Introduction
Esophageal squamous cell carcinoma is a common
malignant tumor (1–3). The initiation and progression of
esophageal cancer is a complicated process that results from the
loss of the normal regulatory pathways controlling cell
proliferation, differentiation and apoptosis. However, present
therapeutic strategies, including chemotherapy, are characterized
by low efficacies (4). Drug
resistance and side effects of chemotherapy drugs are major
barriers to the success of chemotherapy (5). Therefore, the identification of novel
drugs for use in chemotherapy against esophageal squamous cell
carcinoma is required (6).
Certain natural products have been suggested to be
effective agents for cancer prevention, including
epigallocatechin-3-gallate (EGCG). EGCG is the ester form of
epigallocatechin/gallic acid; it is the main catechin in green tea
and contributes to its beneficial therapeutic effects, which
include antioxidant and immunomodulatory effects (7–10). Due to
its reported anti-oxidant and immunomodulatory effects, EGCG has
been extensively investigated against various types of cancer
(11–13). EGCG has not been observed to cause
adverse effects against normal cells and tissues, whereas it has
anti-proliferative, anti-invasive and chemo-preventive effects
against cancer cells (14). However,
the number of investigations concerning the effect of EGCG on
esophageal cancer is limited, and the potential function of EGCG in
esophageal cancer therapy remains poorly understood. Cell growth
and apoptosis are regulated through complex signaling systems in
the human body; their disorder or imbalance may induce the
development of tumors (15). The
efficacy of chemotherapy drugs can be evaluated by their ability to
induce apoptosis. The upregulation of caspase-3 and the reduction
of mitochondrial membrane potential may result in apoptosis, so an
ideal chemotherapy drug would cause these alterations (16,17).
The present study investigated the anticancer
effects of EGCG in esophageal squamous cell carcinoma and the
underlying molecular mechanisms in human esophageal squamous cell
carcinoma cells.
Materials and methods
Cancer cell lines and culture
Human esophageal Eca109 cancer cells were obtained
from the Cancer Institution, The Fourth Hospital of Hebei Medical
University (Shijiazhuang, China). Human esophageal Ec9706 cancer
cells were obtained from the Molecular Oncology State Key
Laboratory Cancer Institute and Hospital of the Chinese Academy of
Medical Sciences (Beijing, China).
Cells were cultured in RPMI-1640 medium supplemented
with 10% fetal bovine serum (both from Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100
µg/ml streptomycin at 37°Cin a humidified atmosphere of 5%
CO2.
Chemicals and reagents
EGCG was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) kit was purchased from Beckman
Coulter, Inc. (Brea, CA, USA). Mouse anti-human caspase-3
monoclonal antibodies (cat. no. sc-7272) were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). FITC-conjugated goat
anti-mouse secondary IgG antibody (cat. no., 115-095-003) was
purchased from Jackson Immuno Research Laboratories, Inc. (West
Grove, PA, USA).
Cytotoxicity assay
The sensitivity of Eca109 and Ec9706 cells to EGCG
was determined using an MTT assay, in which the capacity of viable
cells to metabolize MTT reagent salt to purple formazan crystals
via mitochondrial succinate dehydrogenase was assessed. Cells were
seeded into 96-well culture plates at a density of 5×104
cells/ml. Serial concentrations of EGCG (0, 25, 50, 100, 200 and
400 mg/l) were added in a final volume of 200 µl per well.
Following treatment for 24 or 48 h at 37°C, the medium was replaced
with an equal volume of fresh medium containing 0.5 mg/ml MTT
(Sigma-Aldrich; Merck KGaA) and incubated for 4 h. Then, the medium
was removed and 180 µl DMSO was added and incubated for 10 min at
room temperature. The cytotoxic effects of drugs were determined
according to the OD values using a microplate reader at an
absorption wavelength of 490 nm. Cell viability was expressed as
the relative formazan formation in treated samples when compared
with control cells: Growth inhibitory rate=[(1-A490 treated
cells/A490 control cells)x100%].
Apoptosis analysis
Cultured Eca109 and Ec9706 tumor cells treated with
0, 100, 200 or 300 mg/lEGCG for 24 h at 37°C were harvested. The
cells (1×106) were stained with PI and Annexin V-FITC,
according to the manufacturer's protocol and analyzed using an
Epics-XL flow cytometer (Beckman Coulter, Inc.). Expo32 v1.2
software (Beckman Coulter, Inc.) was used to analyze the flow
cytometric data. Early apoptotic cells were positive for Annexin V
and negative for PI staining, whereas late apoptotic cells
undergoing secondary necrosis were positive for Annexin V and PI
staining.
Analysis of mitochondrial membrane
potential expression level in Eca109 and Ec9706 cells
Cultured Eca109 and Ec9706 tumor cells
(1×106) were harvested following treatment with 0, 100,
200 or 300 mg/l EGCG for 24 hat 37°C. Following two washes with
ice-cold PBS, the cells were dyed in 1 ml of 10 µg/ml Rhodamine 123
dissolved in distilled water. Following incubation for 30 min in
the dark at 37°C and two washes with ice-cold PBS, the stained
cells were resuspended in 1 ml PBS. The stained cells were analyzed
using an Epics-XL flow cytometer. Expo32 v1.2 software was used to
analyze the flow cytometric data.
Analysis of caspase-3 protein
Cultured Eca109 and Ec9706 tumor cells
(1×106) were harvested following treatment with 0, 100,
200 or 300 mg/l EGCG for 24 h at 37°C. Cells were fixed overnight
with 70% ice-cold ethanol. Following two washes with ice-cold PBS,
the fixed cells were resuspended in 1 ml PBS containing caspase-3
antibody (dilution, 1:100) and incubated for 30 min in the dark at
room temperature. Following two washes with PBS, cells were
resuspended in 1 ml PBS containing secondary FITC-conjugated
immunoglobulin G antibodies and incubated for 30 min in the dark at
room temperature. An isotype control group with no primary antibody
was used to exclude nonspecific binding. Following two washes with
PBS, cells were resuspended in 1 ml PBS. The stained cells were
analyzed using an Epics-XL type flow cytometer. Expo32 v1.2
software was used to analysis the flow cytometric data.
Analysis of telomerase activity
Telomerase activity was determined using a telomeric
repeat amplification protocol silver staining kit (cat. no. GMS
20106.1C.1; Genmed Scientifics, Inc., Shanghai, China) according to
the manufacturer's protocol. Subsequent to silver staining, a
positive outcome was bands with a 6 bp interval. Cultured Eca109
and Ec9706 tumor cells (1×106) were harvested following
treatment with 0, 100, 200 or 300 mg/l EGCG for 24 h at 37°C, then
telomerase activity was detected.
Statistical analysis
All data were presented as the mean ± standard
deviation and were statistically analyzed using a one-way analysis
of variance followed by the Newman-Keuls method for post hoc
comparisons, using SPSS 11.5 software (SPSS Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
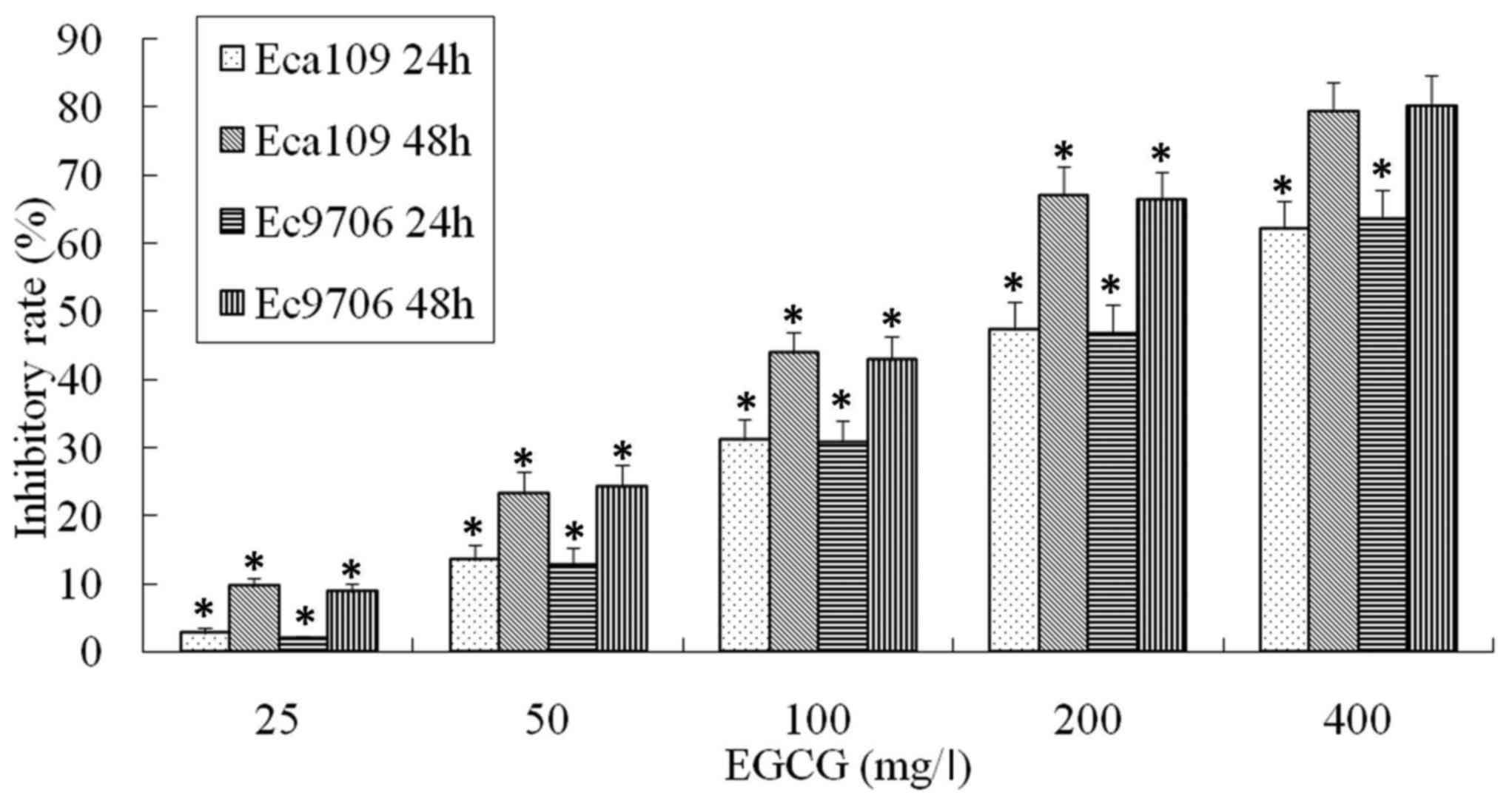
Inhibitory effect of EGCG on Eca109
and Ec9706 cells
The effect of EGCG on the viability of Eca109 and
Ec9706 cells was analyzed by MTT assay. The viability of Eca109 and
Ec9706 cells was significantly inhibited by EGCG in a dose and time
dependent manner (Fig. 1).
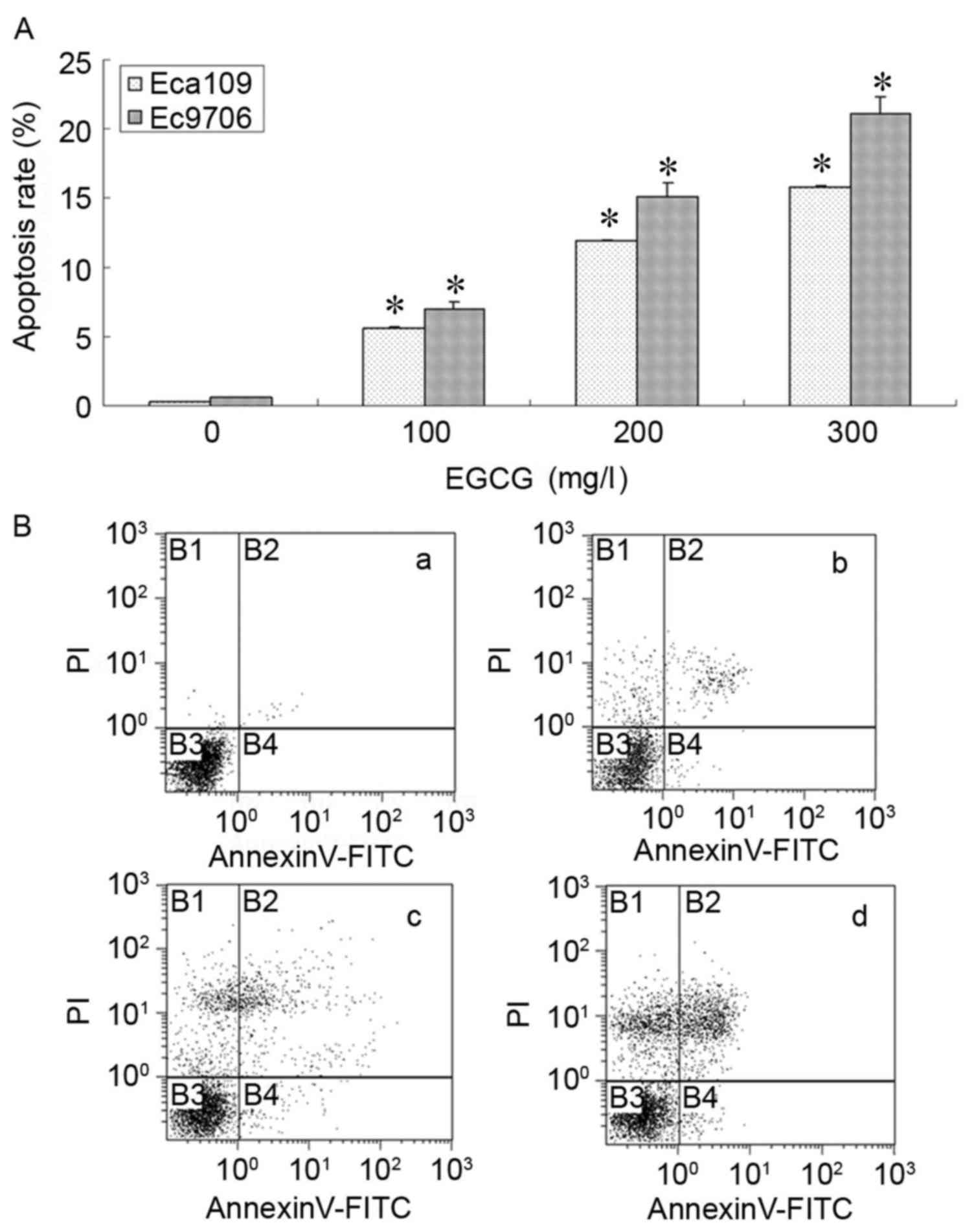
EGCG induced esophageal cancer cell
apoptosis
Experiments were performed using Eca109 and Ec9706
human esophageal cancer cells. Exposure to EGCG for 24 h was
demonstrated to induce apoptosis in a dose-dependent manner in
Eca109 and Ec9706 cells. Annexin V/PI staining revealed that EGCG
treatment induced apoptosis in the range of 100 to 300 mg/l
(Fig. 2).
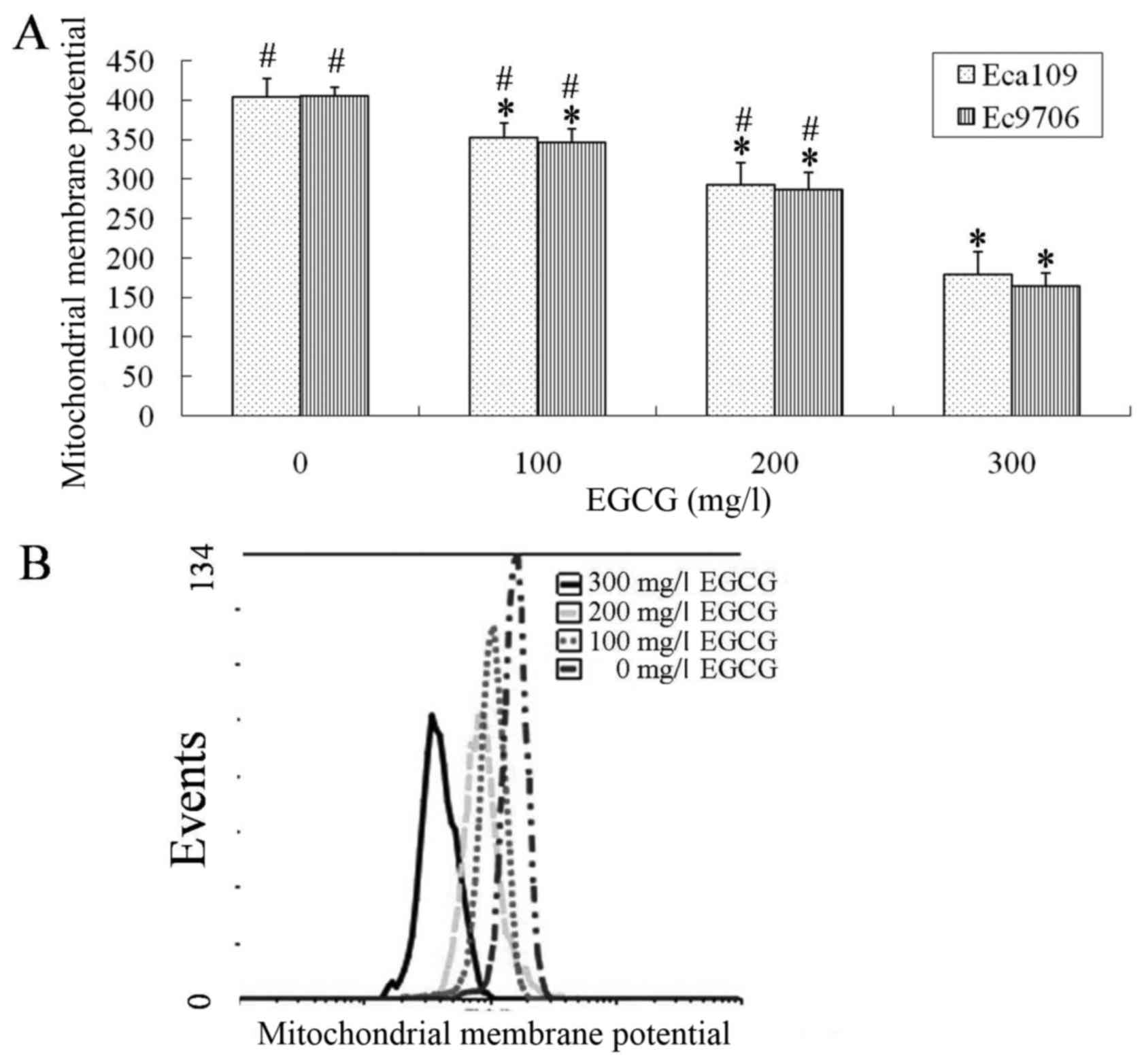
EGCG modulates Eca109 and Ec9706 cell
mitochondrial membrane potential
Eca109 and Ec9706 cells were treated with 0, 100,
200 or 300 mg/l EGCG for 24 h, washed with cold PBS, and flow
cytometry was used to analyze the mitochondrial membrane potential.
The mitochondrial membrane potential in the EGCG-treated groups was
significantly lower compared with the control group (P<0.01;
Fig. 3). The 300 mg/l EGCG group had
significantly lower mitochondrial membrane potential compared with
the100 and 200 mg/l EGCG groups (P<0.01; Fig. 3).
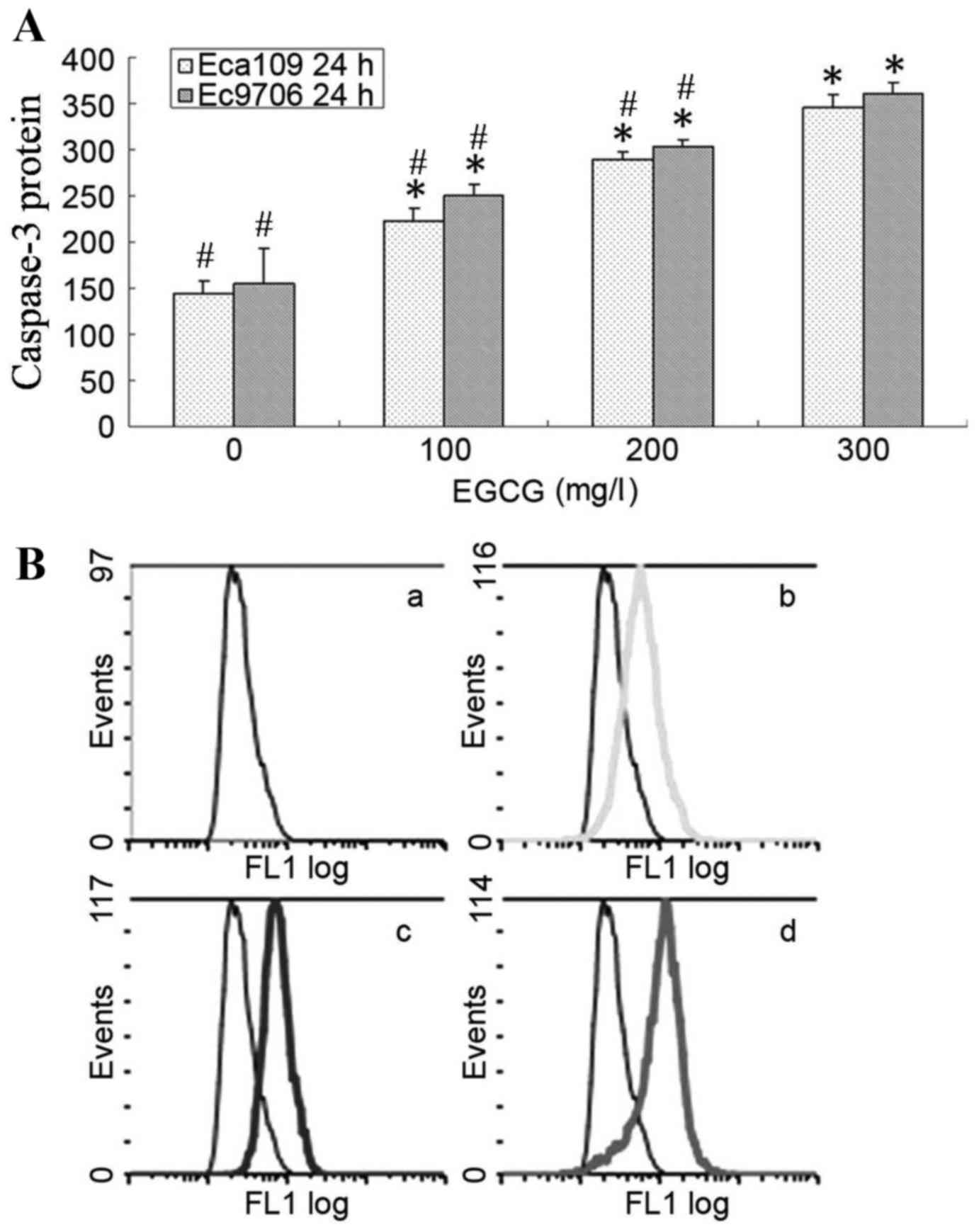
Caspase-3 expression levels were
increased following EGCG treatment in Eca109 and Ec9706 cells
Eca109 and Ec9706 cells were treated with 0, 100,
200 or 300 mg/l EGCG for 24 h, washed with cold PBS, and caspase-3
protein expression was analyzed using flow cytometry. Caspase-3
protein expression level was significantly upregulated in the
treatment groups compared with the controls (P<0.05; Fig. 4). Additionally, caspase-3 protein
expression level was significantly higher in the 300 mg/l EGCG
group compared with the 100 and 200 mg/l EGCG groups
(P<0.05).
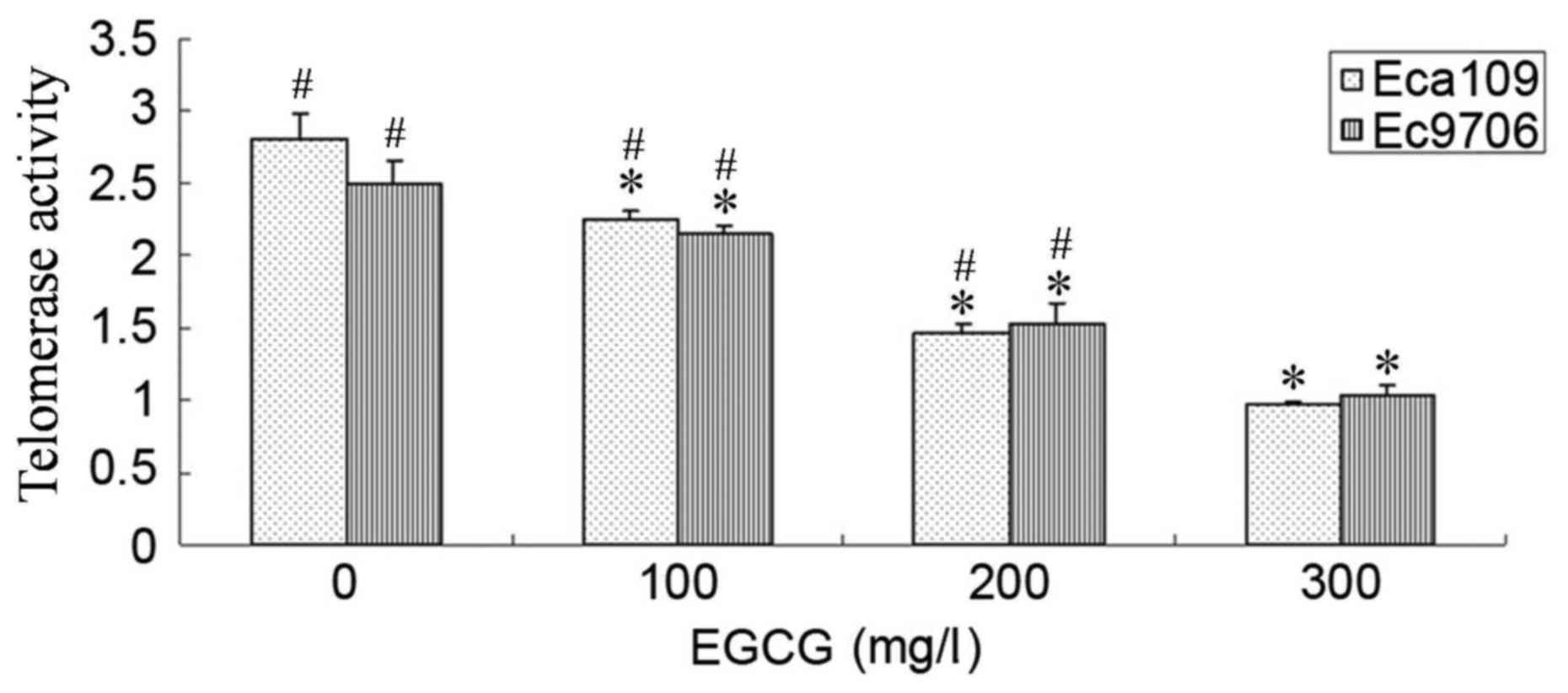
EGCG inhibited telomerase activity in
Eca109 and Ec9706 cells
Following treatment with 100, 200 or 300 mg/l EGCG
for 24 h, the telomerase activity in Eca109 and Ec9706 cells was
significantly inhibited compared with control cells (P<0.05;
Fig. 5). Additionally, telomerase
activity was significantly lower in the 300 mg/l EGCG group
compared with the 100 and 200 mg/l EGCG groups (P<0.05).
Discussion
EGCG is the most abundant catechin in green tea, and
it possesses anti-inflammatory, antioxidant, immunomodulatory and
anticancer functions (18–20). Previous studies have suggested that
EGCG is associated with the potential health benefits attributed to
green tea consumption (21). The
anticancer effect of EGCG has been explored in various tumor cells
(22–24), but there are few articles concerning
the anticancer effect of EGCG on esophageal cancer. In the present
study, EGCG was demonstrated to suppress the viability of
esophageal cancer Eca109 and Ec9706 cells via inducing apoptosis in
an EGCG dose-dependent manner. EGCG has been reported to induce
cancer cell apoptosis through different pathways involving the
pro-oxidant, epigenetic modulation of apoptosis-related genes,
including human telomerase reverse transcriptase, and to reduce
cell proliferation through the modulation of cell cycle progression
(25–27).
In the present study, two esophageal cancer cell
lines, Eca109 and Ec9706, were selected to test the potential
anticancer effects of EGCG on esophageal squamous cell carcinoma
cells. The tumor-suppressive effects of EGCG against esophageal
cancer cells were thus investigated in vitro. The results of
an MTT assay demonstrated that EGCG inhibited the viability of
Eca109 and Ec9706 cells in a dose- and time-dependent manner. Flow
cytometric results indicated that the EGCG also induced apoptosis
in Eca109 and Ec9706 cells in a dose-dependent manner. However, the
molecular mechanisms underlying EGCG-induced apoptosis in
esophageal cancer cells remain poorly understood. In the present
study, EGCG was reported to exert cytotoxic effects on human
esophageal cancer cell lines in vitro. This cytotoxicity was
revealed to be mediated by apoptosis, a conclusion supported by
apoptosis detection and expression of the apoptosis-associated
protein caspase-3. Apoptosis is involved in the maintenance of cell
homeostasis, and dysfunction of apoptotic signaling may result in
serious conditions, including cancer.
At present, apoptosis is the most well-studied
mechanism associated with anticancer therapy. Apoptosis is cell
death under genetic control, involving complicated regulatory
mechanisms. Mitochondrial transmembrane potential loss is able to
induce apoptosis. In the present study, the pro-apoptotic effect of
EGCG on Eca109 and Ec9706 cells was assessed in vitro, with
focus on the mitochondrial pathway. The apoptosis rate was detected
using Annexin V/PI staining and a flow cytometer. Treatment with
various concentrations of EGCG was revealed to promote apoptosis of
Eca109 and Ec9706 cells in a dose-dependent manner. The reduction
of cell proliferation is associated with apoptosis. Mitochondrial
transmembrane potential, an essential effector of the intrinsic
pathway of apoptosis, was downregulated following EGCG treatment.
Mitochondrial transmembrane potential downregulation is associated
with the induction of apoptosis. Caspase-3 protein was upregulated
in a dose-dependent manner in Eca109 and Ec9706 cells following
treatment with EGCG. In the present study, EGCG induced Eca109 and
Ec9706 cell apoptosis by downregulating mitochondrial membrane
potential and upregulating caspase-3 expression levels.
Telomerase activity allows eukaryotic cells to have
unlimited division potential. While functioning, telomerase
synthesizes short DNA repeats at the 3′- end of DNA within
chromosomes, ensuring genome stability during cell division.
Telomerase is active in the majority of cancer cell types.
Meanwhile, telomerase activity is essential for survival of
malignant cells. The present study revealed that telomerase
activity in Eca109 and Ec9706 cells was downregulated following
EGCG treatment.
The specific mechanism inhibiting the growth of
esophageal cancer cells by EGCG was explored in the present study.
EGCG inhibited cell viability and induced esophageal cancer cell
apoptosis, reducing the mitochondrial membrane potential and
telomerase activity while increasing caspase-3 expression levels.
As EGCG has the characteristics of low toxicity and few side
effects, if it can be developed as an anti-tumor drug, it will have
broad application prospects.
References
|
1
|
Gamliel Z: Incidence, epidemiology, and
etiology of esophageal cancer. Chest Surg Clin N Am. 10:441–450.
2000.PubMed/NCBI
|
|
2
|
Holmes RS and Vaughan TL: Epidemiology and
pathogenesis of esophageal cancer. Semin Radiat Oncol. 17:2–9.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wheeler JB and Reed CE: Epidemiology of
esophageal cancer. Surg Clin North Am. 92:1077–1087. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sugimura K, Miyata H, Tanaka K, Takahashi
T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M and Doki
Y: High infiltration of tumor-associated macrophages is associated
with a poor response to chemotherapy and poor prognosis of patients
undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg
Oncol. 111:752–759. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao B, Shi Q and Wang W: Higher expression
of SIRT1 induced resistance of esophageal squamous cell carcinoma
cells to cisplatin. J Thorac Dis. 7:711–719. 2015.PubMed/NCBI
|
|
6
|
Liu L, Zuo LF, Zuo J and Wang J:
Artesunate induces apoptosis and inhibits growth of Eca109 and
Ec9706 human esophageal cancer cell lines in vitro and in
vivo. Mol Med Rep. 12:1465–1472. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang GZ, Wang ZJ, Bai F, Qin XJ, Cao J, Lv
JY and Zhang MS: Epigallocatechin-3-gallate protects HUVECs from
PM2.5-induced oxidative stress injury by activating critical
antioxidant pathways. Molecules. 20:6626–6639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao H, Xie P, Li X, Zhu W, Sun X, Sun X,
Chen X, Xing L and Yu J: A prospective phase II trial of EGCG in
treatment of acute radiation-induced esophagitis for stage III lung
cancer. Radiother Oncol. 114:351–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Xu J, Li J, Du L, Chen T, Liu P,
Peng S, Wang M and Song H: Epigallocatechin-3-gallate attenuates
lipopolysaccharide-induced mastitis in rats via suppressing MAPK
mediated inflammatory responses and oxidative stress. Int
Immunopharmacol. 26:147–152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shankar S, Ganapathy S and Srivastava RK:
Green tea polyphenols: Biology and therapeutic implications in
cancer. Front Biosci. 12:4881–4899. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thangapazham RL, Singh AK, Sharma A,
Warren J, Gaddipati JP and Maheshwari RK: Green tea polyphenols and
its constituent epigallocatechin gallate inhibits proliferation of
human breast cancer cells in vitro and in vivo. Cancer Lett.
245:232–241. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shin YS, Kang SU, Park JK, Kim YE, Kim YS,
Baek SJ, Lee SH and Kim CH: Anti-cancer effect of
(−)-epigallocatechin-3-gallate (EGCG) in head and neck cancer
through repression of transactivation and enhanced degradation of
β-catenin. Phytomedicine. 23:1344–1355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiao Y, Cao J, Xie L and Shi X: Cell
growth inhibition and gene expression regulation by
(−)-epigallocatechin-3-gallate in human cervical cancer cells. Arch
Pharm Res. 32:1309–1315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Min NY, Kim JH, Choi JH, Liang W, Ko YJ,
Rhee S, Bang H, Ham SW, Park AJ and Lee KH: Selective death of
cancer cells by preferential induction of reactive oxygen species
in response to (−)-epigallocatechin-3-gallate. Biochem Biophys Res
Commun. 421:91–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aouacheria A, Cibiel A, Guillemin Y,
Gillet G and Lalle P: Modulating mitochondria-mediated apoptotic
cell death through targeting of Bcl-2 family proteins. Recent Pat
DNA Gene Seq. 1:43–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi LS, Wang H, Wang F, Feng M, Wang M and
Guan WX: Effects of gastrokine-2 expression on gastric cancer cell
apoptosis by activation of extrinsic apoptotic pathways. Mol Med
Rep. 10:2898–2904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi J, Liu F, Zhang W, Liu X, Lin B and
Tang X: Epigallocatechin-3-gallate inhibits nicotine-induced
migration and invasion by the suppression of angiogenesis and
epithelial-mesenchymal transition in non-small cell lung cancer
cells. Oncol Rep. 33:2972–2980. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Irimie AI, Braicu C, Zanoaga O, Pileczki
V, Gherman C, Berindan-Neagoe I and Campian RS:
Epigallocatechin-3-gallate suppresses cell proliferation and
promotes apoptosis and autophagy in oral cancer SSC-4 cells. Onco
Targets Ther. 8:461–470. 2015.PubMed/NCBI
|
|
20
|
Tang G, Zhang Z, Qian H, Chen J, Wang Y,
Chen X, Chen B and Chen Y: (−)-Epigallocatechin-3-gallate inhibits
osteosarcoma cell invasiveness by inhibiting the MEK/ERK signaling
pathway in human osteosarcoma cells. J Environ Pathol Toxicol
Oncol. 34:85–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bao H and Peng A: The green tea
polyphenol(−)-epigallocatechin-3-gallate and its beneficial roles
in chronic kidney disease. J Transl Int Med. 4:99–103. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khan MA, Hussain A, Sundaram MK, Alalami
U, Gunasekera D, Ramesh L, Hamza A and Quraishi U:
(−)-Epigallocatechin-3-gallate reverses the expression of various
tumor-suppressor genes by inhibiting DNA methyltransferases and
histone deacetylases in human cervical cancer cells. Oncol Rep.
33:1976–1984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Xie Y, Feng Y, Zhang L, Huang X,
Shen X and Luo X: (−)-Epigallocatechingallate induces apoptosis in
B lymphoma cells via caspase-dependent pathway and Bcl-2 family
protein modulation. Int J Oncol. 46:1507–1515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li YJ, Wu SL, Lu SM, Chen F, Guo Y, Gan
SM, Shi YL, Liu S and Li SL: (−)-Epigallocatechin-3-gallate
inhibits nasopharyngeal cancer stem cell self-renewal and migration
and reverses the epithelial-mesenchymal transition via NF-κB p65
inactivation. Tumour Biol. 36:2747–2761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang C, Du W and Yang D: Inhibition of
green tea polyphenol EGCG ((−)-epigallocatechin-3-gallate) on the
proliferation of gastric cancer cells by suppressing canonical
wnt/β-catenin signalling pathway. Int J Food Sci Nutr. 67:818–827.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shin YS, Kang SU, Park JK, Kim YE, Kim YS,
Baek SJ, Lee SH and Kim CH: Anti-cancer effect of
(−)-epigallocatechin-3-gallate (EGCG) in head and neck cancer
through repression of transactivation and enhanced degradation of
β-catenin. Phytomedicine. 23:1344–1355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li M, Li JJ, Gu QH, An J, Cao LM, Yang HP
and Hu CP: EGCG induces lung cancer A549 cell apoptosis by
regulating Ku70 acetylation. Oncol Rep. 35:2339–2347. 2016.
View Article : Google Scholar : PubMed/NCBI
|