Introduction
Radiotherapy and adjuvant chemotherapy have become
the standard treatments for multiple types of cancer, including
esophageal and nasopharyngeal carcinoma (1,2). Although
radiotherapy is widely used to treat early-stage tumors, patients
with advanced-stage tumors often experience failure of treatment
mainly due to resistance to radiotherapy, resulting in recurrence
and distant metastases (2–6). Therefore, the development of potent and
reliable radiosensitizers is necessary for improving overall
treatment outcomes in cancer therapy.
Zoledronic acid (ZOL) is a compound containing
nitrogen and bisphosphonates, which harbors anti-reabsorption
effects (7). ZOL is used as therapy
for bone metastasis in malignancy and a number of metabolic
disorders, including bone pain, bone fractures and hypercalcaemia
(8,9).
ZOL inhibits farnesyl pyrophosphate synthase, a key enzyme in the
isoprenoid biosynthetic pathway, and prevents prenylation of small
guanosine triphosphate-binding proteins, including Rho,
p21ras, cell division cycle 42, Rac and Rab, which are
essential for different cellular functions such as signal
transduction and cell adhesion (7–9). ZOL is
considered to possess antitumor activity, particularly in
combination with chemotherapeutic anticancer drugs (10–12). This
effect of ZOL could be observed on cancer cells derived from a
variety of tumors, including breast, prostate and pancreatic
cancers, by inducing cell apoptosis and inhibiting cell invasion,
adhesion and angiogenesis (10–13).
Currently, numerous patients with bone metastases secondary to a
broad range of solid tumors are benefiting from the antitumor
effects of ZOL (14).
Our previous study demonstrated the synergistic
cytotoxic effects of ZOL and ionizing radiation (IR) on esophageal
squamous cell carcinoma and endothelial cells (15). Accordingly, combined treatment with
ZOL plus IR may be an encouraging method to treat cancer with less
side effects and complications, compared with the use of these
agents alone (15–20). However, the molecular mechanism of the
radiosensitizing ability of ZOL in cancer cells remains mostly
unknown. In the present study, ZOL was revealed to enhance the
sensitivity of cancer cells to IR by inducing S-phase arrest in the
cell cycle and subsequently promoting apoptosis, which may be due
to elevated levels of cyclin A and cyclin B in the S and M phases,
as well as decreased expression of the cyclin-dependent kinase
inhibitor p21CIP1.
Materials and methods
Cell culture and reagents
Cancer cell lines (HNE-1 and CNE-2) were gifted from
Miss Jiongyu Chen (Cancer Hospital of Shantou University Medical
College, Shantou, China) and cultured as described previously
(21). The above two cell lines are
identified as mixed cancer types (22,23). ZOL
was kindly supplied by Novartis Pharma AG (Basel, Switzerland) and
diluted in 0.9% saline at a concentration of 10 mM as stock.
Aliquots were stored at −20°C and added to the Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) immediately prior to use.
Cell viability assay
An MTT colorimetric assay was used to measure the
proliferation rate of tumor cells treated with ZOL as described
previously (13,19). Briefly, cells were plated in
triplicate at a density of 4×103 cells per well on
96-well plates (Corning Costar, Cambridge, MA, USA), After 24 h of
culture at 37°C, cells were incubated with ZOL at various
concentrations (2–32 µM) at 37°C for 48 or 72 h, followed by MTT
assays. The purple formazan in each well was dissolved in dimethyl
sulfoxide and measured at 490 nm.
Colony formation assay
A colony formation assay was performed as previously
described (15). Cells were seeded on
6-well plates at various cell densities (100, 200, 400, 600, 800
cells/well) and allowed to grow at 37°C for 12 h. Cells were then
pretreated with ZOL (2 µM) for 6 h followed by transient exposure
to increased doses of irradiation (2–6 Gy, 1 Gy/min) generated by
the Gammacell 3000 Elan system (MDS Nordion, Inc., Kanata, ON,
Canada). At 24 h after radiation exposure, the medium was replaced
with ZOL-free DMEM (Gibco; Thermo Fisher Scientific, Inc.) and
cells were cultured at 37°C for 12–14 days. Cellular colonies were
formed and were stained with 0.5% crystal violet (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) at 37°C for 5 min. Cellular
colonies were counted under a phase-contrast microscope
(magnification, ×100; Carl Zeiss, Thornwood, NY, USA) if >50
cells were in a single colony. The surviving fraction (SF) of each
plate under different radiation dosages was calculated by dividing
the number of cellular colonies by the number of cells plated, and
was normalized to the SF of cells without radiation treatment.
Cell cycle analysis
Flow cytometry was used to analyze the cell cycle
distribution when cells were pretreated with ZOL (2 µM) for 12 h
and then exposed to a single IR dose (4 Gy) for 6 h. Media were
replaced the next day, and cells were harvested at 500 × g for 15
min at 4°C and fixed in 70% ethanol overnight at −20°C. Cells were
resuspended and incubated with 500 µl of a solution containing 10
µg/ml propidium iodide (PI), 100 µg/ml RNase (Sigma-Aldrich; Merck
KGaA) and 20 mM EDTA at 37°C for 1 h, followed by cytometric flow
analysis. DNA content and the percentages of cells in each cell
cycle phase were measured by FACSCalibur (BD Biosciences, Franklin
Lakes, NJ, USA).
Cell invasion assay
This procedure has been described previously
(15,21). Briefly, cells were pretreated with 2
µM ZOL and then plated in 24-well Matrigel-coated Transwell inserts
(EMD Millipore, Billerica, MA, USA) at 5×104 cells per
500 µl of serum-free Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc.). The bottom chamber of the
Transwell insert was filled with 750 µl of DMEM with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.). Irradiation
(4 Gy) was immediately applied to cells. Crystal violet (4%) was
used to stain the migrated cells for 15 min at room temperature,
which were then counted using an inverted microscope at ×100
magnification. Invasion activity was assessed by the mean number of
migrated cells in three microscopic fields, selected randomly.
Western blot analysis
Western blot analysis was performed as described
previously (15,21,24). The
cells were lysed using radioimmunoprecipitation assay lysis buffer
(Tiangen Biotech Co., Ltd., Beijing, China) and ~50 µg of total
protein from each sample was separated by 12% SDS-PAGE, followed by
transfer to a polyvinylidene difluoride membrane (EMD Millipore).
The membrane was blocked overnight at 4°C using 5% bovine serum
albumin (Sigma-Aldrich; Merck Millipore) in PBS. The membrane was
then incubated with the following primary antibodies overnight at
4°C: Rabbit polyclonal antibodies against cyclin A (cat. no.
sc-751; dilution, 1:400; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), cyclin B (cat. no. sc-25764; dilution, 1:500; Santa Cruz
Biotechnology, Inc.), cyclin D1 (cat. no. sc-717; dilution, 1:200;
Santa Cruz Biotechnology, Inc.) and cyclin E (cat. no. sc-717;
dilution, 1:200; Santa Cruz Biotechnology, Inc.), and mouse
monoclonal antibodies against p21CIP1 (cat. no. sc-717;
dilution, 1:200; Santa Cruz Biotechnology, Inc.) and
p27KIP1 (cat. no. sc-817; dilution, 1:500; Santa Cruz
Biotechnology, Inc.). Subsequent to washing with 0.1% Tween-20 in
PBS, the membranes were then incubated with the following
horseradish peroxidase-conjugated secondary antibodies for 1 h at
37°C: Goat anti-rabbit immunoglobulin G (IgG; cat. no. sc-2004;
dilution, 1:2,000; Santa Cruz Biotechnology, Inc.) and rabbit
anti-mouse IgG (cat. no. sc-358914; dilution, 1:2,000; Santa Cruz
Biotechnology, Inc.). Proteins were detected using an enhanced
chemiluminescence kit (GE Healthcare Life Sciences, Chalfont, UK).
Anti-GAPDH monoclonal antibody (cat. no. ab9485; dilution, 1:2,500;
Abcam, Cambridge, MA, USA) was used to assure equal loading of
protein.
Statistical analysis
Statistical analysis was performed using the SPSS
statistical software package (version 17.0; SPSS Inc., Chicago, IL,
USA). Data from at least three independent experiments are
presented as the mean ± standard deviation. Data were analyzed
using a paired t-test with Bonferroni adjustment or one-way
analysis of variance (ANOVA), followed by the Student-Newman-Keuls
post-test for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
ZOL induces anti-proliferative effects
on cancer cells
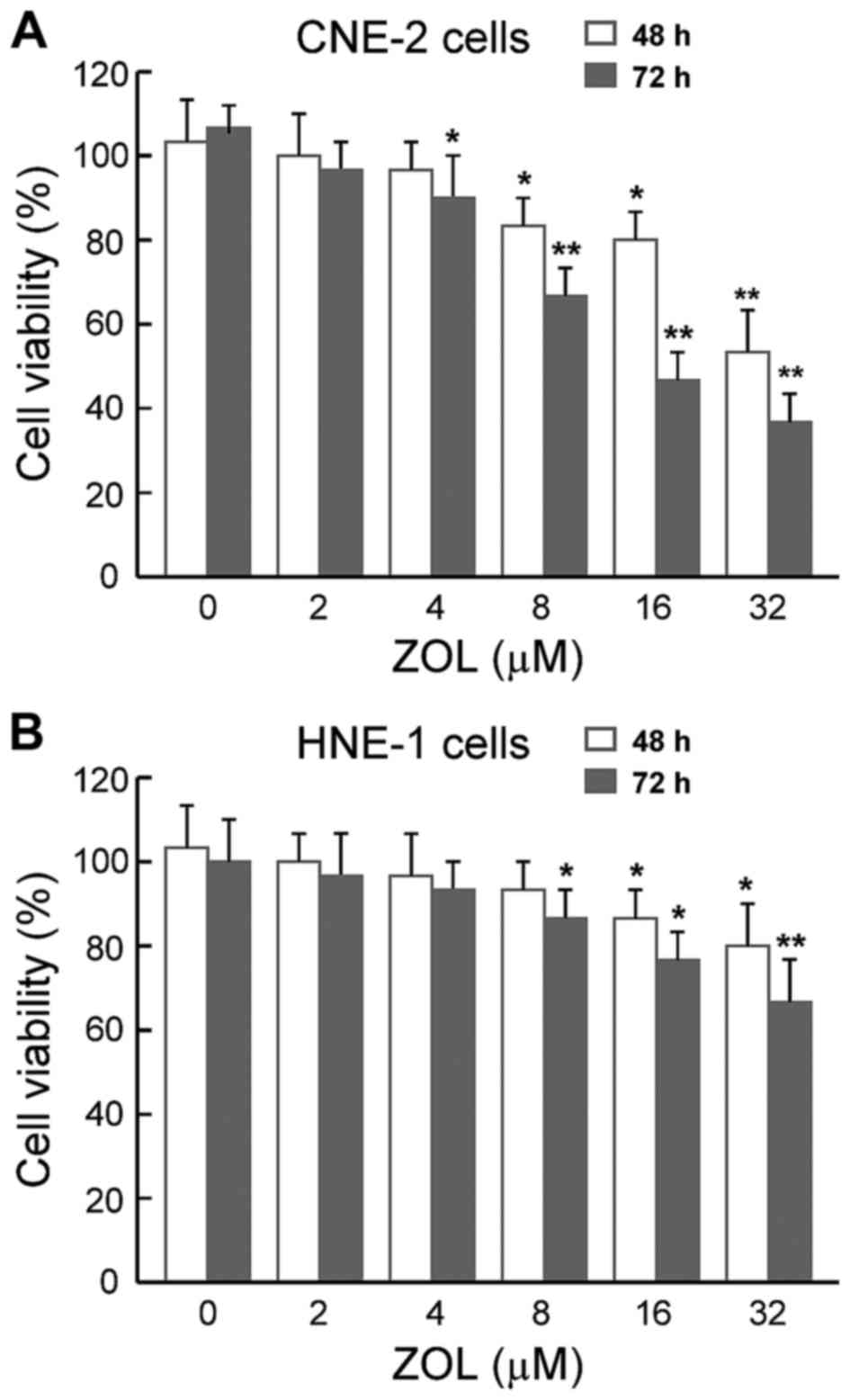
The MTT cytotoxicity assays demonstrated that ZOL
decreased the viability of the two cancer cell lines in a
dose-dependent manner, either after 48 or 72 h of treatment
(Fig. 1). Notably, the
anti-proliferative effects of ZOL on different cell lines were
slightly different, with HNE-1 cells being less sensitive to the
ZOL compared with CNE-2 cells. These data indicated that different
cellular responses to ZOL depend on the intrinsic sensitivity of
each cell line.
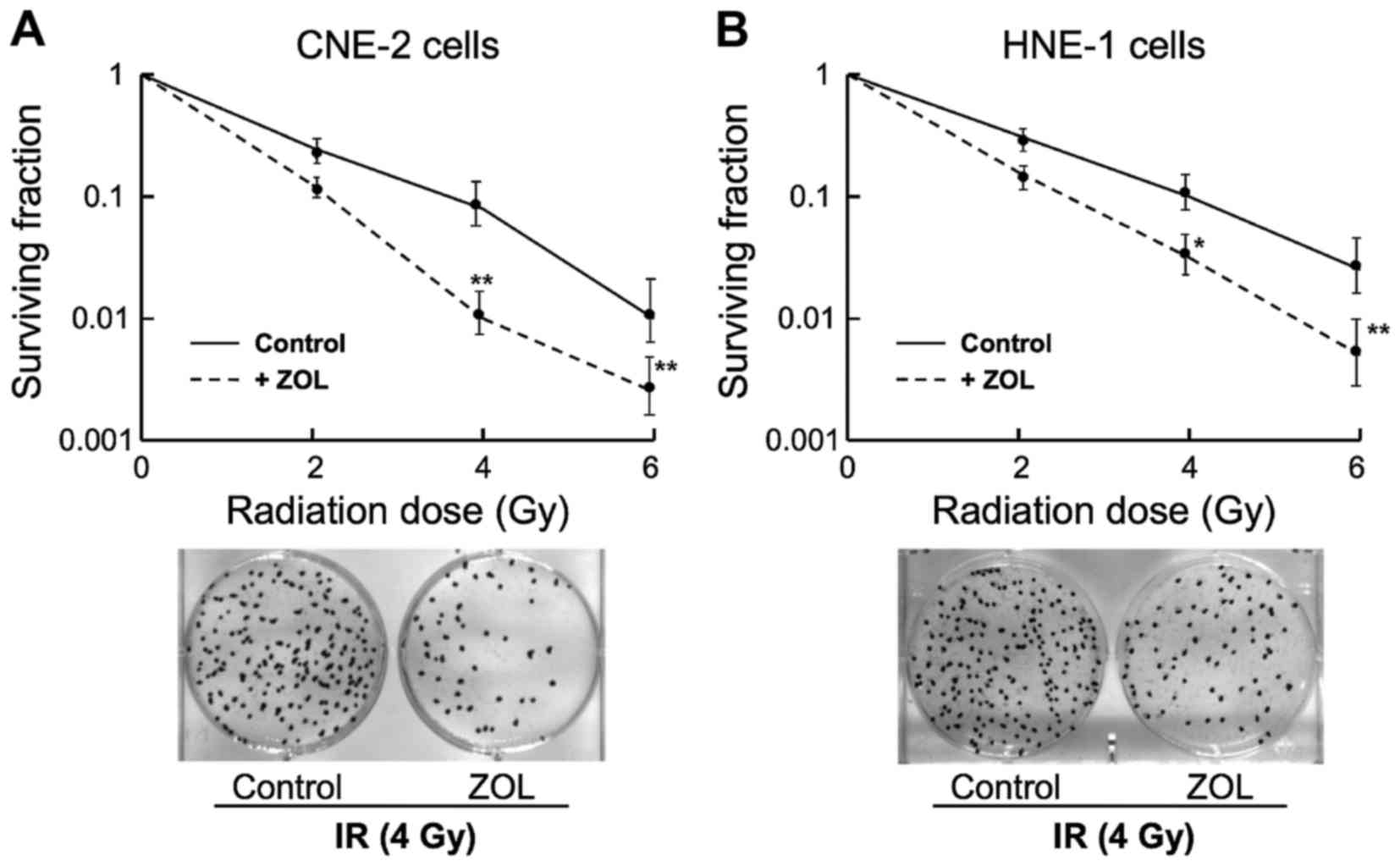
ZOL enhances IR-induced clonogenic
inhibition
Analyses of clonogenicity were then performed to
evaluate the quantities of living cellular clones following
co-treatment of ZOL plus IR. For all subsequent studies, a
relatively low dose of ZOL at 2 µM was applied to cells as
pretreatment prior to IR exposure. As shown in Fig. 2, combined treatment of CNE-2 and HNE-1
cells with ZOL plus IR significantly inhibited the growth of
clonogenic cells as compared with that caused by IR treatment
alone.
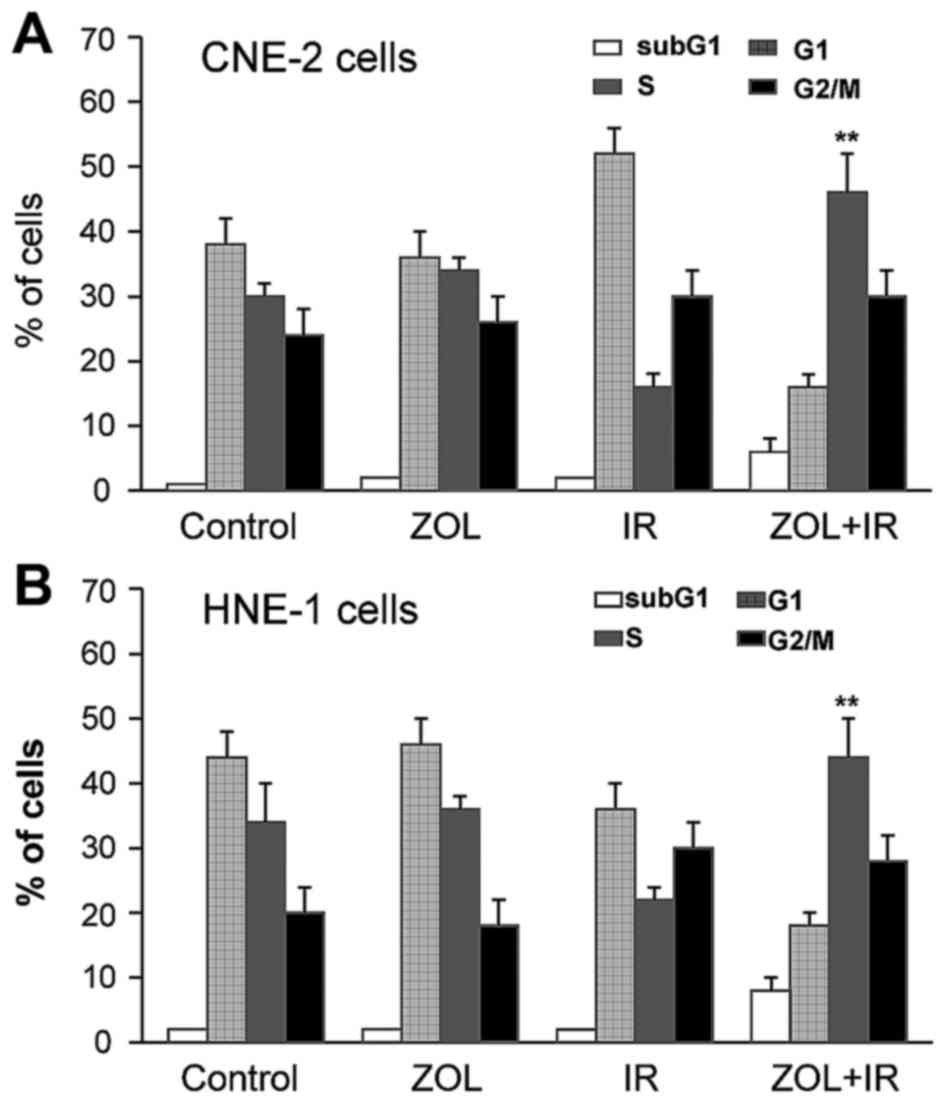
ZOL triggers cell cycle accumulation
in S phase
Flow cytometry was then used to determine whether
the anti-proliferative effects of ZOL plus IR were associated with
cell cycle distribution changes. As shown in Fig. 3, no significant alteration of the cell
cycle was observed in CNE-2 or HNE-1 cells treated with ZOL alone.
However, co-treatment with ZOL (2 µM) plus IR (4 Gy) resulted in
significantly increased S-phase cell proportions (P<0.01). In
addition, subG1-phase cell proportions were slightly higher in the
two cell lines following co-treatment with ZOL plus IR, compared
with ZOL or IR treatment alone.
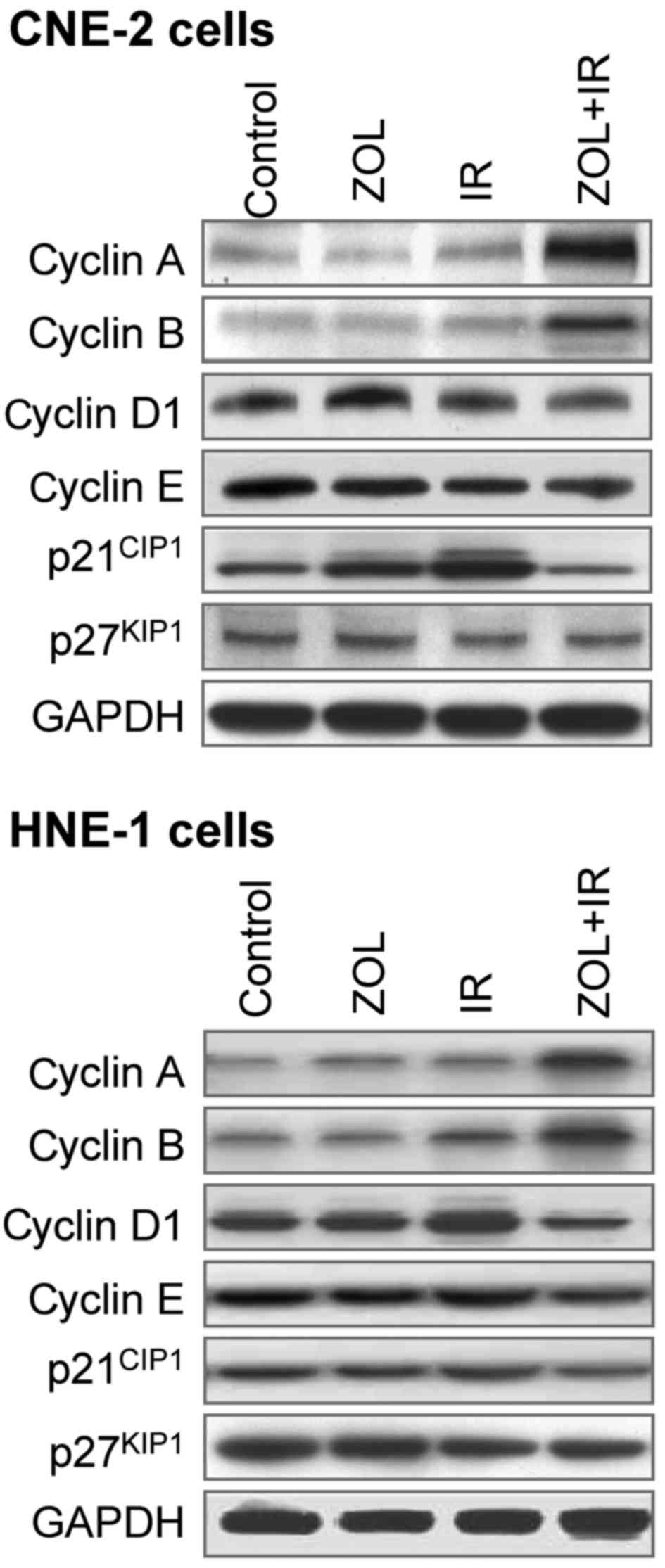
ZOL plus IR elevates the expression
levels of S- and M-phase cyclins
According to previous studies, it is controversial
whether ZOL affects cyclin and cyclin-dependent kinase inhibitor
expression levels in cancer cells (14,25–27). To
elucidate the mechanism by which ZOL causes S-phase arrest in CNE-2
and HNE-1 cells, the expression status of cyclins A, B, D1 and E,
as well as p21CIP1 and p27KIP1, was examined
by western blot analysis (Fig. 4).
Although there was a slight increase in p21CIP1 protein
levels, treatment with ZOL (2 µM) or IR (4 Gy) alone exhibited a
limited effect on expression patterns. By comparison, the combined
treatment led to an increase in cyclin A and B, with a concomitant
faint decrease in cyclins D1 and E. In addition, a decreased level
of p21CIP1 protein was observed, while
p27KIP1 expression was generally unaltered.
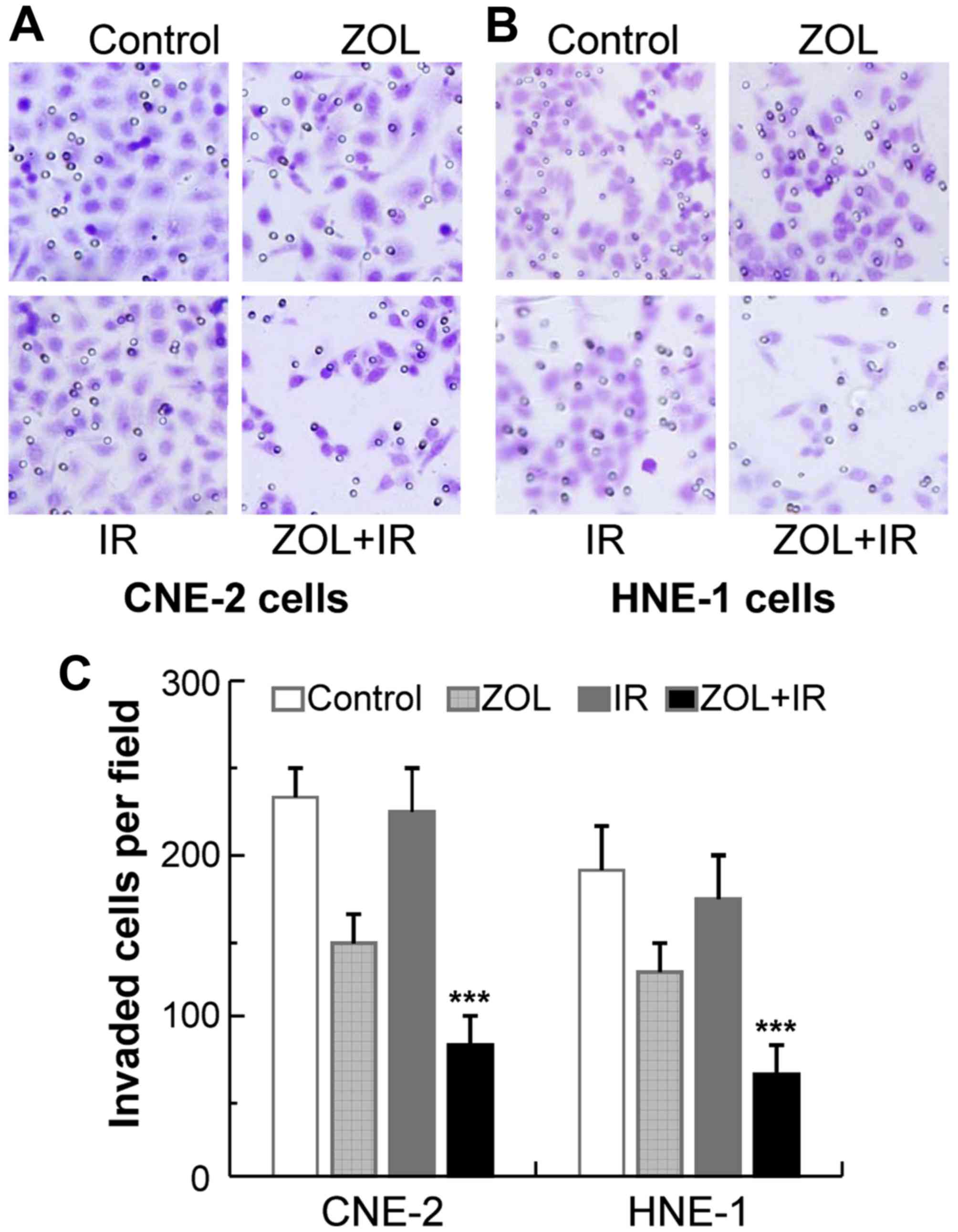
ZOL plus IR results in significant
anti-metastatic effects on cancer cells
Our previous study reported that ZOL may impair
cancer cell migration and invasion via downregulating vascular
endothelial growth factor and matrix metalloproteinase expression
(28). Therefore, the aim of the
present study was to examine whether this anti-metastatic effect
could be augmented by co-treatment with ZOL plus IR. Invasion
assays were performed on CNE-2 and HNE-1 cells treated with ZOL
plus/or IR. ZOL treatment (2 µM) significantly decreased the number
of invaded cells on the membrane coated with Matrigel in the
chambers, as compared with that of the control (Fig. 5). As expected, the anti-metastatic
effects of ZOL were enhanced markedly when combined with IR,
whereas IR treatment alone had marginal effects. These data
suggested that the anti-metastatic effects caused by ZOL and IR
were synergistic, which indicates that the effects induced by the
combination of ZOL and IR are greater than the sum of the effects
caused by ZOL and IR individually.
Discussion
Resistance to chemotherapeutics and radioactive rays
remain the major factors for clinical cancer therapy (3–5).
Therefore, it is important to identify reliable radiosensitizers
that can increase the sensitivity of cancer cells to IR.
Accumulated evidence has revealed that prolonged cell cycle
progression with increased susceptibility to apoptosis and Ras
signaling inhibition in tumor cells may contribute to the cellular
mechanisms of ZOL as a radiosensitizer (17–20). These
results demonstrate the possibility that the radiosensitizing
effects of ZOL and its direct antitumor effects are valuable
characteristics for therapeutic interventions in cancer. In the
present study, a significant synergistic antitumor effect induced
by combination of ZOL plus IR was documented in cancer cells, at
least in part through upregulating S- and M-phase cyclins and
decreasing p21CIP1 levels. In addition, these effects
were accompanied by apoptosis of subG1-phase cells. It was revealed
that co-treatment with ZOL plus IR resulted in augmented inhibition
of cell invasion, over the simple additive effect for each
treatment alone.
It has been reported that the peak concentration of
ZOL in serum, which is maintained only for a few h, is 1–3 µM
(29). This observation indicates
that the optimal serum concentrations of ZOL may not be readily
achieved for its antitumor activity. It is unlikely that ZOL
directly induces apoptotic effects at primary sites of solid
tumors. By contrast, patients with advanced disease states,
including bone metastasis and bone marrow carcinomatosis, may
benefit from ZOL, where ZOL would be able to (or at least
partially) induce apoptosis in tumor cells (8–10). Thus,
ZOL is currently being used to treat cancer, together with other
anticancer drugs, including chemotherapeutic drugs, molecular
targeted drugs and other biological agents (10–12). The
addition of ZOL in cancer therapy not only results in synergistic
anticancer effects with other drugs, but also lowers the toxicity
effects caused by these drugs (10–12).
Similarly, since the present study focused on the anticancer
effects caused by combination with ZOL and IR, a relatively lower
concentration of ZOL (2 µM) was selected, according to the result
from the MTT cytotoxicity assays (Fig.
1). This combination of ZOL plus IR did not expose cells to
excessive drug toxicity and allowed the radiosensitizing effect of
ZOL to be investigated. This was consistent with other previous
studies (15–20), in which ZOL plus IR decreased the drug
concentration and the amount of irradiation to make the combination
safer than when ZOL or radiotherapy were used alone, with less side
effects. According to the current study, treatment of cancer cells
with a low concentration of ZOL (2 µM), which can be achievable
clinically, could enhance the radiotherapeutic effects. These
results suggest that ZOL in combination with IR may be utilized in
clinical use, particularly in patients with cancer.
Generally, tumor cells during late S and G2/M phases
are the most sensitive to radiotherapy (30,31). In
the present study, flow cytometry indicated that the proportion of
cells in the S and subG1 phases was increased following combined
treatment with ZOL plus IR; however, ZOL used alone had no effect
on the cell cycle. These data suggest that co-treatment with ZOL
plus IR may lead to prolonged cell cycle progression and subsequent
apoptosis. The present results are similar to the observations
reported previously on other types of cancer cells (17–20). These
findings suggest that the underlying mechanism of the
radiosensitizing effect may involve not only prolongation of cell
cycle progression but also induction of apoptosis. Notably, p53, a
tumor-suppressor gene involved in numerous intracellular pathways
triggered by IR exposure, is often disrupted in multiple cancers
(32). The p53-independent apoptotic
effect suggests that ZOL may serve as a promising tool to treat
cancers, particularly those with radio or chemoresistance due to
loss of p53 function (25,33).
It has been reported that ZOL may induce cell-cycle
prolongation by altering the expression of certain cyclins and
their associated regulatory proteins (13,24–26). The
present study also revealed that co-treatment with ZOL plus IR
elevated the expression levels of S- and M-phase cyclins, cyclin A
and B, while downregulated the cyclin-dependent kinase inhibitor
p21CIP1, which may lead to cell cycle arrest between
intra-S and M phases. Of note, the expression levels of cyclins D1
and E, as well as p27KIP1, were not significantly
altered upon combined treatment. These results are in accordance,
at least in part, with other cancers reported previously (13,24–26).
In the present study, it was also revealed that
combined treatment with ZOL plus IR exhibited synergism rather than
their individual use, as demonstrated by the anti-invasive effects
against cancer cells. Thus, co-treatment with ZOL plus IR may be
used to increase the anti-proliferative and anti-metastatic
effects, and to decrease side effects and complications. These
findings suggest that ZOL in combination with IR may be a promising
therapy for cancer patients.
Acknowledgements
The authors thank Ms. Jiongyu Chen (Oncological
Research Laboratory, Cancer Hospital of Shantou University Medical
College, Shantou, China) for technical assistance. The present
study was supported in part by the Science and Technology Planning
Project of Henan Province, China (grant no. 142102310464, awarded
to Y. You) and the Key Research Foundation of Higher Education of
Henan Province, China (grant no. 15B320003).
References
|
1
|
You Y, Li H, Chen J, Qin X and Ran Y:
Zoledronic acid reverses cisplatin resistance in nasopharyngeal
carcinoma cells by activating the mitochondrial apoptotic pathway.
Oncol Lett. 13:1840–1846. 2017.PubMed/NCBI
|
|
2
|
Minsky BD, Pajak TF, Ginsberg RJ, Pisansky
TM, Martenson J, Komaki R, Okawara G, Rosenthal SA and Kelsen DP:
INT 0123 (radiation therapy oncology group 94-05) phase III trial
of combined-modality therapy for esophageal cancer: High-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamashita S, Kondo M and Hashimoto S:
Squamous cell carcinoma of the nasopharynx. An analysis of failure
patterns after radiation therapy. Acta Radiol Oncol. 24:315–320.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Sarraf M, LeBlanc M, Giri PG, Fu KK,
Cooper J, Vuong T, Forastiere AA, Adams G, Sakr WA, Schuller DE and
Ensley JF: Chemoradiotherapy versus radiotherapy in patients with
advanced nasopharyngeal cancer: Phase III randomized Intergroup
study 0099. J Clin Oncol. 16:1310–1317. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mao YP, Zhou GQ, Liu LZ, Guo R, Sun Y, Li
L, Lin AH, Zeng MS, Kang TB, Jia WH, et al: Comparison of
radiological and clinical features of temporal lobe necrosis in
nasopharyngeal carcinoma patients treated with 2D radiotherapy or
intensity-modulated radiotherapy. Br J Cancer. 110:2633–2639. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer statistics,
2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dunford JE, Thompson K, Coxon FP, Luckman
SP, Hahn FM, Poulter CD, Ebetino FH and Rogers MJ:
Structure-activity relationships for inhibition of farnesyl
diphosphate synthase in vitro and inhibition of bone resorption in
vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther.
296:235–242. 2001.PubMed/NCBI
|
|
8
|
Yuasa T, Kimura S, Ashihara E, Habuchi T
and Maekawa T: Zoledronic acid - a multiplicity of anti-cancer
action. Curr Med Chem. 14:2126–2135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jantunen E: Bisphosphonate therapy in
multiple myeloma: Past, present, future. Eur J Haematol.
69:257–264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koto K, Murata H, Kimura S, Horie N,
Matsui T, Nishigaki Y, Ryu K, Sakabe T, Itoi M, Ashihara E, et al:
Zoledronic acid inhibits proliferation of human fibrosarcoma cells
with induction of apoptosis, and shows combined effects with other
anticancer agents. Oncol Rep. 24:233–239. 2010.PubMed/NCBI
|
|
11
|
Zhao M, Tominaga Y, Ohuchida K, Mizumoto
K, Cui L, Kozono S, Fujita H, Maeyama R, Toma H and Tanaka M:
Significance of combination therapy of zoledronic acid and
gemcitabine on pancreatic cancer. Cancer Sci. 103:58–66. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumoto S, Kimura S, Segawa H, Kuroda J,
Yuasa T, Sato K, Nogawa M, Tanaka F, Maekawa T and Wada H: Efficacy
of the third-generation bisphosphonate, zoledronic acid alone and
combined with anti-cancer agents against small cell lung cancer
cell lines. Lung Cancer. 47:31–39. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ge XY, Yang LQ, Jiang Y, Yang WW, Fu J and
Li SL: Reactive oxygen species and autophagy associated apoptosis
and limitation of clonogenic survival induced by zoledronic acid in
salivary adenoid cystic carcinoma cell line SACC-83. PLoS One.
9:e1012072014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Di Salvatore M, Orlandi A, Bagalà C,
Quirino M, Cassano A, Astone A and Barone C: Anti-tumour and
anti-angiogenetic effects of zoledronic acid on human
non-small-cell lung cancer cell line. Cell Prolif. 44:139–146.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
You Y, Liu J, Wang Z, Zhang Y, Ran Y, Guo
X, Liu H and Wang H: The enhancement of radiosensitivity in human
esophageal squamous cell carcinoma cells by zoledronic acid and its
potential mechanism. Cytotechnology. 66:17–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Algur E, Macklis RM and Häfeli UO:
Synergistic cytotoxic effects of zoledronic acid and radiation in
human prostate cancer and myeloma cell lines. Int J Radiat Oncol
Biol Phys. 61:535–542. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ryu K, Murata H, Koto K, Horie N, Matsui
T, Nishigaki Y, Sakabe T, Takeshita H, Itoi M, Kimura S, et al:
Combined effects of bisphosphonate and radiation on osteosarcoma
cells. Anticancer Res. 30:2713–2720. 2010.PubMed/NCBI
|
|
18
|
Lopez Jornet P, Susana SC, Rosario TM and
Alvaro PF: Zoledronic acid and irradiation in oral squamous cell
carcinoma. J Oral Pathol Med. 44:103–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ural AU and Avcu F: Radiosensitizing
effect of zoledronic acid in small cell lung cancer. Lung Cancer.
50:271–272. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ural AU, Avcu F, Candir M, Guden M and
Ozcan MA: In vitro synergistic cytoreductive effects of zoledronic
acid and radiation on breast cancer cells. Breast Cancer Res.
8:R522006. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
You Y, Yang W, Qin X, Wang F, Li H, Lin C,
Li W, Gu C, Zhang Y and Ran Y: ECRG4 acts as a tumor suppressor and
as a determinant of chemotherapy resistance in human nasopharyngeal
carcinoma. Cell Oncol (Dordr). 38:205–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan SY, Choy KW, Tsao SW, Tao Q, Tang T,
Chung GT and Lo KW: Authentication of nasopharyngeal carcinoma
tumor lines. Int J Cancer. 122:2169–2171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ye F, Chen C, Qin J, Liu J and Zheng C:
Genetic profiling reveals an alarming rate of cross-contamination
among human cell lines used in China. FASEB J. 29:4268–4272. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
You Y, Yang W, Wang Z, Zhu H, Li H, Lin C
and Ran Y: Promoter hypermethylation contributes to the frequent
suppression of the CDK10 gene in human nasopharyngeal carcinomas.
Cell Oncol (Dordr). 36:323–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuroda J, Kimura S, Segawa H, Sato K,
Matsumoto S, Nogawa M, Yuasa T, Kobayashi Y, Yoshikawa T, Ottmann
OG and Maekawa T: p53-independent anti-tumor effects of the
nitrogen-containing bisphosphonate zoledronic acid. Cancer Sci.
95:186–192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li YY, Chang JW, Liu YC, Wang CH, Chang
HJ, Tsai MC, Su SP and Yeh KY: Zoledronic acid induces cell-cycle
prolongation in murine lung cancer cells by perturbing cyclin and
Ras expression. Anticancer Drugs. 22:89–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mani J, Vallo S, Barth K, Makarević J,
Juengel E, Bartsch G, Wiesner C, Haferkamp A and Blaheta RA:
Zoledronic acid influences growth, migration and invasive activity
of prostate cancer cells in vitro. Prostate Cancer Prostatic Dis.
15:250–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li XY, Lin Y, Huang W, Hong CQ, Chen JY,
You YJ and Li WB: Zoledronic acid inhibits proliferation and
impairs migration and invasion through downregulating VEGF and MMPs
expression in human nasopharyngeal carcinoma cells. Med Oncol.
29:714–720. 2013. View Article : Google Scholar
|
|
29
|
Skerjanec A, Berenson J, Hsu C, Major P,
Miller WH Jr, Ravera C, Schran H, Seaman J and Waldmeier F: The
pharmacokinetics and pharmacodynamics of zoledronic acid in cancer
patients with varying degrees of renal function. J Clin Pharmacol.
43:154–162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sinclair WK: Cyclic x-ray responses in
mammalian cells in vitro. Radiat Res. 33:620–643. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Milas L, Hunter NR, Mason KA, Kurdoglu B
and Peters LJ: Enhancement of tumor radioresponse of a murine
mammary carcinoma by paclitaxel. Cancer Res. 54:3506–3510.
1994.PubMed/NCBI
|
|
32
|
Yip KW, Shi W, Pintilie M, Martin JD,
Mocanu JD, Wong D, MacMillan C, Gullane P, O'Sullivan B,
Bastianutto C and Liu FF: Prognostic significance of the
Epstein-Barr virus, p53, Bcl-2, and survivin in nasopharyngeal
cancer. Clin Cancer Res. 12:5726–5732. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ory B, Blanchard F, Battaglia S, Gouin F,
Rédini F and Heymann D: Zoledronic acid activates the DNA S-phase
checkpoint and induces osteosarcoma cell death characterized by
apoptosis-inducing factor and endonuclease-G translocation
independently of p53 and retinoblastoma status. Mol Pharmacol.
71:333–343. 2007. View Article : Google Scholar : PubMed/NCBI
|