Introduction
Nitric oxide is a multifunctional biological
mediator and an active participant in proinflammatory and
bronchodilatory biological processes (1). The measurement of exhaled nitric oxide
(eNO) levels is a simple, non-invasive technique; it is used to
monitor airway inflammation in patients with asthma and as an
indicator for steroid usage (2,3). eNO
assessment is also useful in other diseases, e.g., chronic
obstructive pulmonary disease (4),
pulmonary hypertension (3) and
scleroderma (5). We previously
hypothesized that eNO may be a predictor for radiation pneumonitis
(RP) following the thoracic radiotherapy (RT) of patients with lung
cancer (6). However, when the
original study was performed, two-dimensional RT planning and a
large eNO assay-machine were utilized. The equipment required a
long time for initial calibration, making the task impractical;
this ultimately resulted in the discontinuation of the study. In
the 21st century, three-dimensional computed tomography (CT)-guided
conformal radiation therapy (3D-CRT) has become a standard
treatment. Additionally, advanced RT technology, including
stereotactic body RT (SBRT), is now available in clinical settings.
Improved, small-size equipment for measuring eNO concentrations has
been developed and is also available in clinical practice. These
developments made it possible to re-examine eNO levels using the
new equipment in order to observe the effects of modern RT
techniques. Additionally, there are several biomarkers proposed for
RP monitoring, including surfactant protein D (SP-D) (7,8). A
simultaneous, prospective examination of the levels of this
biomarker was performed. The overall aim of the study was to
confirm the results of our previous study and examine the possible
extension of the methods for monitoring RP after advanced RT
treatments.
Materials and methods
Patients
Between February 2012 and July 2015, the eNO levels
from the 34 thoracic RT treatments of 33 patients (25 male, 8
female), with a median age of 78 (range, 37–87), were assessed in
the Department of Radiology, Graduate School of Medical Science,
Kyoto Prefectural University of Medicine (Kyoto, Japan). Patients
were staged according to the Union for International Cancer
Control, 7th edition (9). A total of
16 patients received 3D-CRT (including patients at primary lung
cancer stage T1N0, 2; T1N1, 1; T1N2, 1; T2N0, 1; T3N0, 1; T3N1 1;
T3N3, 1; T4N2, 1; T4N3, 1; rN1 2 and rN3 1, in addition to 1 with
rN1 esophageal cancer and 2 with metastatic lung cancer) and 18
received SBRT (including 13 with primary lung cancer, including
T1N0, 8; T2N0, 5; 6 with metastatic lung cancer). One patient
underwent thoracic RT twice, including initial 3D-CRT for primary
esophageal cancer and SBRT for metastatic lung cancer. Patients
were ineligible if they had an Eastern Cooperative Oncology Group
performance status >2 (10),
active systemic or pulmonary infection, asthma or synchronous
malignancy within 2 years of enrollment.
3D-CRT procedure
For 3D-CRT, the patients were placed in a supine
position with arms above the head. Target volumes were delineated
on a planning CT scan with 2.5 mm slices. The gross tumor volume
(GTV), clinical target volume (CTV), and planning target volume
(PTV) were obtained according to the definitions of the
International Commission on Radiation Units and Measurements
(11). The GTV included tumor and
lymph node tissue visualized using a CT and positron emission
tomography (PET)-CT scan. PTV, including set-up margin and internal
target volume, was obtained using a 2–10 mm 3D-expansion from CTV.
The median prescribed dose was 60 Gy in 30 fractions. Concurrent
chemotherapy (cisplatin with pemetrexed, 5 patients; carboplatin
with paclitaxel, 2 patients; cisplatin with fluorouracil, 1
patient) was administered only for patients receiving 3D-CRT.
SBRT procedure
SBRT was performed using an immobilization device
Body FIX® Vacuum Pump P2 system (Medical Intelligence
Medizintechnik GmbH, Schwabmünchen, Germany), with the patient in a
supine position. All patients underwent 3 simulation CT scans
(Aquilion 64; Toshiba Medical Systems Ltd., Tokyo, Japan) in three
phases, i.e., free breathing, inhalation and exhalation during
shallow breathing. The GTV in each phase was delineated using lung
CT (window level, 550 HU; width, 1,600 HU) and a radiation
treatment contouring system (XiO; Elekta AB, Stockholm, Sweden).
GTVs of the 3 phases were fused and expanded as a PTV with a margin
of 5 mm in the anteroposterior and right-left directions and 5–10
mm in the superior-inferior and anterior-posterior directions
(typically 5 mm). The following structures were contoured as organs
at risk: Spinal cord, esophagus, lung and others as required by the
situation (e.g., the heart, ipsilateral bronchus, liver and
bowels). The treatment was generally delivered so that >95% of
the PTV received the prescribed dose of 50 Gy. The median
prescribed dose was 48 Gy in 4 fractions.
Irradiation was applied using 4-, 6-, and 10-mV
photon beams from accelerators (Elekta Synergy; Elekta AB) with
multileaf linear collimators. Lung toxicity grade 2 (symptomatic RP
with image abnormality) or higher, according to Common Terminology
Criteria of Adverse Events v4.0 (12), was regarded as RP; one patient
exhibited moderate fibrotic lung but did not suffer symptomatic
radiation pneumonitis. The toxicity assessment was performed by
physicians from several departments, including a diagnostic
radiologist, pulmonologist and radiation oncologist. All patients
had signed an informed consent before registration in the
observational study, which was approved by the Institutional Review
Board of Kyoto Prefectural University of Medicine (approval no.
PBMR-C-988-1).
eNO measurements
eNO levels were measured using a chemiluminescence
analyzer (Aerocrine NIOX; Circassia AB, Uppsala, Sweden). The
results were expressed as the mean of triplicate measurements. eNO
measurements were performed prior to RT, immediately subsequent to
RT, every week during RT, and 1, 3, 6, 9 and 12 months later.
Additional measurements were conducted if RP was suspected. The eNO
ratio was calculated as current eNO value/minimum eNO value during
RT. Serum SP-D level was also examined prior to RT and 1, 3, 6, and
12 months after RT as described previously (7,8). To obtain
control eNO values, the eNO levels of 17 healthy volunteers were
assessed.
Statistical analysis
All statistical analyses were performed using
StatView 5.0 statistical software (SAS Institute, Inc., Cary, NC,
USA). Percentages were compared using the χ2 test;
comparisons between time points within the same group were compared
using Student's paired t-test and the Mann-Whitney U test was used
for other comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
eNO
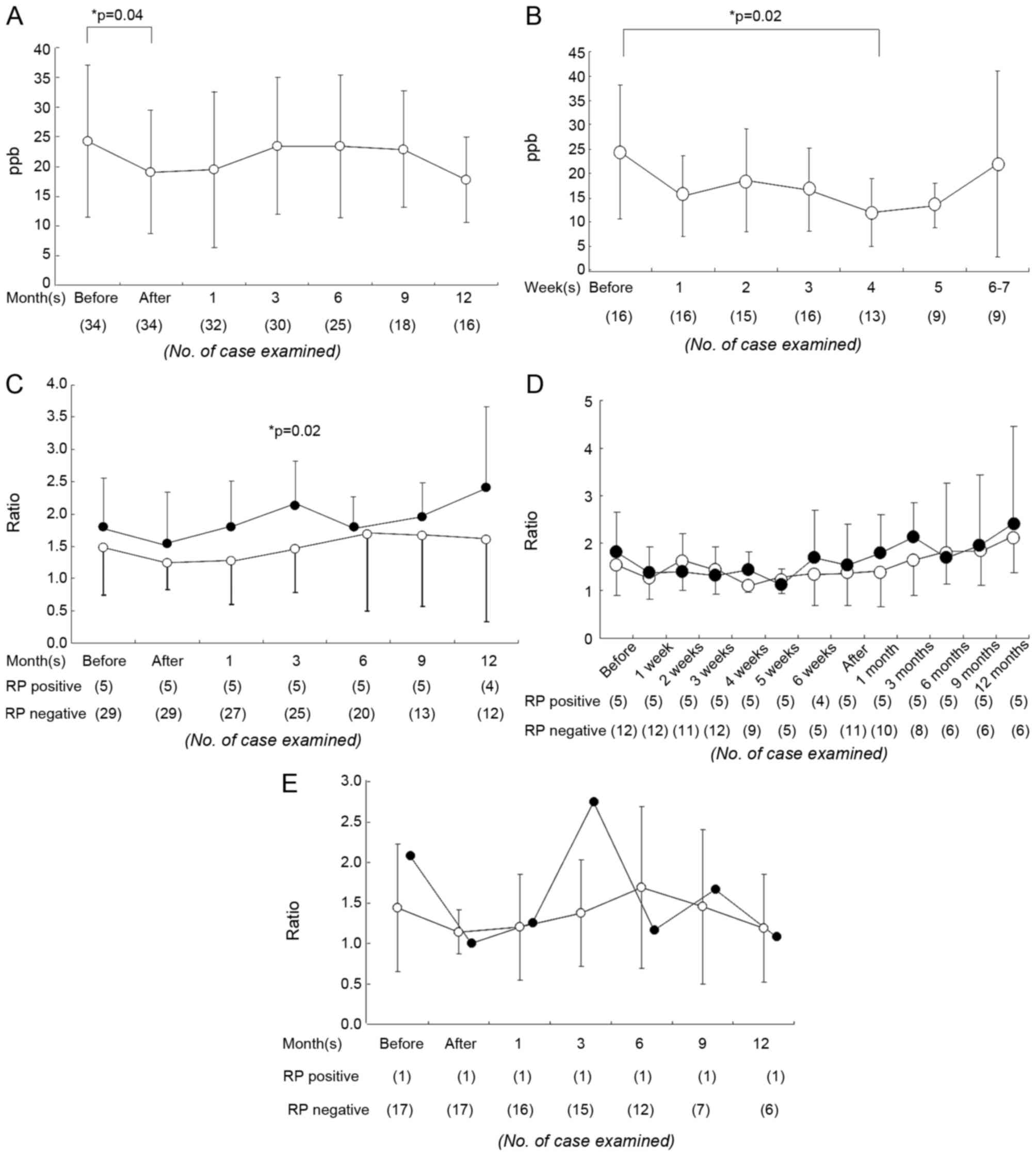
The mean ± standard deviation eNO level prior to RT
was 24.3±12.8 ppb; it decreased to 19.0±10.4 ppb following
treatment (immediately subsequent to RT, P=0.04; Fig. 1A). In the 3D-CRT group, a
statistically significant difference between the initial value and
the value at 4 weeks during RT was identified (Fig. 1B). Patients with a primary lung tumor
(n=25) exhibited an initial eNO level (23.4±13.0 ppb) higher than
healthy volunteers (15.7±8.4 ppb) to an extent that bordered on
significance (P=0.06). As the RT treatment reduced eNO levels, the
eNO ratio was used instead of the crude eNO value to examine the
variation thereafter.
A total of 5 patients (14%) exhibited RP of grade 2
or higher, including 1 patient receiving SBRT, which developed 3–5
months after RT (median 3 months). A comparison of basic patient
characteristics for RP+ and RP− patients is
presented in Table I. In patients
receiving 3D-CRT, the RP+ group exhibited a higher lung
irradiation dose (including mean lung dose and V20 values) than the
RP− group (P<0.01; Table
I). There was no difference between eNO levels in the
RP+ group (19.7±6.68 ppb prior to RT, 15.93±8.98 ppb
immediately subsequent to RT) and the RP− group
(24.4±13.7 ppb prior to RT, 19.6±10.7 ppb immediately subsequent to
RT) during the examined periods. The eNO ratio increased at the
onset of symptomatic RP; the symptomatic RP+ group
exhibited a 2.1±0.68-fold elevation in the eNO ratio at 3–5 months,
compared with a 1.4±0.6-fold elevation in the RP− group
(P=0.02; Fig. 1C). However, in
RP+ patients, there was no predictive elevation in the
crude eNO values or eNO ratio prior to the onset of RP.
Accordingly, an eNO ratio of 1.4 was used as the threshold ratio
for RP; this was associated with 100% sensitivity and 52%
specificity for indicating the occurrence of RP.
 | Table I.Characteristics and treatments of the
patients. |
Table I.
Characteristics and treatments of the
patients.
|
| Radiation pneumonitis
status |
|
|---|
|
|
|
|
|---|
| Characteristic | All patients | Negative | Positive | P-value |
|---|
| Total patients,
n | 33a | 28a | 5 |
|
| Age, median (range)
years | 78 (37–87) | 76 (62–80) | 78 (37–87) | 0.63 |
| Sex, n (%) |
|
|
| 0.41 |
|
Female | 8
(24) | 8
(29) | 0 (0) |
|
|
Male | 25 (76) | 20 (71) | 5
(100) |
|
| Disease, n (%) |
|
|
| 0.85 |
| Primary
lung cancer | 25 (76) | 22 (79) | 1
(20) |
|
|
Adenocarcinoma | 17 (51) |
|
|
|
|
Squamous cell
carcinoma | 2 (6) |
|
|
|
|
Others | 6
(18) |
|
|
|
|
Esophageal cancer | 2 (6) | 2 (7) | 0 (0) |
|
|
Metastatic lung cancer | 5
(15) | 5
(18) | 0 (0) |
|
| Treatment, n
(%) |
|
|
|
|
|
SBRT | 18 (53) | 17 (59) | 1
(20) | 0.26 |
|
48 Gy in 4
fractions | 15 (44) |
|
|
|
|
50 Gy in 5
fractions | 2 (6) |
|
|
|
|
60 Gy in 10
fractions | 1 (3) |
|
|
|
|
3D-CRT | 16 (47) | 12 (41) | 4
(80) |
|
|
60 Gy in 20
fractions | 2 (6) |
|
|
|
|
69 Gy in 23
fractions | 2 (6) |
|
|
|
|
66 Gy in 12
fractions | 1 (3) |
|
|
|
|
50 Gy in 25
fractions | 1 (3) |
|
|
|
|
54 Gy in 27
fractions | 1 (3) |
|
|
|
|
60 Gy in 30
fractions | 4 (12) |
|
|
|
|
65 Gy in 30
fractions | 1 (3) |
|
|
|
|
67.2 Gy in 34
fractions | 1 (3) |
|
|
|
|
64 Gy in 40
fractions | 2 (6) |
|
|
|
|
70 Gy in 42
fractions | 1 (3) |
|
|
|
| Concurrent
chemotherapy, n (%) |
|
|
| 0.71 |
|
Yes |
| 6
(21) | 2
(40) |
|
| No |
| 23 (79) | 3
(60) |
|
| Exhaled NO, median
(range) ppb |
| 19
(13–27) | 22
(8–60) | 0.77 |
| Krebs von den
Lungen-6, median (range) U/ml |
| 298 (173–567) | 372
(154–2,066) | 0.84 |
| Surfactant
protein-D, median (range) ng/ml |
| 47.6
(17.2–207) | 95.8
(55.1–167) | 0.03 |
| Mean lung dose,
median (range) Gy |
|
|
|
|
|
3D-CRT |
| 8.55
(1.84–13.38) | 14.49
(9.92–16.05) | 0.01 |
|
SBRT |
| 3.08
(1.42–5.81) | 3.58 | 0.77 |
| V20lung,
median (range) Gy |
|
|
|
|
|
3D-CRT |
| 14.49
(9.92–16.05) | 28.3
(17.6–31.8) | 0.01 |
|
SBRT |
| 3.21
(1.24–10.09) | 6.01 | 0.20 |
Serum SP-D
Serum SP-D level was also examined to assess its
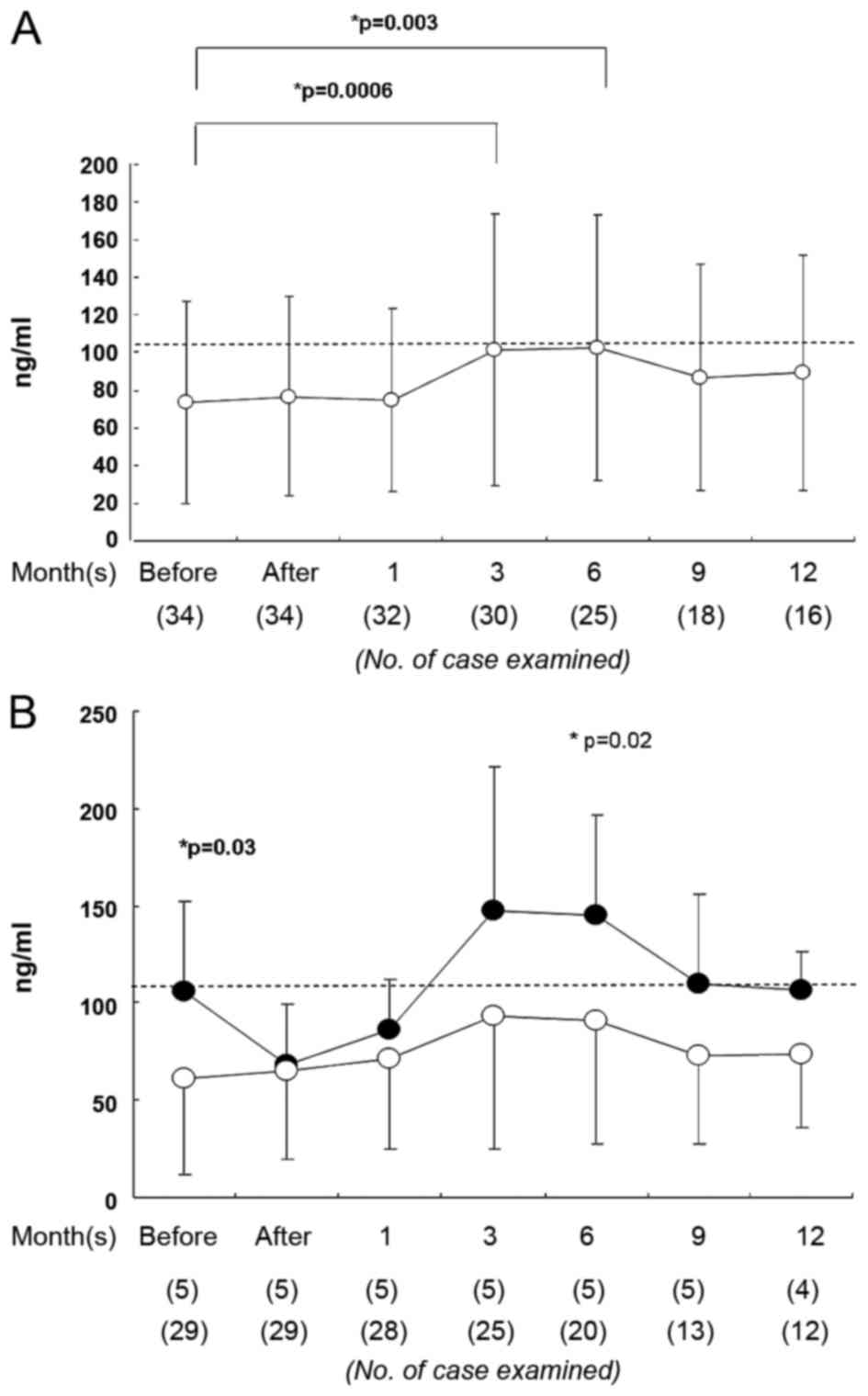
association with RP. The RT treatment did not significantly alter
serum SP-D levels (67.8±49.4 ng/ml prior to RT, 67.5±37.8 ng/ml
immediately subsequent to RT; Fig.
2A). However, the SP-D level was elevated at 3–6 months after
RT (Fig. 2A). The RP+ group exhibited
a significantly higher serum SP-D level than the RP- group prior to
RT (RP+, 105±46 ng/ml, RP-, 61±47 ng/ml; P=0.03; Fig. 2B). At 6 months later, the levels of
serum SP-D remained significantly different between these groups
(P=0.02; Fig. 2B). Serum SP-D level
prior to RT exhibited 83% specificity and 40% sensitivity for RP
prediction (Table II). None of the
patients with a serum SP-D level of ≤50 ng/ml prior to RT
experienced RP (Fig. 3). The criteria
of an eNO ratio <1.4 and SP-D >109 ng/ml provided 100%
sensitivity and 48% specificity for the prediction of RP, whereas
the criteria of an eNO ratio <1.4 or SP-D >109 ng/ml provided
40% sensitivity and 100% specificity.
 | Table II.Stratification based on serum SP-D
level prior to RT. |
Table II.
Stratification based on serum SP-D
level prior to RT.
|
|
| RP+ | RP− |
|---|
|
|
|
|
|
|---|
| Serum SP-D level
prior to RT | All patients,
n | n | Observation of all
cases of this type | n | Observation of all
cases of this type |
|---|
| High (>109
ng/ml) | 6 | 2 | Transient decrease
with resurgence at 1 month | 4 | High level
throughout the examination period |
| Low (≤109
ng/ml) | 28 | 3 | Gradual
increase | 25 | N/A |
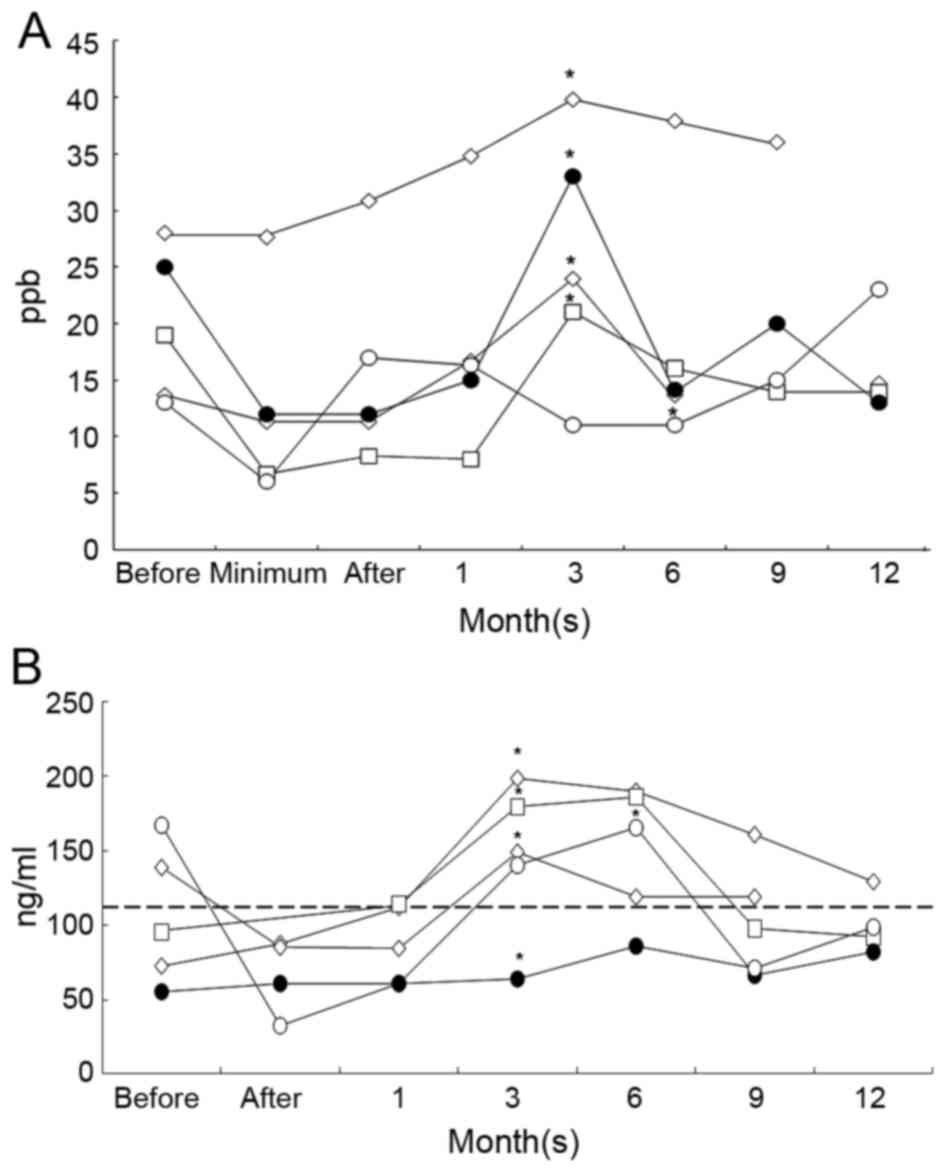
Among the 5 RP cases, 2 of the patients exhibited a
unique pattern of serum SP-D expression. The patients exhibited
high serum SP-D level prior to RT; those levels were initially
reduced by RT and surged to abnormally high values at the onset of
RP at 3 months (Fig. 3B). Of the 3
RP+ patients with low initial SP-D levels, 2 exhibited
an increase in serum SP-D to an abnormal level (>109 ng/ml) a
month subsequent to RT. The RT+ patient receiving SBRT (presenting
with large cell lung cancer, cT1N0M0) exhibited a gradual elevation
in serum SPD and eNO levels with patchy consolidation in CT images
immediately prior to RP onset. Following the remission of RP, eNO
level decreased to 14 ppb at 6 months.
Discussion
eNO levels increase in patients with Hodgkin
lymphoma and normalize following remission (13,14). A
similar effect has been reported for patients with lung cancer,
which exhibit increased eNO levels, including in our previous study
(6,15). It is established that tumors and
surrounding tissues produce NO. In the present study, eNO levels in
the patients with primary lung cancer was higher than in normal
volunteers to an extent which bordered on significance. This may be
explained by the characteristics of the patients in the present
study, as they were predominantly patients with early stage lung
cancer. Our previous study was concerning predominantly patients
with advanced cancer (6).
Serial measurements have demonstrated that eNO
diminishes subsequent to chemotherapy (16) and RT (6). A previous study demonstrated that at 8
days subsequent to carboplatin or cisplatin-based chemotherapy, the
eNO level decreased by 3.8 ppb in patients with lung cancer and
returned to the baseline value after 15 days (16). We previously reported that subsequent
to RT or combined chemo-RT in 29 patients, the eNO level had
decreased by 35% immediately subsequent to the completion of 40 Gy
RT (6). The decrease in the eNO level
observed following the treatment of chest malignancies is caused by
cell death in the tumor and the surrounding tissues; this may also
be accompanied by a reduction in tumor-associated inflammation,
including cytokine production (17).
The present study confirmed our previous data, demonstrating that
RT reduced the levels of eNO.
RP remains a dose-limiting side effect of RT,
impairing the quality of life of patients and potentially leading
to mortality (18). The level of the
inducible NO synthase (iNOS) expression in alveolar macrophages is
associated with the level of eNO (11). Immunohistochemical studies indicate
that alveolar macrophages are the major cellular site for NO
production (15,16). In mice, the alveolar macrophages
produce NO, and the expression of iNOS in alveolar macrophages and
epithelial cells is increased, subsequent to irradiation (15). Our previous study demonstrated that
irradiation itself does not increase the iNOS mRNA levels in
vitro; however, it enhances the expression of iNOS in the
presence of cytokines [tumor necrosis factor α and interleukin
(IL)-6] (16,19). Additionally, the progression of RP can
be reduced by treatment with an iNOS inhibitor (16,20).
A number of studies have demonstrated the value of
eNO measurements for predicting RP. In our previous study, a
three-fold increase in the eNO levels during RT treatment was
reported in 5 patients. A total of 3/5 of these patients developed
RP requiring steroid medication (6).
McCurdy et al (20) and
Guerrero et al (21) examined
28 patients with esophageal cancer and identified that a 1.5-fold
increase in eNO exhibited 100% sensitivity and specificity for the
prediction of RP (18,20). In another study by the same group, the
ratio of eNO (the level at the end of RT/pre-RT level) was
calculated for 50 lung or esophageal cancer patients. A threshold
of 1.4 could perfectly distinguish symptomatic and asymptomatic
patients (21). In contrast, Enache
et al (18) identified that
although the specificity of a 10 ppb increase in eNO level was
relatively specific (specificity, 83%) for predicting RP symptoms
in lung cancer patients, the sensitivity was low (18%). The data of
the present study supports their conclusion that 10-ppb elevation
from the initial eNO level does not always indicate RP. Their
results indicated that the basal level of eNO (i.e., prior to RT)
was not a good basis for RP prediction, and that the crude eNO
value was not suitable for such estimates (18). Moré et al (22) reported that acute changes in eNO
level, defined as percent changes between each pre-fraction and
post-fraction measurement, were significantly smaller in
RP+ than in RP− patients. The observations
from the present study confirm that eNO variation may be meaningful
in the prediction of RP occurrence. The minimum value during
treatment was used as the control level, rather than the initial
value. The minimum value may be the result of a reduction in
tumor-associated inflammation. In our previous study, RP occurred
earlier during RT (<2 months) with a larger extent of eNO level
variation; >3-fold elevation in comparison with the initial
level was identified (6). However, in
the present study, RP was observed at 3–5 months with a smaller,
1.4-fold increase from the minimum level.
The eNO level could not be used for the prediction
of RP in the present study. In the 20th century, RT using 2D
planning caused large volumes of tissue to be irradiated and
resulted in a RP rate of ~30%. This rate has been reduced to ~10%
with 3D-CRT and SBRT, which irradiate a reduced volume (23). It remains difficult to predict RP by
monitoring only during RT or the following month. The monitoring
period for these biomarkers should be extended to ≥2 months after
RT treatment. Data from ‘before’, at 4–5 weeks during RT or ‘after’
may all be candidates for the minimum value, as in the present
study, minimum values occurred at those time points. The most
common timing for minimum eNO as determined from the present study
was 4 weeks according to Fig. 1B;
calculating eNO ratio by measuring at that time point may be
useful.
There are a number of reports that serum SP-D is a
useful biomarker for RP prediction (7,8,24–26).
Yamashita et al (8,19) have reported that patients presenting
with an interstitial pneumonitis shadow in CT imaging and a high
value of the serum SP-D prior to SBRT treatment develop severe RP
(grade 4 or 5) at a relatively high rate. There was no correlation
between dose-volume histogram parameters and severe RP events
(24,25). The present study identified that an
increase in the serum SP-D level was associated with the occurrence
of RP. However, grade 2–3 RP was also significantly associated with
dose-volume histogram parameters, including mean lung dose and V20,
in the present study. This data suggests that grade 2–3 RP are
associated with dose-volume histogram parameters, whereas fatal RP
(grade 5) is not. This may vary depending on other patient
characteristics, including the interstitial lung condition
reflected by the increased SP-D level. RP grade >3 was not
encountered in the present study; however, it may be important to
monitor SP-D to prevent severe adverse reactions.
Sasaki et al (7) hypothesized that serum SP-D monitoring is
a practical and useful method for the early detection of RP.
However, its use is limited to patients with normal levels of serum
SP-D prior to the initiation of RT, because it was not predictive
for patients with values outside of the normal range (7). We concur that measuring the eNO levels
may also be valuable, particularly for high-risk patients with high
levels of SP-D and/or an interstitial pneumonitis shadow (15). For example, in our previous study,
lethal RP occurred in a patient with squamous cell lung cancer
(T2N1M0) and idiopathic interstitial pneumonitis (15). The patient exhibited an elevation in
eNO levels to 30–50 Gy, followed by a decrease to 10–20 Gy, twice
the minimum value. The patient also exhibited an abnormal shadow on
CT examination at 50 Gy. RT treatment was discontinued; however,
the patient developed fatal RP (15).
Sasaki et al (7) have also
identified a notable pattern of SP-D variation. In 3 patients with
primary lung adenocarcinoma that developed RP, the initial serum
SP-D levels were over twice the upper normal limit, but decreased
following RT. A similar phenomena was observed in the present study
in 2 patients with high serum SPD level prior to RT. The level was
reduced by RT and then resurged to abnormally high values after a
month; the RP was then observed after three months (Fig. 3). A potential hypothesis from this
observation is that the hyperproduction of SP-D, the interstitial
lung high-risk factor for RP, may be suppressed by RT and become
resurgent following the onset of RP.
As eNO is a non-specific inflammatory marker,
numerous other factors, including corticosteroid use, smoking and
diet, may have effects on eNO, causing difficulty in the
interpretation of eNO variations. The response of the lung to
radiation may also vary between patients. Zhang et al
(27) demonstrated that genetic
variants of NOS2 may serve as reliable predictors for RP.
The study genotyped a cohort of 301 patients for 21 single
nucleotide polymorphisms (SNPs) in the NOS2 gene. The
multivariate analysis identified that 3 SNPs (rs2297518, rs1137933
and rs16949) in NOS2 were significantly associated with the
risk of RP. Thus, a multiple biomarker analysis (including SP-D,
Krebs von den Lungen-6, transforming growth factor-β 1, IL-6 and
eNO) including genotype analysis could be a successful approach for
further study.
There are several limitations to the present study.
Firstly, it was a small study concerning a small number of patients
with a limited follow-up duration. Secondly, as no single biomarker
was adequate for the prediction of RP, only the best timing for
assessment and the best combination of biomarkers could be
explored. Finally, a good method for identifying patients with a
high risk of RP is required for serial biomarker monitoring.
In conclusion, obtaining the eNO ratio is a useful
RP monitoring tool, but does not predict the occurrence of RP in
the present setting. Serum SP-D level may be a potential predictor
for the detection of RP risk.
Acknowledgements
The present study was supported in part by a
Research Grant for Science and Cancer from the Ministry of
Education, Culture, Sports, Science and Technology, Japan (Kiban C
grant no. JP24591848).
References
|
1
|
Hart CM: Nitric oxide in adult lung
disease. Chest. 115:1407–1417. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alving K, Weitzberg E and Lundberg JM:
Increased amount of nitric oxide in exhaled air of asthmatics. Eur
Respir J. 6:1368–1370. 1993.PubMed/NCBI
|
|
3
|
Dweik RA, Boggs PB, Erzurum SC, Irvin CG,
Leigh MW, Lundberg JO, Olin AC, Plummer AL and Taylor DR: American
Thoracic Society Committee on Interpretation of Exhaled Nitric
Oxide Levels (FENO) for Clinical Applications: An official ATS
clinical practice guideline: Interpretation of exhaled nitric oxide
levels (FENO) for clinical applications. Am J Respir Crit Care Med.
184:602–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dummer JF, Epton MJ, Cowan JO, Cook JM,
Condliffe R, Landhuis CE, Smith AD and Taylor DR: Predicting
corticosteroid response in chronic obstructive pulmonary disease
using exhaled nitric oxide. Am J Respir Crit Care Med. 18:846–852.
2009. View Article : Google Scholar
|
|
5
|
Tiev KP, Hua-Huy T, Kettaneh A, Allanore
Y, Le-Dong NN, Duong-Quy S, Cabane J and Dinh-Xuan AT: Alveolar
concentration of nitric oxide predicts pulmonary function
deterioration in scleroderma. Thorax. 67:157–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koizumi M, Yamazaki H, Toyokawa K,
Yoshioka Y, Suzuki G, Ito M, Shinkawa K, Nishino K, Watanabe Y,
Inoue T, et al: Influence of thoracic radiotherapy on exhaled
nitric oxide levels in patients with lung cancer. Jpn J Clin Oncol.
31:142–146. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasaki R, Soejima T, Matsumoto A, Maruta
T, Yamada K, Ota Y, Kawabe T, Nishimura H, Sakai E, Ejima Y and
Sugimura K: Clinical significance of serum pulmonary surfactant
proteins A and D for the early detection of RP. Int J Radiat Oncol
Biol Phys. 50:301–307. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamashita H, Kobayashi-Shibata S, Terahara
A, Okuma K, Haga A, Wakui R, Ohtomo K and Nakagawa K: Prescreening
based on the presence of CT-scan abnormalities and biomarkers (KL-6
and SP-D) may reduce severe radiation pneumonitis after
stereotactic radiotherapy. Radiat Oncol. 5:322010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
International Union Against Cancer: TNM
Classification of Malignant Tumours. Sobin LH, Gospodarowicz MK and
Wittekind CH: 7th. Wiley-Blackwell; Hoboken, NJ: 2009
|
|
10
|
Cancer Therapy Evaluation Program: Common
Toxicity Criteria. Version 2.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdfAccessed.
April 30–1999.
|
|
11
|
Oxford University Press, . Prescribing,
recording, and reporting photon-beam intensity-modulated radiation
therapy (IMRT). J ICRU. 10:Report 83. 2010.
|
|
12
|
Cancer Therapy Evaluation Program: Common
Terminology Criteria for Adverse Events. CTCAEv 4.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htmAccessed.
September 14–2016.
|
|
13
|
Guida G, Culla B, Scirelli T, Bellone G,
Sciascia S, Brussino L, Novero D, Palestro G, Heffler E, Gavarotti
P, et al: Exhaled nitric oxide and nitric oxide synthase expression
in Hodgkin's disease. Int J Immunopathol Pharmacol. 22:1027–1034.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holmkvist T, Erlanson M, Meriläinen P and
Högman M: Exhaled nitric oxide is highly increased in a case of
Hodgkin's disease. Acta Oncol (Madr). 42:788–789. 2003. View Article : Google Scholar
|
|
15
|
Wewel AR, Crusius JA, Gatzemeier U,
Heckmayr M, Becher G, Magnussen H, Jörres RA and Holz O: Time
course of exhaled hydrogen peroxide and nitric oxide during
chemotherapy. Eur Respir J. 27:1033–1039. 2006.PubMed/NCBI
|
|
16
|
Liu CY, Wang CH, Chen TC, Lin HC, Yu CT
and Kuo HP: Increased level of exhaled nitric oxide and
up-regulation of inducible nitric oxide synthase in patients with
primary lung cancer. Br J Cancer. 78:534–541. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Enache I, Noel G, Jeung MY, Meyer N,
Oswald-Mammosser M, Urban-Kraemer E, Schumacher C, Geny B, Quoix E
and Charloux A: Can exhaled NO fraction predict
radiotherapy-induced lung toxicity in lung cancer patients? Radiat
Oncol. 7:1172012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toyokawa K, Yamazaki H, Koizuni M and
Inoue T and Inoue T: Assessment of exhaled NO concentration in
monitoring RP in patient who underwent thoracic radiotherapy for
lung cancer. Nihon Igaku Hoshasen Gakkai Zasshi. 61:347–349.
2001.(In Japanese). PubMed/NCBI
|
|
19
|
Koizumi M, Yamazaki H, Toyokawa K, Ozeki
S, Matsumura S and Inoue T and Inoue T: Influence of in vitro
radiation on changes in nitric oxide in rat macrophages and smooth
muscle cells. Anticancer Res. 23:331–334. 2003.PubMed/NCBI
|
|
20
|
McCurdy MR, Wazni MW, Martinez J, McAleer
MF and Guerrero T: Exhaled nitric oxide predicts RP in esophageal
and lung cancer patients receiving thoracic radiation. Radiother
Oncol. 101:443–448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guerrero T, Martinez J, McCurdy MR, Wolski
M and McAleer MF: Elevation in exhaled nitric oxide predicts for
radiation pneumonitis. Int J Radiat Oncol Biol Phys. 82:981–988.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moré JM, Eclov NC, Chung MP, Wynne JF,
Shorter JH, Nelson DD Jr, Hanlon AL, Burmeister R, Banos P, Maxim
PG, et al: Feasibility and potential utility of multicomponent
exhaled breath analysis for predicting development of radiation
pneumonitis after stereotactic ablative radiotherapy. J Thorac
Oncol. 9:957–964. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamazaki H, Tang JT and Inoue T, Teshima
T, Ohtani M, Ikeda H, Itou M, Takeuchi E and Inoue T: Radiographic
changes following radiotherapy in the patients with lung cancer. Is
the irradiated area of the mediastinum in the simulation film a
significant factor? Strahlenther Onkol. 171:272–277.
1995.PubMed/NCBI
|
|
24
|
Yamashita H, Takahashi W, Haga A and
Nakagawa K: Radiation pneumonitis after stereotactic radiation
therapy for lung cancer. World J Radiol. 6:708–715. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsuno Y, Satoh H, Ishikawa H, Kodama T,
Ohtsuka M and Sekizawa K: Simultaneous measurements of KL-6 and
SP-D in patients undergoing thoracic radiotherapy. Med Oncol.
23:75–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takahashi H, Imai Y, Fujishima T,
Shiratori M, Murakami S, Chiba H, Kon H, Kuroki Y and Abe S:
Diagnostic significance of surfactant proteins A and D in sera from
patients with radiation pneumonitis. Eur Respir J. 17:481–487.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Li B, Ding X, Sun M, Li H, Yang
M, Zhou C, Yu H, Liu H and Yu G: Genetic variants in inducible
nitric oxide synthase gene are associated with the risk of
radiation-induced lung injury in lung cancer patients receiving
definitive thoracic radiation. Radiother Oncol. 111:194–198. 2014.
View Article : Google Scholar : PubMed/NCBI
|