Introduction
Thyroid cancer is the most common endocrine system
malignancy and accounts for ~1% of all cancer diagnoses, with an
incidence that increases each year (1). Among thyroid malignancies, papillary
thyroid cancer (PTC) is the most common histological type,
accounting for 80–85% of all thyroid cancer and presenting with a
10-year survival rate of >90% (2).
PTC is not a unitary carcinoma; this malignancy includes several
sub-histological variants, including classical variant PTC (CVPTC)
and follicular variant PTC (FVPTC). Tall cell, diffuse sclerosing
and poorly differentiated PTC subtypes are categorized as
aggressive variant PTC (AVPTC). Although patients with PTC
typically demonstrate an excellent prognosis, cervical lymph node
metastasis (LNM) can be identified in 40–90% of cases at the time
of the first surgery (3). The most
typical LNM site is the central neck compartment (level VI). LNM
has been confirmed to be an independent risk factor for regional
recurrence (4–6) and decreased survival (7–9). The
American Joint Committee on Cancer advocates the surgical removal
of suspected lymph nodes detected by preoperative ultrasonography
(10). However, a routine central
neck lymph node dissection (LND) in patients with subclinical nodal
disease (cN0) is controversial. On one hand, LNM occurs in 60% of
patients with PTC on average (11)
and metastasis has been reported in ≤50% of dissected lymph nodes
in cN0 patients (12). Prophylactic
LND can remove subclinical metastatic lymph nodes, thus avoiding
potential recurrence in addition to aiding in disease staging for
radioiodine therapy (4,13). On the other hand, previous studies
have reported that prophylactic LND in cN0 patients increases the
rate of recurrent laryngeal nerve injury and hypoparathyroidism,
which would be disadvantageous for patients with PTC (14,15).
The T1799A nucleotide transversion in the B-Raf
proto-oncogene (BRAF) gene (NM_004333) causes a V600E amino acid
substitution and leads to constitutive activation of the
mitogen-activated protein kinase signaling pathway (16). Previous studies have demonstrated that
BRAFV600E is the most frequent genetic change in PTC,
with prevalence ranging from 27 to 83% (17,18). The
association between BRAFV600E and central LNM has been
widely investigated. The majority of studies support that
BRAFV600E is associated with the presence of LNM and the
probability of recurrence (18–20).
Certain studies considered that patients with the
BRAFV600E mutation should receive LND in the central
compartment (18,21,22).
However, other studies reported that the correlation between
BRAFV600E and tumor aggressiveness, involving LNM, was
not evident (23,24). Thus, this divergence in the
association between BRAFV600E and more aggressive
clinicopathological characteristics deserves further in-depth
investigation.
To date, to the best of our knowledge, just one
previous study has investigated the clinical significance of
BRAFV600E for central LNM based on preoperative central
lymph node status and various histological subtypes of PTC
(25). Therefore, the present study
was designed to separately assess the clinical significance of
BRAFV600E in patients with cN0 and cN1 PTC with
different histological subtypes.
Methods and patients
Patients
A total of 793 patients accepted thyroidectomy in
Department of Surgical Oncology of the First Affiliated Hospital of
Wenzhou Medical University (Wenzhou, China) between January 2007
and April 2011. These patients were diagnosed with PTC during the
final pathological examination. All patients came from the eastern
coastal regions in China. Eligibility criteria were as follows:
Patients' acceptance of total thyroidectomy plus central LND,
absence of a history of neck surgery or radiotherapy on the
thyroid, and absence of other types of head and neck cancer. A
total of 397 patients were excluded from the present study as they
only underwent thyroidectomy, 93 were disqualified for having a
previous history of neck surgery or radiotherapy on the thyroid
gland, and another 16 were omitted for their history of other types
of head and neck cancer. Finally, 287 patients with PTC with total
thyroidectomy plus central LND were enrolled.
Clinicopathological data included patients' age at
diagnosis, sex, tumor size, Hashimoto's thyroiditis, multifocality,
extrathyroidal extension, thyroid capsular invasion, central LNM
and tumor histological subtype. If >1 malignant nodule existed
in the thyroid gland, the largest nodule was analyzed. cN0 patients
were those patients without preoperative clinical or ultrasonic
evidence of central LNM and without suspicious lymph nodes
identified during surgery; otherwise, the patients were categorized
as cN1 patients. The Institutional Review Board of Wenzhou Medical
University approved the present study, and written informed consent
was obtained from all patients included.
DNA extraction and BRAF mutation
analysis
Genomic DNA was isolated from formalin-fixed
paraffin-embedded 7-µm tissue sections using a QIAamp DNA FFPE
Tissue kit (Qiagen GmbH, Hilden, Germany) according to the
manufacturer's protocol. BRAF exon 15 was amplified via polymerase
chain reaction (PCR) with the following primers: Forward,
5′-TCATAATGCTTGCTCTGATAGGA-3′ and reverse,
5′-GGCCAAAAATTTAATCAGTGGA-3′. A 3-µl sample of the DNA template was
added into a 50 µl (total volume) reaction containing 25 µl 2X PCR
Reagent (containing 0.1 U/µl Taq polymerase, 500 µM dNTP each, 20
mM Tris-HCl, 100 mM KCl, 3 mM MgCl2), 2 µl of each
primer, and 18 µl dH2O (all Tiangen Biotech Co., Ltd.,
Beijing, China). The PCR conditions were 94°C for 3 min, followed
by 35 cycles of 94°C for 30 sec, 55°C for 30 sec, 72°C for 1 min
and 72°C for 5 min. The quality of the PCR products was confirmed
by 2% agarose gel electrophoresis. Each test was repeated in
triplicate, with dH2O used as the negative control. The
PCR products were sequenced using the BigDye Terminator v3.1 Cycle
Sequencing kit (Applied Biosystems, Foster City, USA) on an ABI
PRISM 3730XL DNA Analyzer (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) to identify the mutation.
Statistical analysis
Statistical analysis was performed using IBM SPSS
software (version 19.0; IBM Corp., Armonk, NY, USA). A univariate
analysis was conducted to reveal the risk factors associated with
central LNM in different histological subtypes of PTC. Factors that
demonstrated significant differences were further tested through
multivariate analysis. Data were described as the number of cases
for categorical variables. Confrontations between different groups
were performed using a χ2 test. When the sample size was
<40 or with a theoretical frequency of <1, Fisher's exact
test was used instead. For continuous variables, data were
described as the mean ± standard deviation. A multivariate analysis
was performed using binary logistic regression. The results were
presented as odds ratios (ORs) with 95% confidence intervals (CIs).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient clinicopathological
characteristics
A total of 287 patients with PTC were included in
the present study. Patient ages ranged between 9 and 77 years, with
a mean of 43.82±11.96 years. The female-to-male ratio was 4.86 (49
males vs. 238 females). A total of 1,985 lymph nodes from the
central compartment were dissected, among which 837 were identified
as cancer metastases by pathologists (2.9 metastatic lymph nodes to
6.9 total lymph nodes dissected from each patient on average).
Central LNM was identified in 189 patients (65.85%), and underlying
Hashimoto's thyroiditis was identified in 112 patients (39.02%).
Other characteristics such as multifocality, extrathyroidal
extension and thyroid capsular invasion were identified in 85
(29.62%), 87 (30.31%) and 109 (37.98%) patients, respectively.
Among the patients, 172 (59.93%) were patients with cN0 PTC,
whereas the other 115 (40.07%) were patients with cN1 PTC.
According to the histological subtype classification, 177 (61.67%)
and 94 (32.75%) patients were diagnosed with CVPTC and FVPTC,
respectively. The remaining 16 patients (5.57%) were grouped under
AVPTC, which included tall cell, diffuse sclerosing and poorly
differentiated PTC subtypes.
Distribution of central LNM and
BRAFV600E
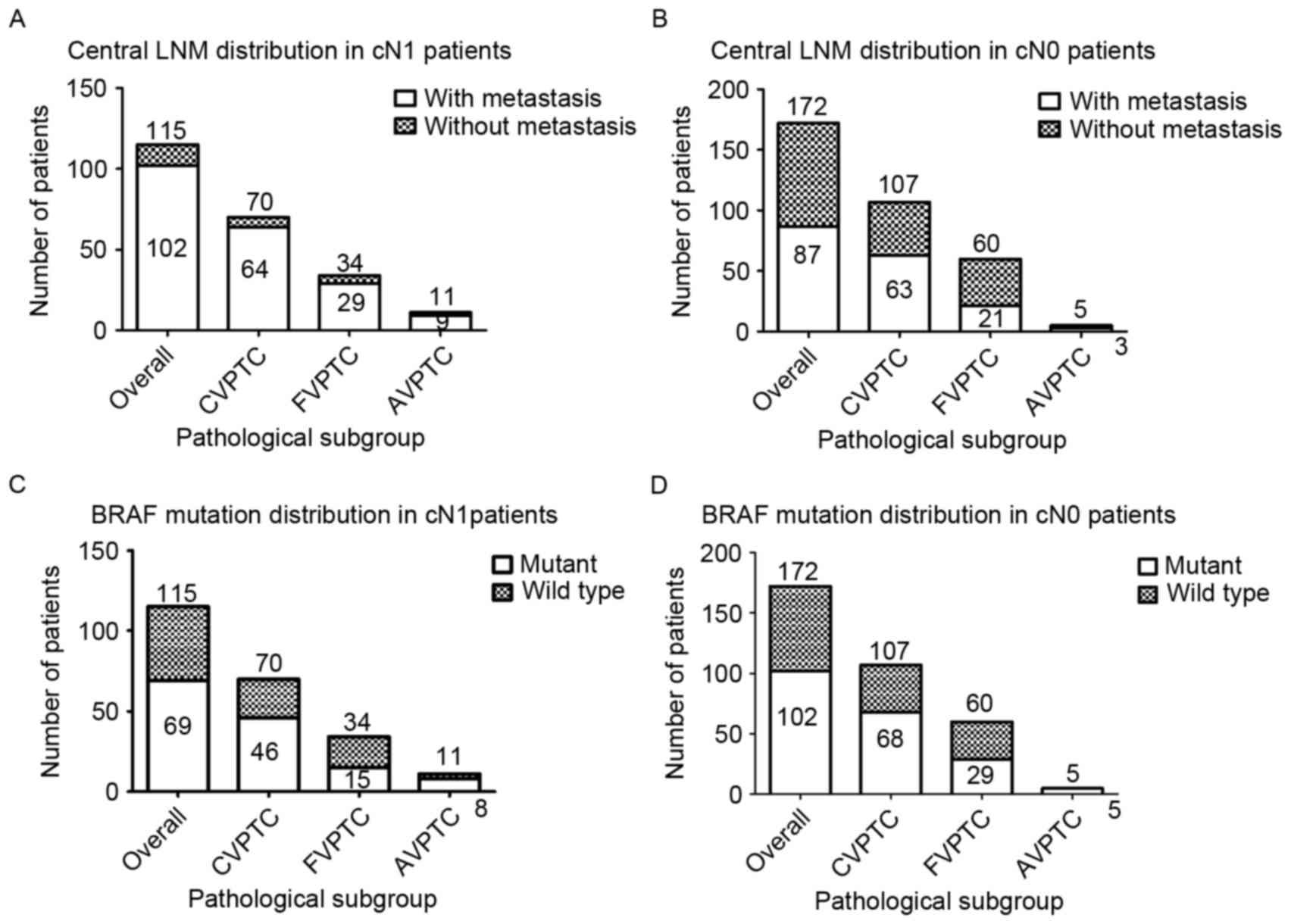
Among the patients with cN0 PTC, LNM could be
identified in 63 patients (58.88%) in the CVPTC group, 21 patients
(35.00%) in the FVPTC group and 3 patients (60.00%) in the AVPTC
group; the proportion in the cN1 group was 64 (91.43%), 29 (85.29%)
and 9 (81.82%) patients in the CVPTC, FVPTC and AVPTC groups,
respectively (Fig. 1A and B).
Similarly, among all patients with cN0 PTC, BRAFV600E
was detected in 68 patients (63.55%) in the CVPTC group, 29
patients (48.33%) in the FVPTC group and all 5 patients (100.00%)
in the AVPTC group; the proportion in the cN1 group was 46
(65.71%), 15 (44.12%) and 8 (72.72%) patients in the CVPTC, FVPTC,
and AVPTC groups, respectively (Fig. 1C
and D).
Univariate and multivariate analyses
in all patients with PTC
Among all patients with PTC (Table I), BRAFV600E was associated
with a higher rate of central LNM (73.10 vs. 55.17%; P=0.002).
Underlying Hashimoto's thyroiditis was negatively correlated with
central LNM (58.93 vs. 70.29%, P=0.048). In addition, central LNM
occurred more frequently in patients with larger tumor size,
extrathyroidal extension, thyroid capsular invasion and
BRAFV600E (82.56 vs. 58.71%, P<0.001; 78.16 vs.
60.50%, P=0.004; and 76.15 vs. 56.08%, P=0.004, respectively).
These significantly different factors were then analyzed by
multivariate logistic regression. The results demonstrated that
BRAFV600E was independently associated with central LNM
with an OR value of 1.708 (95% CI, 1.004–2.094; P=0.048). In
addition, large tumor size was an independent risk factor for
central LNM with an OR value of 2.621 (95% CI, 1.365–5.034;
P=0.004).
 | Table I.Univariate and multivariate analyses
of associations between central LNM and clinicopathological
characteristics in overall patients with PTC. |
Table I.
Univariate and multivariate analyses
of associations between central LNM and clinicopathological
characteristics in overall patients with PTC.
|
|
| Univariate
analyses |
|
|
|---|
|
|
|
|
|
|
|---|
|
|
| Central LNM | Multivariate
analyses |
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | Total, n | +, n (%) | −, n (%) | P-value | OR (95% CI) | P-value |
|---|
| Total number | 287 | 189 | 98 |
|
|
|
| Age at diagnosis,
years |
|
|
|
|
|
|
|
≥45 | 129 | 87 (67.44) | 42 (32.56) | 0.608 |
|
|
<45 | 158 | 102 (64.56) | 56 (35.44) |
|
|
| Sex |
|
|
|
|
|
|
|
Female | 238 | 155 (65.13) | 83 (34.83) | 0.567 |
|
|
|
Male | 49 | 34 (69.39) | 15 (30.61) |
|
|
|
| Pathological tumor
size, cm |
|
|
|
|
|
|
|
>2 | 86 | 71 (82.56) | 15 (17.44) | 0.000 |
2.621(1.365–5.034) | 0.004 |
| ≤2 | 201 | 118 (58.71) | 83 (41.29) |
|
|
|
| Underlying
Hashimoto's thyroiditis |
|
|
|
|
|
|
|
Present | 112 | 66 (58.93) | 46 (41.07) | 0.048 | 0.652
(0.396–1.099) | 0.108 |
|
Absent | 175 | 123 (70.29) | 52 (29.71) |
|
|
|
| Multifocality |
|
|
|
|
|
|
|
Present | 85 | 63 (74.12) | 22 (25.88) | 0.055 |
|
|
| Absent | 202 | 126 (62.38) | 76 (37.62) |
|
|
|
| Extrathyroidal
extension |
|
|
|
|
|
|
|
Present | 87 | 68 (78.16) | 19 (21.84) | 0.004 | 1.391
(0.702–2.757) | 0.344 |
|
Absent | 200 | 121 (60.50) | 79 (39.50) |
|
|
|
| Thyroid capsular
invasion |
|
|
|
|
|
|
|
Present | 109 | 83 (76.15) | 26 (23.85) | 0.004 | 1.477
(0.794–2.747) | 0.218 |
|
Absent | 178 | 106 (56.08) | 72 (73.47) |
|
|
|
| BRAF mutation |
|
|
|
|
|
|
|
Positive | 171 | 125 (73.10) | 46 (26.90) | 0.002 | 1.708
(1.004–2.094) | 0.048 |
|
Negative | 116 | 64 (55.17) | 52 (44.83) |
|
|
|
Among all patients with cN1 PTC (Table II), BRAFV600E was the only
factor correlated with central LNM (94.20 vs. 80.43%; P=0.022).
Only 13 patients did not exhibit central LNM in this cohort.
Central LNM had a higher prevalence in patients with large tumor
size (95.24 vs. 84.93%; P=0.169), extrathyroidal extension (95.56
vs. 84.29%; P=0.063) and thyroid capsular invasion (93.62 vs.
85.29%, P=0.166); however, these results were not statistically
significant.
 | Table II.Univariate analyses of associations
between central LNM and clinicopathological features in overall
patients with cN1 PTC. |
Table II.
Univariate analyses of associations
between central LNM and clinicopathological features in overall
patients with cN1 PTC.
| Clinicopathological
characteristic | Total, n | Central LNM(+), n
(%) | Central LNM(−), n
(%) | P-value |
|---|
| Total number | 115 | 102 | 13 |
|
| Age at diagnosis,
years |
|
|
|
|
|
≥45 | 60 | 53 (88.33) | 7 (11.67) | 0.898 |
|
<45 | 55 | 49 (89.09) | 6 (10.90) |
|
| Sex |
|
|
|
|
|
Female | 90 | 78 (86.67) | 12 (13.33) | 0.344a |
|
Male | 25 | 24 (96.00) | 1 (4.00) |
|
| Pathological tumor
size, cm |
|
|
|
|
|
>2 | 42 | 40 (95.24) | 2 (4.76) | 0.169a |
| ≤2 | 73 | 62 (84.93) | 11 (15.07) |
|
| Underlying
Hashimoto's thyroiditis |
|
|
|
|
|
Present | 42 | 37 (88.10) | 5 (11.90) | 1.000a |
|
Absent | 73 | 65 (89.04) | 8 (10.96) |
|
| Multifocality |
|
|
|
|
|
Present | 44 | 38 (86.36) | 6 (13.64) | 0.750a |
| Absent | 71 | 64 (90.14) | 7 (9.86) |
|
| Extrathyroidal
extension |
|
|
|
|
|
Present | 45 | 43 (95.56) | 2 (4.44) | 0.063 |
|
Absent | 70 | 59 (84.29) | 11 (15.71) |
|
| Thyroid capsular
invasion |
|
|
|
|
|
Present | 47 | 44 (93.62) | 3 (6.38) | 0.166 |
|
Absent | 68 | 58 (85.29) | 10 (14.71) |
|
| BRAF mutation |
|
|
|
|
|
Positive | 69 | 65 (94.20) | 4 (5.80) | 0.022 |
|
Negative | 46 | 37 (80.43) | 9 (19.57) |
|
Among all patients with cN0 PTC (Table III), central LNM was identified in
58.82% of BRAFV600E-positive patients compared with
38.57% of BRAFV600E-negative patients (P=0.009).
Additionally, a higher rate of central LNM was revealed in patients
with larger tumor size and thyroid capsular invasion (70.45 vs.
43.75%, P=0.002; and 62.90 vs. 43.64%, P=0.015, respectively).
Underlying Hashimoto's thyroiditis was negatively correlated with
central LNM (41.43 vs. 56.86%, P=0.047). These four significantly
different factors were further examined using multivariate
analysis. Large tumor size was the only independent risk factor for
central LNM with an OR value of 2.633 (95% CI, 1.189–5.832;
P=0.017). However, BRAFV600E was not associated with
central LNM in the multivariate analysis (P=0.059), which was
inconsistent with the overall result for the patients with PTC in
the present study.
 | Table III.Univariate and multivariate analyses
of associations between central LNM and clinicopathological
features in overall patients with cN0 PTC. |
Table III.
Univariate and multivariate analyses
of associations between central LNM and clinicopathological
features in overall patients with cN0 PTC.
|
|
| Univariate
analyses |
|
|
|---|
|
|
|
|
|
|
|---|
|
|
| Central LNM | Multivariate
analyses |
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | Total, n | +, n (%) | −, n (%) | P-value | OR (95% CI) | P-value |
|---|
| Total number | 172 | 87 | 85 |
|
|
|
| Age at diagnosis,
years |
|
|
|
|
|
|
|
≥45 | 69 | 34 (49.28) | 35 (50.72) | 0.779 |
|
|
|
<45 | 103 | 53 (51.46) | 50 (48.54) |
|
|
|
| Sex |
|
|
|
|
|
|
|
Female | 148 | 77 (52.03) | 71 (47.97) | 0.346 |
|
|
|
Male | 24 | 10 (41.67) | 14 (58.33) |
|
|
|
| Pathological tumor
size, cm |
|
|
|
|
>2 | 44 | 31 (70.45) | 13 (29.55) | 0.002 | 2.633
(1.189–5.832) | 0.017 |
| ≤2 | 128 | 56 (43.75) | 72 (56.25) |
|
|
|
| Underlying
Hashimoto's thyroiditis |
|
|
|
|
|
|
|
Present | 70 | 29 (41.43) | 41 (58.57) | 0.047 | 0.559
(0.288–1.086) | 0.086 |
|
Absent | 102 | 58 (56.86) | 44 (43.14) |
|
|
|
| Multifocality |
|
|
|
|
|
|
|
Present | 41 | 25 (60.98) | 16 (39.02) | 0.127 |
|
|
|
Absent | 131 | 62 (47.33) | 69 (52.67) |
|
|
|
| Extrathyroidal
extension |
|
|
|
|
|
|
|
Present | 42 | 25 (59.52) | 17 (40.48) | 0.182 |
|
|
|
Absent | 130 | 62 (47.69) | 68 (52.31) |
|
|
|
| Thyroid capsular
invasion |
|
|
|
|
|
|
|
Present | 62 | 39 (62.90) | 23 (37.10) | 0.015 | 1.692
(0.850–3.370) | 0.134 |
|
Absent | 110 | 48 (43.64) | 62 (56.36) |
|
|
|
| BRAF mutation |
|
|
|
|
|
|
|
Positive | 102 | 60 (58.82) | 42 (41.18) | 0.009 | 1.882
(0.976–3.629) | 0.059 |
|
Negative | 70 | 27 (38.57) | 43 (61.43) |
|
|
|
Univariate and multivariate analyses
of patients with CVPTC
A total of 177 patients were diagnosed with CVPTC in
the present study; 70 (39.55%) of these patients were cN1, and 107
(60.45%) were cN0. Tumor size >2 cm (87.27 vs. 64.75%; P=0.002),
underlying Hashimoto's thyroiditis (60.81 vs. 79.61%; P=0.006),
extrathyroidal extension (86.00 vs. 66.14%; P=0008), thyroid
capsular invasion (87.93 vs. 63.87%; P=0.001) and
BRAFV600E (79.82 vs. 57.14%; P=0.001) were all
correlated with central LNM in the univariate analysis (Table IV). The aforementioned factors were
then included in the multivariate analysis. Tumor size >2 cm
(OR, 2.656; 95% CI, 1.049–6.724; P=0.039), underlying Hashimoto's
thyroiditis (OR, 0.410; 95% CI, 0.200–0.841; P=0.015) and thyroid
capsular invasion (OR, 2.941; 95% CI, 1.054–8.202; P=0.039) were
all independently associated with central LNM. Notably,
BRAFV600E was independently associated with central LNM
in patients with CVPTC (OR, 2.243; 95% CI, 1.080–4.658;
P=0.030).
 | Table IV.Univariate and multivariate analyses
of associations between central LNM and clinicopathological
features in patients with CVPTC. |
Table IV.
Univariate and multivariate analyses
of associations between central LNM and clinicopathological
features in patients with CVPTC.
|
|
| Univariate
analyses |
|
|
|---|
|
|
|
|
|
|
|---|
|
|
| Central LNM | Multivariate
analyses |
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | Total, n | +, n (%) | −, n (%) | P-value | OR (95% CI) | P-value |
|---|
| Total number | 177 | 127 | 50 |
|
|
|
| Age at diagnosis,
years |
|
|
|
|
|
|
|
≥45 | 75 | 56 (74.67) | 19 (25.33) | 0.460 |
|
|
|
|
<45 | 102 | 71 (69.61) | 31 (3.39) |
|
|
|
| Sex |
|
|
|
|
|
|
|
Female | 148 | 103 (69.59) | 45 (30.41) | 0.150 |
|
|
|
Male | 29 | 24 (82.76) | 5 (17.24) |
|
|
|
| Pathological tumor
size, cm |
|
|
|
|
|
|
|
>2 | 55 | 48 (87.27) | 7 (12.73) | 0.002 | 2.656
(1.049–6.724) | 0.039 |
| ≤2 | 122 | 79 (64.75) | 43 (35.25) |
|
|
|
| Underlying
Hashimoto's thyroiditis |
|
|
|
|
|
|
|
Present | 74 | 45 (60.81) | 29 (39.19) | 0.006 | 0.410
(0.200–0.841) | 0.015 |
|
Absent | 103 | 82 (79.61) | 21 (20.39) |
|
|
|
| Multifocality |
|
|
|
|
|
|
|
Present | 50 | 41 (82.00) | 9 (18.00) | 0.057 |
|
|
|
Absent | 127 | 86 (67.72) | 41 (32.28) |
|
|
|
| Extrathyroidal
extension |
|
|
|
|
|
|
|
Present | 50 | 43 (86.00) | 7 (14.00) | 0.008 | 1.358
(0.470–3.926) | 0.572 |
|
Absent | 127 | 84 (66.14) | 43 (33.86) |
|
|
|
| Thyroid capsular
invasion |
|
|
|
|
|
|
|
Present | 58 | 51 (87.93) | 7 (12.07) | 0.001 | 2.941
(1.054–8.202) | 0.039 |
|
Absent | 119 | 76 (63.87) | 43 (36.13) |
|
|
|
| BRAF mutation |
|
|
|
|
|
|
|
Positive | 114 | 91 (79.82) | 23 (20.18) | 0.001 | 2.243
(1.080–4.658) | 0.030 |
|
Negative | 63 | 36 (57.14) | 27 (42.86) |
|
|
|
Similar analyses were performed on 70 patients with
cN1 CVPTC (Table V). The results
revealed that only thyroid capsular invasion was associated with
central LNM (100.00 vs. 85.37%; P=0.038). Other factors, including
BRAFV600E (95.65 vs. 83.33%, P=0.171) were not
statistically different between the central LNM-positive and
-negative groups.
 | Table V.Univariate analyses of associations
between central LNM and clinicopathological features in patients
with cN1 CVPTC. |
Table V.
Univariate analyses of associations
between central LNM and clinicopathological features in patients
with cN1 CVPTC.
| Clinicopathological
characteristic | Total, n | Central LNM (+), n
(%) | Central LNM (−), n
(%) | P-value |
|---|
| Total number | 70 | 64 | 6 |
|
| Age at diagnosis,
years |
|
|
|
|
|
≥45 | 37 | 35 (94.59) | 2 (5.41) | 0.411a |
|
<45 | 33 | 29 (87.88) | 4 (12.12) |
|
| Sex |
|
|
|
|
|
Female | 53 | 48 (90.67) | 5 (9.43) | 1.000a |
|
Male | 17 | 16 (94.12) | 1 (5.88) |
|
| Pathological tumor
size, cm |
|
|
|
|
|
>2 | 27 | 27 (100.00) | 0 (0.00) | 0.075a |
| ≤2 | 43 | 37 (86.05) | 6 (13.95) |
|
| Underlying
Hashimoto's thyroiditis |
|
|
|
|
|
Present | 25 | 22 (88.00) | 3 (12.00) | 0.659a |
|
Absent | 45 | 42 (93.33) | 3 (6.67) |
|
| Multifocality |
|
|
|
|
|
Present | 26 | 24 (92.31) | 2 (7.69) | 1.000a |
|
Absent | 44 | 40 (90.91) | 4 (9.09) |
|
| Extrathyroidal
extension |
|
|
|
|
|
Present | 27 | 27 (100.00) | 0 (0.00) | 0.075a |
|
Absent | 43 | 37 (86.05) | 6 (13.95) |
|
| Thyroid capsular
invasion |
|
|
|
|
|
Present | 29 | 29 (100.00) | 0 (0.00) | 0.038a |
|
Absent | 41 | 35 (85.37) | 6 (14.63) |
|
| BRAF mutation |
|
|
|
|
|
Positive | 46 | 44 (95.65) | 2 (4.35) | 0.171a |
|
Negative | 24 | 20 (83.33) | 4 (16.67) |
|
Among the 107 patients with cN0 CVPTC (Table VI), central LNM was correlated with
BRAFV600E (69.12 vs. 41.03%; P=0.004), tumor size >2
cm (75.00 vs. 53.16%; P=0.044) and thyroid capsular invasion (75.86
vs. 52.56%; P=0.029). Underlying Hashimoto's thyroiditis was
negatively associated with central LNM (46.94 vs. 68.97%, P=0.021).
When these factors were included in the multivariate analysis,
BRAFV600E was identified to be an independent risk
factor for central LNM (P=0.032), with an OR value of 2.586 (95%
CI, 1.087–6.151), and underlying Hashimoto's thyroiditis was an
independent negative factor in this cohort (P=0.042), with an OR
value of 0.411 (95% CI, 0.174–0.970).
 | Table VI.Univariate and multivariate analyses
of associations between central LNM and clinicopathological
features in patients with cN0 CVPTC. |
Table VI.
Univariate and multivariate analyses
of associations between central LNM and clinicopathological
features in patients with cN0 CVPTC.
|
|
| Univariate
analyses |
|
|
|---|
|
|
|
|
|
|
|---|
|
|
| Central LNM | Multivariate
analyses |
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | Total, n | +, n (%) | −, n (%) | P-value | OR (95% CI) | P-value |
|---|
| Total number | 107 | 63 | 44 |
|
|
|
| Age at diagnosis,
years |
|
|
|
|
|
|
|
≥45 | 38 | 21 (55.26) | 17 (44.74) | 0.573 |
|
|
|
<45 | 69 | 42 (60.87) | 27 (39.13) |
|
|
|
| Sex |
|
|
|
|
|
|
|
Female | 95 | 55 (57.89) | 40 (42.11) | 0.787a |
|
|
|
Male | 12 | 8 (66.67) | 4 (33.33) |
|
|
|
| Pathological tumor
size, cm |
|
|
|
|
|
|
|
>2 | 28 | 21 (75.00) | 7 (25.00) | 0.044 | 2.261
(0.790–6.468) | 0.128 |
| ≤2 | 79 | 42 (53.16) | 37 (46.84) |
|
|
|
| Underlying
Hashimoto's thyroiditis |
|
|
|
|
|
|
|
Present | 49 | 23 (46.94) | 26 (53.06) | 0.021 | 0.411
(0.174–0.970) | 0.042 |
|
Absent | 58 | 40 (68.97) | 18 (31.03) |
|
|
|
| Multifocality |
|
|
|
|
|
|
|
Present | 24 | 17 (70.83) | 7 (29.17) | 0.177 |
|
|
|
Absent | 83 | 46 (55.42) | 37 (44.58) |
|
|
|
| Extrathyroidal
extension |
|
|
|
|
|
|
|
Present | 23 | 16 (69.57) | 7 (30.43) | 0.240 |
|
|
|
Absent | 84 | 47 (55.95) | 37 (44.05) |
|
|
|
| Thyroid capsular
invasion |
|
|
|
|
|
|
|
Present | 29 | 22 (75.86) | 7 (24.14) | 0.029 | 2.512
(0.885–7.135) | 0.084 |
|
Absent | 78 | 41 (52.56) | 37 (47.44) |
|
|
|
| BRAF mutation |
|
|
|
|
|
|
|
Positive | 68 | 47 (69.12) | 21 (30.88) | 0.004 | 2.586
(1.087–6.151) | 0.032 |
|
Negative | 39 | 16 (41.03) | 23 (58.97) |
|
|
|
Univariate and multivariate analyses
of patients with FVPTC
The aforementioned factors were analyzed in a total
of 94 patients with FVPTC (Table
VII), which comprised 34 patients with cN1 FVPTC (Table VIII) and 60 patients with cN0 FVPTC
(Table IX). However, central LNM was
only associated with thyroid capsular invasion in the patients with
cN0 FVPTC (P=0.015). None of the other factors were associated with
central LNM regardless of preoperative lymph node status. Central
LNM rate was not significantly different between
BRAFV600E-positive and -negative patients (54.55 vs.
52.00%, P=0.805 in all patients with FVPTC; 93.33 vs. 78.95%,
P=0.355 in cN1 patients; 34.48 vs. 35.48%, P=0.935 in cN0
patients).
 | Table VII.Univariate analyses of associations
between central LNM and clinicopathological characteristics in
patients with FVPTC. |
Table VII.
Univariate analyses of associations
between central LNM and clinicopathological characteristics in
patients with FVPTC.
| Clinicopathological
characteristic | Total, n | Central LNM (+), n
(%) | Central LNM (−), n
(%) | P-value |
|---|
| Total number | 94 | 50 | 44 |
|
| Age at diagnosis,
years |
|
|
|
|
|
≥45 | 49 | 27 (55.10) | 22 (44.90) |
|
|
<45 | 45 | 23 (51.11) | 22 (48.89) | 0.689 |
| Sex |
|
|
|
|
|
Female | 78 | 42 (53.85) | 36 (46.15) | 0.779 |
|
Male | 16 | 8 (50.00) | 8 (50.18) |
|
| Pathological tumor
size, cm |
|
|
|
|
|
>2 | 24 | 16 (66.67) | 8 (33.33) | 0.125 |
| ≤2 | 70 | 34 (48.57) | 36 (51.43) |
|
| Underlying
Hashimoto's thyroiditis |
|
|
|
|
|
Present | 31 | 16 (51.61) | 15 (48.39) | 0.83 |
|
Absent | 63 | 34 (53.97) | 29 (46.03) |
|
| Multifocality |
|
|
|
|
|
Present | 28 | 17 (60.71) | 11 (39.29) | 0.341 |
|
Absent | 66 | 33 (50.00) | 33 (50.00) |
|
| Extrathyroidal
extension |
|
|
|
|
|
Present | 32 | 21 (65.63) | 11 (34.37) | 0.083 |
|
Absent | 62 | 29 (46.77) | 33 (53.23) |
|
| Thyroid capsular
invasion |
|
|
|
|
|
Present | 46 | 28 (60.87) | 18 (39.13) | 0.144 |
|
Absent | 48 | 22 (45.83) | 26 (54.17) |
|
| BRAF mutation |
|
|
|
|
|
Positive | 44 | 24 (54.55) | 20 (45.45) | 0.805 |
|
Negative | 50 | 26 (52.00) | 24 (48.00) |
|
 | Table VIII.Univariate analyses of associations
between central LNM and clinicopathological characteristics in
patients with cN1 FVPTC. |
Table VIII.
Univariate analyses of associations
between central LNM and clinicopathological characteristics in
patients with cN1 FVPTC.
| Clinicopathological
characteristic | Total, n | Central LNM (+), n
(%) | Central LNM (−), n
(%) | P-value |
|---|
| Total number | 34 | 29 | 5 |
|
| Age at diagnosis,
years |
|
|
|
|
|
≥45 | 20 | 16 (80.00) | 4 (20.00) |
|
|
<45 | 14 | 13 (92.86) | 1 (7.14) | 0.298a |
| Sex |
|
|
|
|
|
Female | 28 | 23 (82.14) | 5 (17.86) | 0.559a |
|
Male | 6 | 6 (100.00) | 0 (0.00) |
|
| Pathologic tumor
size, cm |
|
|
|
|
| ≤2 | 24 | 21 (87.50) | 3 (12.50) | 0.618a |
|
>2 | 10 | 8 (80.00) | 2 (20.00) |
|
| Underlying
Hashimoto's thyroiditis |
|
|
|
|
|
Present | 12 | 11 (91.67) | 1 (8.33) | 0.635a |
|
Absent | 22 | 18 (81.82) | 4 (18.18) |
| Multifocality |
|
|
|
|
|
Present | 12 | 10 (83.33) | 2 (16.67) | 1.000a |
|
Absent | 22 | 19 (86.36) | 3 (13.64) |
|
| Extrathyroidal
extension |
|
|
|
|
|
Present | 15 | 13 (86.67) | 2 (13.33) | 1.000a |
|
Absent | 19 | 16 (84.21) | 3 (15.79) |
|
| Thyroid capsular
invasion |
|
|
|
|
|
Present | 16 | 13 (81.25) | 3 (18.75) | 1.000a |
|
Absent | 18 | 16 (88.89) | 2 (11.11) |
|
| BRAF mutation |
|
|
|
|
|
Positive | 15 | 14 (93.33) | 1 (6.67) | 0.355a |
|
Negative | 19 | 15 (78.95) | 4 (21.05) |
|
 | Table IX.Univariate analyses of associations
between of central LNM and clinicopathological features in patients
with cN0 FVPTC. |
Table IX.
Univariate analyses of associations
between of central LNM and clinicopathological features in patients
with cN0 FVPTC.
| Clinicopathological
characteristic | Total, n | Central LNM (+), n
(%) | Central LNM (−), n
(%) | P-value |
|---|
| Total number | 60 | 21 | 39 |
|
| Age at diagnosis,
years |
|
|
|
|
|
≥45 | 29 | 11 (37.93) | 18 (62.07) |
|
|
<45 | 31 | 10 (32.26) | 21 (67.74) | 0.645 |
| Sex |
|
|
|
|
|
Female | 50 | 19 (38.00) | 31 (62.00) | 0.468a |
|
Male | 10 | 2 (20.00) | 8 (80.00) |
|
| Pathological tumor
size, cm |
|
|
|
|
|
>2 | 14 | 8 (57.14) | 6 (42.86) | 0.096a |
| ≤2 | 46 | 13 (28.26) | 33 (71.74) |
|
| Underlying
Hashimoto's thyroiditis |
|
|
|
|
|
Present | 19 | 5 (26.32) | 14 (73.68) | 0.337 |
|
Absent | 41 | 16 (39.02) | 25 (60.98) |
|
| Multifocality |
|
|
|
|
|
Present | 16 | 7 (43.75) | 9 (56.25) | 0.392 |
|
Absent | 44 | 14 (31.82) | 30 (68.18) |
| Extrathyroidal
extension |
| Present | 17 | 8 (47.06) | 9 (52.94) | 0.218 |
| Absent | 43 | 13 (30.23) | 30 (69.77) |
|
| Thyroid capsular
invasion |
|
|
|
|
|
Present | 30 | 15 (50.00) | 15 (50.00) | 0.015 |
|
Absent | 30 | 6 (20.00) | 24 (80.00) |
|
| BRAF mutation |
|
|
|
|
|
Positive | 29 | 10 (34.48) | 19 (65.52) | 0.935 |
|
Negative | 31 | 11 (35.48) | 20 (64.52) |
|
Univariate and multivariate analyses
of patients with AVPTC
Given that the patient number in this cohort was
limited to 12 and 4 patients in the central LNM-positive and
-negative groups, respectively, the risk factors were analyzed in
all patients with AVPTC together (Table
X) instead of analyzing them separately by preoperative lymph
node status. Central LNM rate demonstrated a higher trend in
patients with extrathyroidal extension, thyroid capsular invasion
(80.00 vs. 72.73% for the two factors) and BRAFV600E
(76.92 vs. 66.67%). However, none of these factors were
significantly different.
 | Table X.Univariate analyses of associations
between central LNM and clinicopathological characteristics in
patients with AVPTC. |
Table X.
Univariate analyses of associations
between central LNM and clinicopathological characteristics in
patients with AVPTC.
| Clinicopathological
characteristic | Total, n | Central LNM (+), n
(%) | Central LNM (−), n
(%) | P-value |
|---|
| Total number | 16 | 12 | 4 |
| Age at diagnosis,
years |
|
|
|
|
|
≥45 | 5 | 4 (80.00) | 1 (20.00) |
|
|
<45 | 11 | 8 (72.73) | 3 (27.27) | 0.755a |
| Sex |
|
|
|
|
|
Female | 12 | 10 (83.33) | 2 (16.67) | 0.245a |
|
Male | 4 | 2 (50.00) | 2 (50.00) |
|
| Pathologic tumor
size, cm |
|
|
|
|
|
>2 | 7 | 7 (100.00) | 0 (0.00) | 0.088a |
| ≤2 | 9 | 5 (55.56) | 4 (44.44) |
|
| Underlying
Hashimoto's thyroiditis |
|
|
|
|
|
Present | 7 | 5 (71.43) | 2 (28.57) | 1.000a |
|
Absent | 9 | 7 (77.78) | 2 (22.22) |
|
| Multifocality |
|
|
|
|
|
Present | 7 | 5 (71.43) | 2 (28.57) | 1.000a |
|
Absent | 9 | 7 (77.78) | 2 (22.22) |
|
| Extrathyroidal
extension |
|
|
|
|
|
Present | 5 | 4 (80.00) | 1 (20.00) | 1.000a |
|
Absent | 11 | 8 (72.73) | 3 (27.27) |
|
| Thyroid capsular
invasion |
|
|
|
|
|
Present | 5 | 4 (80.00) | 1 (20.00) | 1.000a |
|
Absent | 11 | 8 (72.73) | 3 (27.27) |
|
| BRAF mutation |
|
|
|
|
|
Positive | 13 | 10 (76.92) | 3 (23.08) | 1.000a |
|
Negative | 3 | 2 (66.67) | 1 (33.33) |
|
Discussion
Risk factors associated with central LNM in patients
with PTC have been widely studied; tumor size, extrathyroidal
extension, thyroid capsular invasion, underlying Hashimoto's
thyroiditis and BRAFV600E have been reported to be
associated with LNM (26–28). BRAFV600E has been
demonstrated to be a good risk factor for cervical LNM and was
significantly correlated with recurrence (18,19).
Nevertheless, certain studies have revealed no correlation between
BRAFV600E and cervical LNM (29,30).
PTC is composed of several distinct histological
subtypes, including CVPTC, FVPTC and AVPTC; AVPTC includes tall
cell PTC, diffuse sclerosing PTC and poorly differentiated PTC,
which all exhibit more aggressive biological behavior (19). In the present study, 287 patients with
PTC, comprising 177 patients with CVPTC, 94 patients with FVPTC and
16 patients with AVPTC, were analyzed. Multivariate regression
analysis revealed that BRAFV600E was independently
associated with central LNM in the overall PTC group, which is in
accordance with previous reports (18,19).
However, similar results cannot be drawn from FVPTC or AVPTC, as
the BRAF mutation rate was not significantly different between
these two PTC subtypes. Importantly, BRAFV600E in CVPTC,
which is the most common subtype, was a potent independent
indicator for central LNM in addition to the overall PTC group.
FVPTC presented some similarities with follicular thyroid
carcinoma, and BRAFV600E exhibited a relatively low
prevalence in this PTC subtype (31).
The results from the present study are in agreement with studies by
Walts et al (32) and Li et
al (33), which stated that
BRAFV600E was not associated with LNM in FVPTC. The
difference in BRAFV600E mutation rate was not
significant in patients with AVPTC who had a high incidence of
BRAFV600E between LNM-positive (83.33%) and -negative
(75.00%) groups. The significance of the association between
BRAFV600E and LNM in AVPTC may be concealed by such a
high rate of BRAFV600E. As a consequence, BRAF mutation
may perform differently in diverse subtypes. Thus, the divergence
in the association between BRAF mutation and central LNM in
previous studies may be a result of the different histological
subtypes of PTC.
Although previous studies have demonstrated that
cervical LNM is associated with local recurrence and even
disease-specific survival in patients with PTC (7,34),
prophylactic central LND in patients with cN0 PTC remains
controversial in thyroid cancer surgery. In the systematic review
by Mulla and Schulte (35), cancerous
lymph nodes were detected in 46.15% of 1,946 patients with cN0 PTC.
Therefore, Mulla and Schulte (35)
advocated for prophylactic central LND to be performed on patients
with PTC. By contrast, a previous study by Conzo et al
(15) of 752 patients with cN0 PTC
revealed a greater rate of surgical complications, including
permanent hypoparathyroidism (3.6 vs. 1%; P=0.018) and temporary
unilateral vocal cord palsy (3.5% vs. 1.2; P=0.039). Therefore,
Conzo et al (15) stated that
prophylactic central LND in patients with cN0 PTC should be used
selectively.
To improve the pertinence of prophylactic central
LND in patients with cN0 PTC and to avoid potential surgical
morbidity, the data was further analyzed based on preoperative
central lymph node status; the analyses were performed separately
for patients with cN0 and cN1 PTC. Univariate analysis of all
patients with PTC revealed that BRAFV600E was associated
with central LNM in the 115 patients with cN1 PTC and the 172
patients with cN0 PTC. However, in the multivariate regression
analysis, BRAFV600E was significant in neither patients
with cN0 PTC nor in patients with cN1 PTC, suggesting that the
effect of BRAFV600E as an indicator for performing
prophylactic central LND was limited in this cohort of
patients.
When the patients were subdivided into variant
histological subtypes, in the univariate analysis
BRAFV600E was a potent indicator for central LNM in
patients with CVPTC, in addition to patients with cN0 CVPTC, but
not for cN1 patients of this variant. In the multivariate
regression, BRAFV600E was independently correlated with
central LNM in all patients with CVPTC and patients with cN0 CVPTC,
but not in cN1 patients of this subtype.
The aforementioned research demonstrating a
correlation between BRAFV600E and central LNM (26–28) may
not be representative of all patients with PTC. As demonstrated in
the present study, BRAFV600E was independently
associated with central LNM in overall patients with PTC, but when
subdivided by histological variant, BRAFV600E was only
significantly associated with the CVPTC subtype. In addition, when
categorized by preoperative central lymph node status,
BRAFV600E was only independently associated with central
LNM in patients with cN0 CVPTC instead of in all patients with cN0
PTC. For patients with cN1 CVPTC, suspected LNM was already
detected on preoperative ultrasonography and surgical removal of
regional lymph nodes was typically recommended, thus the clinical
significance of BRAFV600E or other clinicopathological
features was limited. As a consequence, BRAFV600E was
only associated with central LNM in a selected cohort of patients
instead of all patients with PTC.
Other clinicopathological factors have been
associated with central LNM. Tumor size has been demonstrated to be
an important risk factor for central LNM in numerous studies
(28,33). In the present study, tumor size was
significantly different between central LNM-positive or -negative
patients in overall patients with PTC and patients with CVPTC, in
addition to in the univariate analysis. Furthermore, tumor size was
an independent factor in the multivariate analysis. Upon further
investigation using surgical approaches, tumor size was revealed to
be associated with central LNM in overall patients with cN0 PTC and
in patients with cN0 CVPTC, and was the only independent risk
factor associated with central LNM in overall patients with cN0
PTC. Additionally, no significant differences were identified
between patients with FVPTC and patients with AVPTC with or without
LNM. Thyroid capsular invasion is another widely investigated risk
factor for central LNM (26,27). The data from the present study
demonstrated that thyroid capsular invasion was associated with
central LNM in a number of comparison groups, as revealed by the
univariate analysis results. Thyroid capsular invasion was the only
significantly different factor for central LNM in patients with cN1
CVPTC.
Previous studies have demonstrated that underlying
Hashimoto's thyroiditis has a protective effect for patients with
PTC, and that it is associated with smaller tumor size, fewer LNM
and improved prognosis (36,37). The data from the present study
demonstrated that underlying Hashimoto's thyroiditis was negatively
correlated with central LNM in overall patients with PTC and
patients with CVPTC. When further analyzed, the difference was only
significant in overall patients with cN0 PTC and in patients with
cN0 CVPTC. Underlying Hashimoto's thyroiditis serves as an
independent factor only in patients with cN0 CVPTC. Considering
that a great fraction of patients with cN1 who received therapeutic
LND had central LNM, the suspected regional lymph nodes should be
routinely removed. Aggressive features, including larger tumor
size, multifocality, extrathyroidal extension, thyroid capsular
invasion and BRAFV600E became less important under such
a high ratio of central LNM.
The present study has certain limitations. The
sample size was not big enough, particularly for patients with s;
only 16 patients with AVPTC in total were analyzed in the present
study, which may affect the statistics. Additionally, on account of
insufficient numbers of patients, the analyses of patients with cN0
and cN1 AVPTC were not performed separately. The association
between BRAFV600E and other risk factors with long-term
follow-up data in different subgroups require further investigation
in order to unveil their prognostic function in different
histological subtypes and preoperative central lymph node
statuses.
In conclusion, the clinical significance of
BRAFV600E for central LNM depends not only on PTC
histological subtype, but also on preoperative central lymph node
status, with the greatest significance in patients with cN0 CVPTC
instead of all patients with PTC. Furthermore, BRAFV600E
alone may not be able to accurately indicate the central lymph node
status. Hence, other associated factors should also be
recognized.
Acknowledgements
The present study was supported by the National High
Technology Research and Development Program of China (863 Program)
(grant no. 2012AA02A210) and the Zhejiang Provincial Natural
Science Foundation of Zhejiang (China) (grant no. Y13H16011).
Glossary
Abbreviations
Abbreviations:
|
PTC
|
papillary thyroid cancer
|
|
LNM
|
lymph node metastasis
|
|
LND
|
lymph node dissection
|
|
CVPTC
|
classical variant PTC
|
|
FVPTC
|
follicular variant PTC
|
|
AVPTC
|
aggressive variant PTC
|
References
|
1
|
Martinez Trufero J, Capdevilla J, Cruz JJ
and Isla D: SEOM clinical guidelines for the treatment of thyroid
cancer. Clin Transl Oncol. 13:574–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lundgren CI, Hall P, Dickman PW and
Zedenius J: Clinically significant prognostic factors for
differentiated thyroid carcinoma: A population-based, nested
case-control study. Cancer. 106:524–531. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moo TA, McGill J, Allendorf J, Lee J,
Fahey T III and Zarnegar R: Impact of prophylactic central neck
lymph node dissection on early recurrence in papillary thyroid
carcinoma. World J Surg. 34:1187–1191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scheumann GF, Gimm O, Wegener G,
Hundeshagen H and Dralle H: Prognostic significance and surgical
management of locoregional lymph node metastases in papillary
thyroid cancer. World J Surg. 18:559–567. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mazzaferri EL and Kloos RT: Clinical
review 128: Current approaches to primary therapy for papillary and
follicular thyroid cancer. J Clin Endocrinol Metab. 86:1447–1463.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suh YJ, Kwon H, Kim SJ, Choi JY, Lee KE,
Park YJ, Park DJ and Youn YK: Factors affecting the locoregional
recurrence of conventional papillary thyroid carcinoma after
surgery: A retrospective analysis of 3381 Patients. Ann Surg Oncol.
22:3543–3549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Podnos YD, Smith D, Wagman LD and
Ellenhorn JD: The implication of lymph node metastasis on survival
in patients with well-differentiated thyroid cancer. Am Surg.
71:731–734. 2005.PubMed/NCBI
|
|
8
|
Randolph GW, Duh QY, Heller KS, LiVolsi
VA, Mandel SJ, Steward DL, Tufano RP and Tuttle RM: American
Thyroid Association Surgical Affairs Committee's Taskforce on
Thyroid Cancer Nodal Surgery: The prognostic significance of nodal
metastases from papillary thyroid carcinoma can be stratified based
on the size and number of metastatic lymph nodes, as well as the
presence of extranodal extension. Thyroid. 22:1144–1152. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aboelnaga EM and Ahmed RA: Difference
between papillary and follicular thyroid carcinoma outcomes: An
experience from Egyptian institution. Cancer Biol Med. 12:53–59.
2015.PubMed/NCBI
|
|
10
|
Cooper DS, Doherty GM, Haugen BR, Kloos
RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI and
Tuttle RM: American Thyroid Association Guidelines Taskforce:
Management guidelines for patients with thyroid nodules and
differentiated thyroid cancer. Thyroid. 16:109–142. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rotstein L: The role of lymphadenectomy in
the management of papillary carcinoma of the thyroid. J Surg Oncol.
99:186–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu XM, Wan Y, Sippel RS and Chen H: Should
all papillary thyroid microcarcinomas be aggressively treated? An
analysis of 18,445 cases. Ann Surg. 254:653–660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sawka AM, Brierley JD, Tsang RW, Thabane
L, Rotstein L, Gafni A, Straus S and Goldstein DP: An updated
systematic review and commentary examining the effectiveness of
radioactive iodine remnant ablation in well-differentiated thyroid
cancer. Endocrinol Metab Clin North Am. 37457–480. (x)2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giordano D, Valcavi R, Thompson GB,
Pedroni C, Renna L, Gradoni P and Barbieri V: Complications of
central neck dissection in patients with papillary thyroid
carcinoma: Results of a study on 1087 patients and review of the
literature. Thyroid. 22:911–917. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Conzo G, Calò PG, Sinisi AA, De Bellis A,
Pasquali D, Iorio S, Tartaglia E, Mauriello C, Gambardella C,
Cavallo F, et al: Impact of prophylactic central compartment neck
dissection on locoregional recurrence of differentiated thyroid
cancer in clinically node-negative patients: A retrospective study
of a large clinical series. Surgery. 155:998–1005. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davies H, Bignell GR, Cox C, Stephens P,
Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W,
et al: Mutations of the BRAF gene in human cancer. Nature.
417:949–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JH, Lee ES and Kim YS:
Clinicopathologic significance of BRAF V600E mutation in papillary
carcinomas of the thyroid: A meta-analysis. Cancer. 110:38–46.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK,
Lee YJ, Kim KW, Hahn SK, Youn YK, Kim KH, et al: The association of
the BRAF(V600E) mutation with prognostic factors and poor clinical
outcome in papillary thyroid cancer: A meta-analysis. Cancer.
118:1764–1773. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xing M, Westra WH, Tufano RP, Cohen Y,
Rosenbaum E, Rhoden KJ, Carson KA, Vasko V, Larin A, Tallini G, et
al: BRAF mutation predicts a poorer clinical prognosis for
papillary thyroid cancer. J Clin Endocrinol Metab. 90:6373–6379.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Basolo F, Torregrossa L, Giannini R,
Miccoli M, Lupi C, Sensi E, Berti P, Elisei R, Vitti P, Baggiani A
and Miccoli P: Correlation between the BRAF V600E mutation and
tumor invasiveness in papillary thyroid carcinomas smaller than 20
millimeters: Analysis of 1060 cases. J Clin Endocrinol Metab.
95:4197–4205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tufano RP, Teixeira GV, Bishop J, Carson
KA and Xing M: BRAF mutation in papillary thyroid cancer and its
value in tailoring initial treatment: A systematic review and
meta-analysis. Medicine. 91:274–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alzahrani AS and Xing M: Impact of lymph
node metastases identified on central neck dissection (CND) on the
recurrence of papillary thyroid cancer: Potential role of BRAFV600E
mutation in defining CND. Endocr Relat Cancer. 20:13–22. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ito Y, Yoshida H, Maruo R, Morita S,
Takano T, Hirokawa M, Yabuta T, Fukushima M, Inoue H, Tomoda C, et
al: BRAF mutation in papillary thyroid carcinoma in a Japanese
population: Its lack of correlation with high-risk
clinicopathological features and disease-free survival of patients.
Endocr J. 56:89–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fugazzola L, Mannavola D, Cirello V,
Vannucchi G, Muzza M, Vicentini L and Beck-Peccoz P: BRAF mutations
in an Italian cohort of thyroid cancers. Clin Endocrinol (Oxf).
61:239–243. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Walts AE, Mirocha JM and Bose S:
Follicular variant of papillary thyroid carcinoma (FVPTC):
Histological features, BRAF V600E mutation, and lymph node status.
J Cancer Res Clin Oncol. 141:1749–1756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang W, Gu J, Shang J and Wang K:
Correlation analysis on central lymph node metastasis in 276
patients with cN0 papillary thyroid carcinoma. Int J Clin Exp
Pathol. 6:510–515. 2013.PubMed/NCBI
|
|
27
|
Blanchard C, Brient C, Volteau C, Sebag F,
Roy M, Drui D, Hamy A, Mathonnet M, Henry JF and Mirallié E:
Factors predictive of lymph node metastasis in the follicular
variant of papillary thyroid carcinoma. Br J Surg. 100:1312–1317.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Chen C, Chen Z, Jiang J, Chen Y,
Jin L, Guo G, Zhang X and Ye T: Prediction of central compartment
lymph node metastasis in papillary thyroid microcarcinoma. Clin
Endocrinol (Oxf). 81:282–288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sheu SY, Grabellus F, Schwertheim S,
Handke S, Worm K and Schmid KW: Lack of correlation between BRAF
V600E mutational status and the expression profile of a distinct
set of miRNAs in papillary thyroid carcinoma. Horm Metab Res.
41:482–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Givens DJ, Buchmann LO, Agarwal AM,
Grimmer JF and Hunt JP: BRAF V600E does not predict aggressive
features of pediatric papillary thyroid carcinoma. Laryngoscope.
124:E389–E393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xing M: BRAF mutation in thyroid cancer.
Endocr Relat Cancer. 12:245–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Walts AE, Mirocha JM and Bose S:
Follicular variant of papillary thyroid carcinoma (FVPTC):
Histological features, BRAF V600E mutation, and lymph node status.
J Cancer Res Clin Oncol. 141:1749–1756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li C, Aragon Han P, Lee KC, Lee LC, Fox
AC, Beninato T, Thiess M, Dy BM, Sebo TJ, Thompson GB, et al: Does
BRAF V600E mutation predict aggressive features in papillary
thyroid cancer? Results from four endocrine surgery centers. J Clin
Endocrinol Metab. 98:3702–3712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zaydfudim V, Feurer ID, Griffin MR and
Phay JE: The impact of lymph node involvement on survival in
patients with papillary and follicular thyroid carcinoma. Surgery.
144:1070–1078. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mulla M and Schulte KM: Central cervical
lymph node metastases in papillary thyroid cancer: A systematic
review of imaging-guided and prophylactic removal of the central
compartment. Clin Endocrinol (Oxf). 76:131–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jara SM, Carson KA, Pai SI, Agrawal N,
Richmon JD, Prescott JD, Dackiw A, Zeiger MA, Bishop JA and Tufano
RP: The relationship between chronic lymphocytic thyroiditis and
central neck lymph node metastasis in North American patients with
papillary thyroid carcinoma. Surgery. 154:1272–1282. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lun Y, Wu X, Xia Q, Han Y, Zhang X, Liu Z,
Wang F, Duan Z, Xin S and Zhang J: Hashimoto's thyroiditis as a
risk factor of papillary thyroid cancer may improve cancer
prognosis. Otolaryngol Head Neck Surg. 148:396–402. 2013.
View Article : Google Scholar : PubMed/NCBI
|