1343–1350. 2014.
View Article : Google Scholar
|
|
50
|
Zhang K, Tian F, Zhang Y, Zhu Q, Xue N,
Zhu H, Wang H and Guo X: MACC1 is involved in the regulation of
proliferation, colony formation, invasion ability, cell cycle
distribution, apoptosis and tumorigenicity by altering Akt
signaling pathway in human osteosarcoma. Tumor Biol. 35:2537–2548.
2014. View Article : Google Scholar
|
|
51
|
Zhu M, Xu Y, Mao X, Gao Y, Shao L and Yan
F: Overexpression of metastasis-associated in colon cancer-1
associated with poor prognosis in patients with esophageal cancer.
Pathol Oncol Res. 19:749–753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang JJ, Hong Q, Hu CG, Fang YJ and Wang
Y: Significance of MACC1 protein expression in esophageal
carcinoma. Zhonghua yi xue za zhi. 93:2584–2586. 2013.(In Chinese).
PubMed/NCBI
|
|
53
|
Meng FJ, Li H, Shi H, Yang Q, Zhang F,
Yang Y, Kang L, Zhen T, Dong DY and Han A: MACC1 down-regulation
inhibits proliferation and tumourigenicity of nasopharyngeal
carcinoma cells through Akt/β-catenin signaling pathway. PLoS One.
8:e608212013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang G, Kang MX, Lu WJ, Chen Y, Zhang B
and Wu YL: MACC1: A potential molecule associated with pancreatic
cancer metastasis and chemoresistance. Oncol Lett. 4:783–791.
2012.PubMed/NCBI
|
|
55
|
Lederer A, Herrmann P, Seehofer D, Dietel
M, Pratschke J, Schlag P and Stein U: Metastasis-associated in
colon cancer 1 is an independent prognostic biomarker for survival
in klatskin tumor patients. Hepatology. 62:841–850. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li H, Liao X, Liu Y, Shen Z, Gan X, Li H
and Huang Z: The expression of MACC1 and its role in the
proliferation and apoptosis of salivary adenoid cystic carcinoma. J
Oral Pathol Med. 44:810–817. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guo L, Lu W, Zhang X, Luo D and Zhang H:
Metastasis-associated colon cancer-1 is a novel prognostic marker
for cervical cancer. Int J Clin Exp Pathol. 7:4150–4155.
2014.PubMed/NCBI
|
|
58
|
Shirahata A, Shinmura K, Kitamura Y,
Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K,
Kigawa G, et al: MACC1 as a marker for advanced colorectal
carcinoma. Anticancer Res. 30:2689–2692. 2010.PubMed/NCBI
|
|
59
|
Lang AH, Geller-Rhomberg S, Winder T,
Stark N, Gasser K, Hartmann B, Kohler B, Grizelj I, Drexel H and
Muendlein A: A common variant of the MACC1 gene is significantly
associated with overall survival in colorectal cancer patients. BMC
Cancer. 12:202012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Schmid F, Burock S, Klockmeier K, Schlag
PM and Stein U: SNPs in the coding region of the
metastasis-inducing gene MACC1 and clinical outcome in colorectal
cancer. Mol Cancer. 11:492012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Stein U, Burock S, Herrmann P, Wendler I,
Niederstrasser M, Wernecke KD and Schlag PM: Circulating MACC1
transcripts in colorectal cancer patient plasma predict metastasis
and prognosis. PLos One. 7:e492492012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Isella C, Mellano A, Galimi F, Petti C,
Capussotti L, De Simone M, Bertotti A, Medico E and Muratore A:
MACC1 mRNA levels predict cancer recurrence after resection of
colorectal cancer liver metastases. Ann Surg. 257:1089–1095. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ren B, Zakharov V, Yang Q, McMahon L, Yu J
and Cao W: MACC1 is related to colorectal cancer initiation and
early-stage invasive growth. Am J Clin Pathol. 140:701–707. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Harpaz N, Taboada S, Ko HM, Yu J, Yang Q,
Xu H and Cao W: Expression of MACC1 and MET in inflammatory bowel
disease-associated colonic neoplasia. Inflamm Bowel Dis.
20:703–711. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yamamoto H, Miyoshi N, Mimori K, Hitora T,
Tokuoka M, Fujino S, Ellis HL, Ishii H, Noura S, Ohue M, et al:
MACC1 expression levels as a novel prognostic marker for colorectal
cancer. Oncol Lett. 8:2305–2309. 2014.PubMed/NCBI
|
|
66
|
Ilm K, Kemmner W, Osterland M, Burock S,
Koch G, Herrmann P, Schlag PM and Stein U: High MACC1 expression in
combination with mutated KRAS G13 indicates poor survival of
colorectal cancer patients. Mol Cancer. 14:382015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Koelzer VH, Herrmann P, Zlobec I,
Karamitopoulou E, Lugli A and Stein U: Heterogeneity analysis of
metastasis associated in colon cancer 1 (MACC1) for survival
prognosis of colorectal cancer patients: A retrospective cohort
study. BMC Cancer. 15:1602015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhen T, Dai S, Li H, Yang Y, Kang L, Shi
H, Zhang F, Yang D, Cai S, He Y, et al: MACC1 promotes
carcinogenesis of colorectal cancer via β-catenin signaling
pathway. Oncotarget. 5:3756–3769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kawamura M, Saigusa S, Toiyama Y, Tanaka
K, Okugawa Y, Hiro J, Uchida K, Mohri Y, Inoue Y and Kusunoki M:
Correlation of MACC1 and MET expression in rectal cancer after
neoadjuvant chemoradiotherapy. Anticancer Res. 32:1527–1531.
2012.PubMed/NCBI
|
|
70
|
Ge Y, Meng X, Zhou Y, Zhang J and Ding Y:
Positive MACC1 expression correlates with invasive behaviors and
postoperative liver metastasis in colon cancer. Int J Clin Exp Med.
8:1094–1100. 2015.PubMed/NCBI
|
|
71
|
Galimi F, Torti D, Sassi F, Isella C, Corà
D, Gastaldi S, Ribero D, Muratore A, Massucco P, Siatis D, et al:
Genetic and expression analysis of MET, MACC1, and HGF in
metastatic colorectal cancer: Response to met inhibition in patient
xenografts and pathologic correlations. Clin Cancer Res.
17:3146–3156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Boardman LA: Overexpression of MACC1 leads
to downstream activation of HGF/MET and potentiates metastasis and
recurrence of colorectal cancer. Genome Med. 1:362009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Nitsche U, Rosenberg R, Balmert A,
Schuster T, Slotta-Huspenina J, Herrmann P, Bader FG, Friess H,
Schlag PM, Stein U and Janssen KP: Integrative marker analysis
allows risk assessment for metastasis in stage II colon cancer. Ann
Surg. 256:763–771. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Stroun M, Anker P, Maurice P, Lyautey J,
Lederrey C and Beljanski M: Neoplastic characteristics of the DNA
found in the plasma of cancer patients. Oncology. 46:318–322. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kopreski MS, Benko FA, Kwak LW and Gocke
CD: Detection of tumor messenger RNA in the serum of patients with
malignant melanoma. Clin Cancer Res. 5:1961–1965. 1999.PubMed/NCBI
|
|
76
|
Silva JM, Dominguez G, Silva J, Garcia JM,
Sanchez A, Rodriguez O, Provencio M, España P and Bonilla F:
Detection of epithelial messenger RNA in the plasma of breast
cancer patients is associated with poor prognosis tumor
characteristics. Clin Cancer Res. 7:2821–2825. 2001.PubMed/NCBI
|
|
77
|
Silva JM, Rodriguez R, Garcia JM, Muñoz C,
Silva J, Dominguez G, Provencio M, España P and Bonilla F:
Detection of epithelial tumour RNA in the plasma of colon cancer
patients is associated with advanced stages and circulating tumour
cells. Gut. 50:530–534. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Garcia V, Garcia JM, Silva J, Martin P,
Peña C, Dominguez G, Diaz R, Herrera M, Maximiano C, Sabin P, et
al: Extracellular tumor-related mRNA in plasma of lymphoma patients
and survival implications. PLoS One. 4:e81732009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Stein U, Burock S, Herrmann P, Wendler I,
Niederstrasser M, Wernecke KD and Schlag PM: Diagnostic and
prognostic value of metastasis inducer S100A4 transcripts in plasma
of colon, rectal, and gastric cancer patients. J Mol Diagn.
13:189–198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Mohammed N, Rodriguez M, Garcia V, Garcia
JM, Dominguez G, Peña C, Herrera M, Gomez I, Diaz R, Soldevilla B,
et al: EPAS1 mRNA in plasma from colorectal cancer patients is
associated with poor outcome in advanced stages. Oncol Lett.
2:719–724. 2011.PubMed/NCBI
|
|
81
|
Shen J, Wei J, Guan W, Wang H, Ding Y,
Qian X, Yu L, Zou Z, Xie L, Costa C, et al: Plasma mRNA expression
levels of BRCA1 and TS as potential predictive biomarkers for
chemotherapy in gastric cancer. J Transl Med. 12:3552014.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kishikawa T, Otsuka M, Ohno M, Yoshikawa
T, Takata A and Koike K: Circulating RNAs as new biomarkers for
detecting pancreatic cancer. World J Gastroenterol. 21:8527–8540.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
García JM, García V, Peña C, Domínguez G,
Silva J, Diaz R, Espinosa P, Citores MJ, Collado M and Bonilla F:
Extracellular plasma RNA from colon cancer patients is confined in
a vesicle-like structure and is mRNA-enriched. RNA. 14:1424–1432.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zheng Z, Gao S, Yang Z, Xie H, Zhang C,
Lin B, Wu L, Zheng S and Zhou L: Single nucleotide polymorphisms in
the metastasis-associated in colon cancer-1 gene predict the
recurrence of hepatocellular carcinoma after transplantation. Int J
Med Sci. 11:142–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Siravegna G, Mussolin B, Buscarino M,
Corti G, Cassingena A, Crisafulli G, Ponzetti A, Cremolini C, Amatu
A, Lauricella C, et al: Clonal evolution and resistance to EGFR
blockade in the blood of colorectal cancer patients. Nat Med.
21:795–801. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mouliere F, Robert B, Arnau Peyrotte E,
Del Rio M, Ychou M, Molina F, Gongora C and Thierry AR: High
fragmentation characterizes tumour-derived circulating DNA. PLoS
One. 6:e234182011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bettegowda C, Sausen M, Leary RJ, Kinde I,
Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, et al:
Detection of circulating tumor DNA in early- and late-stage human
malignancies. Sci Transl Med. 6:224ra2242014. View Article : Google Scholar
|
|
88
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
96:(Suppl). R40–R44. 2007.PubMed/NCBI
|
|
89
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang Y, Wang ZQ, Chen M, Peng L, Wang X,
Ma Q, Ma F and Jiang B: MicroRNA-143 targets MACC1 to inhibit cell
invasion and migration in colorectal cancer. Mol Cancer. 11:232012.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Huang N, Wu Z, Lin L, Zhou M, Wang L, Ma
H, Xia J, Bin J, Liao Y and Liao W: MiR-338-3p inhibits
epithelial-mesenchymal transition in gastric cancer cells by
targeting ZEB2 and MACC1/Met/Akt signaling. Oncotarget.
6:15222–15234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Feng J, Wang J, Chen M, Chen G, Wu Z, Ying
L, Zhuo Q, Zhang J and Wang W: miR-200a suppresses cell growth and
migration by targeting MACC1 and predicts prognosis in
hepatocellular carcinoma. Oncol Rep. 33:713–720. 2015.PubMed/NCBI
|
|
93
|
Xue Q, Sun K, Deng HJ, Lei ST, Dong JQ and
Li GX: MicroRNA-338-3p inhibits colorectal carcinoma cell invasion
and migration by targeting smoothened. Jpn J Clin Oncol. 44:13–21.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sun K, Su G, Deng H, Dong J, Lei S and Li
G: Relationship between miRNA-338-3p expression and progression and
prognosis of human colorectal carcinoma. Chin Med J (Engl).
127:1884–1890. 2014.PubMed/NCBI
|
|
95
|
Sheng XJ, Li Z, Sun M, Wang ZH, Zhou DM,
Li JQ, Zhao Q, Sun XF and Liu QC: MACC1 induces metastasis in
ovarian carcinoma by upregulating hepatocyte growth factor receptor
c-MET. Oncol Lett. 8:891–897. 2014.PubMed/NCBI
|
|
96
|
Pichler M, Ress AL, Winter E, Stiegelbauer
V, Karbiener M, Schwarzenbacher D, Scheideler M, Ivan C, Jahn SW,
Kiesslich T, et al: MiR-200a regulates epithelial to mesenchymal
transition-related gene expression and determines prognosis in
colorectal cancer patients. Br J Cancer. 110:1614–1621. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lin L, Huang H and Liao W, Ma H, Liu J,
Wang L, Huang N, Liao Y and Liao W: MACC1 supports human gastric
cancer growth under metabolic stress by enhancing the Warburg
effect. Oncogene. 34:2700–2710. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hara S, Nakashiro K, Klosek SK, Ishikawa
T, Shintani S and Hamakawa H: Hypoxia enhances c-Met/HGF receptor
expression and signaling by activating HIF-1alpha in human salivary
gland cancer cells. Oral Oncol. 42:593–598. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chen HH, Su WC, Lin PW, Guo HR and Lee WY:
Hypoxia-inducible factor-1alpha correlates with MET and metastasis
in node-negative breast cancer. Breast Cancer Res Treat.
103:167–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Comito G, Calvani M, Giannoni E, Bianchini
F, Calorini L, Torre E, Migliore C, Giordano S and Chiarugi P:
HIF-1α stabilization by mitochondrial ROS promotes Met-dependent
invasive growth and vasculogenic mimicry in melanoma cells. Free
Radic Biol Med. 51:893–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ide T, Kitajima Y, Miyoshi A, Ohtsuka T,
Mitsuno M, Ohtaka K and Miyazaki K: The hypoxic environment in
tumor-stromal cells accelerates pancreatic cancer progression via
the activation of paracrine hepatocyte growth factor/c-Met
signaling. Ann Surg Oncol. 14:2600–2607. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kim BW, Cho H, Chung JY, Conway C, Ylaya
K, Kim JH and Hewitt SM: Prognostic assessment of hypoxia and
metabolic markers in cervical cancer using automated digital image
analysis of immunohistochemistry. J Transl Med. 11:1852013.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chen Z, He X, Xia W, Huang Q, Zhang Z, Ye
J, Ni C, Wu P, Wu D, Xu J, et al: Prognostic value and
clinicopathological differences of HIFs in colorectal cancer:
Evidence from meta-analysis. PLoS One. 8:e803372013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hardie DG: The AMP-activated protein
kinase pathway-new players upstream and downstream. J Cell Sci.
117:5479–5487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Loo JM, Scherl A, Nguyen A, Man FY,
Weinberg E, Zeng Z, Saltz L, Paty PB and Tavazoie SF: Extracellular
metabolic energetics can promote cancer progression. Cell.
160:393–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Fűri I, Kalmár A, Wichmann B, Spisák S,
Schöller A, Barták B, Tulassay Z and Molnár B: Cell free DNA of
tumor origin induces a ‘metastatic’ expression profile in HT-29
cancer cell line. PLoS One. 10:e01316992015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Fűri I, Sipos F, Germann TM, Kalmár A,
Tulassay Z, Molnár B and Műzes G: Epithelial toll-like receptor 9
signaling in colorectal inflammation and cancer: Clinico-pathogenic
aspects. World J Gastroenterol. 19:4119–4126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Juneja M, Ilm K, Schlag PM and Stein U:
Promoter identification and transcriptional regulation of the
metastasis gene MACC1 in colorectal cancer. Mol Oncol. 7:929–943.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Stein U, Smith J, Walther W and Arlt F:
MACC1 controls Met: What a difference an Sp1 site makes. Cell
Cycle. 8:2467–2469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang L, Lin L, Chen X, Sun L, Liao Y,
Huang N and Liao W: Metastasis-associated in colon cancer-1
promotes vasculogenic mimicry in gastric cancer by upregulating
TWIST1/2. Oncotarget. 6:11492–11506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Migliore C, Martin V, Leoni VP, Restivo A,
Atzori L, Petrelli A, Isella C, Zorcolo L, Sarotto I, Casula G, et
al: MiR-1 downregulation cooperates with MACC1 in promoting MET
overexpression in human colon cancer. Clin Cancer Res. 18:737–747.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Shang C, Hong Y, Guo Y, Liu YH and Xue YX:
Influence of the MACC1 gene on sensitivity to chemotherapy in human
U251 glioblastoma cells. Asian Pac J Cancer Prev. 16:195–199. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Yang WJ, Song MJ, Park EY, Lee JJ, Park
JH, Park K, Park JH and Kim HP: Transcription factors Sp1 and Sp3
regulate expression of human ABCG2 gene and chemoresistance
phenotype. Mol Cells. 36:368–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Gao Y, Liu Z, Zhang X, He J, Pan Y, Hao F,
Xie L, Li Q, Qiu X and Wang E: Inhibition of cytoplasmic GSK-3β
increases cisplatin resistance through activation of Wnt/β-catenin
signaling in A549/DDP cells. Cancer Lett. 336:231–239. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wei Y, Shen N, Wang Z, Yang G, Yi B, Yang
N, Qiu Y and Lu J: Sorafenib sensitizes hepatocellular carcinoma
cell to cisplatin via suppression of Wnt/β-catenin signaling. Mol
Cell Biochem. 381:139–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhao H, Wei W, Sun Y, Gao J, Wang Q and
Zheng J: Interference with the expression of β-catenin reverses
cisplatin resistance in A2780/DDP cells and inhibits the
progression of ovarian cancer in mouse model. DNA Cell Biol.
34:55–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Xia Y, He Z, Liu B, Wang P and Chen Y:
Downregulation of Meg3 enhances cisplatin resistance of lung cancer
cells through activation of the WNT/β-catenin signaling pathway.
Mol Med Rep. 12:4530–4537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Nagaraj AB, Joseph P, Kovalenko O, Singh
S, Armstrong A, Redline R, Resnick K, Zanotti K, Waggoner S and
DiFeo A: Critical role of Wnt/β-catenin signaling in driving
epithelial ovarian cancer platinum resistance. Oncotarget.
6:23720–23734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kim YH, Kim G, Kwon CI, Kim JW, Park PW
and Hahm KB: TWIST1 and SNAI1 as markers of poor prognosis in human
colorectal cancer are associated with the expression of ALDH1 and
TGF-β1. Oncol Rep. 31:1380–1388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yu H, Jin GZ, Liu K, Dong H, Yu H, Duan
JC, Li Z, Dong W, Cong WM and Yang JH: Twist2 is a valuable
prognostic biomarker for colorectal cancer. World J Gastroenterol.
19:2404–2411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Di Renzo MF, Olivero M, Giacomini A, Porte
H, Chastre E, Mirossay L, Nordlinger B, Bretti S, Bottardi S,
Giordano S, et al: Overexpression and amplification of the met/HGF
receptor gene during the progression of colorectal cancer. Clin
Cancer Res. 1:147–154. 1995.PubMed/NCBI
|
|
122
|
Mao W, Irby R, Coppola D, Fu L, Wloch M,
Turner J, Yu H, Garcia R, Jove R and Yeatman TJ: Activation of
c-Src by receptor tyrosine kinases in human colon cancer cells with
high metastatic potential. Oncogene. 15:3083–3090. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zeng Z, Weiser MR, D'Alessio M, Grace A,
Shia J and Paty PB: Immunoblot analysis of c-Met expression in
human colorectal cancer: Overexpression is associated with advanced
stage cancer. Clin Exp Metastasis. 21:409–417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zeng ZS, Weiser MR, Kuntz E, Chen CT, Khan
SA, Forslund A, Nash GM, Gimbel M, Yamaguchi Y, Culliford AT IV, et
al: c-Met gene amplification is associated with advanced stage
colorectal cancer and liver metastases. Cancer Lett. 265:258–269.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Osada S, Matsui S, Komori S, Yamada J,
Sanada Y, Ihawa A, Tanaka Y, Tokuyama Y, Okumura N, Nonaka K, et
al: Effect of hepatocyte growth factor on progression of liver
metastasis in colorectal cancer. Hepatogastroenterology. 57:76–80.
2010.PubMed/NCBI
|
|
126
|
Matsui S, Osada S, Tomita H, Komori S,
Mori R, Sanada Y, Takahashi T, Yamaguchi K and Yoshida K: Clinical
significance of aggressive hepatectomy for colorectal liver
metastasis, evaluated from the HGF/c-Met pathway. Int J Oncol.
37:289–297. 2010.PubMed/NCBI
|
|
127
|
Sun YL, Liu WD, Ma GY, Gao DW, Jiang YZ,
Liu Q and Du JJ: Expression of HGF and Met in human tissues of
colorectal cancers: Biological and clinical implications for
synchronous liver metastasis. Int J Med Sci. 10:548–559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Abou-Bakr AA and Elbasmi A: c-MET
overexpression as a prognostic biomarker in colorectal
adenocarcinoma. Gulf J Oncolog. 1:28–34. 2013.PubMed/NCBI
|
|
129
|
Sueta A, Yamamoto Y, Yamamoto-Ibusuki M,
Hayashi M, Takeshita T, Yamamoto S, Omoto Y and Iwase H:
Differential role of MACC1 expression and its regulation of the
HGF/cMet pathway between breast and colorectal cancer. Int J Oncol.
46:2143–2153. 2015.PubMed/NCBI
|
|
130
|
Pichorner A, Sack U, Kobelt D, Smith J,
Walther W, Schlag PM and Stein U: In vivo imaging of MACC1 induced
metastasis formation in a xenograft mouse model based on an
IRES-vector harboring the reporter luciferase and the gene MACC1:
PO125. Onkologie. 33:652010.PubMed/NCBI
|