Introduction
Gastric cancer is the fifth most common form of
malignant tumor and the third leading cause of cancer-associated
mortality worldwide (1). Due to the
aggressiveness of gastric cancer biology and the limited
effectiveness of current therapeutic modalities with advanced
disorders, further studies are required in order to understand the
underlying molecular mechanisms of gastric cancer growth and
identify the potential of novel targets for therapeutic
intervention.
Obesity, a worldwide epidemic (2), is associated with an increased risk of
numerous types of cancer including colorectal, postmenopausal
breast, prostate and renal cancer (3), and esophageal and gastric adenocarcinoma
(4). Obesity may promote the growth
of gastric cancer through complex molecular mechanisms, which
require further investigation. The molecular mechanisms by which
obesity affects cancer growth are currently being investigated;
adipokine, hormonal, inflammatory and immunological changes may
contribute in part to altered tumor biology (5). The most abundant adipokines derived from
adipose tissue have been implicated as mediators of the effects of
obesity on cancer development (6).
The level of one such adipokine, visfatin, increases in obese
individuals and contributes to a general pro-inflammatory state in
the peripheral organs (7). This may
prove to be an important mechanistic link in the network of factors
affecting obesity-associated tumor growth. Visfatin acts as a NAD
biosynthetic enzyme similar to nicotinamide phosphoribosyl
transferase (Nampt) (8), functioning
as an enzyme involved in the NAD+ salvage pathway, which
was demonstrated to be upregulated in numerous types of human
malignant tumors (9), including
gastric cancer (10).
Silent mating-type information regulation 2 homolog
1 (Sirt1), originally identified as a longevity gene, is induced by
caloric restriction and regulates various cellular functions,
including DNA repair, metabolism and cell survival under genotoxic
and oxidative stress (11). Sirt1
functions as a NAD+-dependent histone deacetylase, and
deacetylates a number of key cell cycle proteins and apoptosis
regulatory molecules, including tumor protein p53 (12,13). The
role of Sirt1 in cancers has been studied previously; however,
whether Sir1 serves a role as a tumor suppressor or tumor promoter
remains unclear, since it seems to depend on the cellular context,
its targets in specific signaling pathways or the specific type of
cancer (11). Previous reports have
demonstrated that Sirt1 is involved in carcinogenesis and enhances
the growth, metastasis and chemotherapy resistance of a number of
cancers through its anti-apoptotic activity, including colon
(14), breast (15) and gastric cancer (16). Elevated Sirt1 deacetylates activated
p53 (17) and this allows cells with
damaged DNA to proliferate, promoting tumor development (18).
Yes-associated protein (YAP), a transcriptional
co-activator that acts downstream of the Hippo signaling pathway,
regulates multiple cellular processes and is associated with tumor
growth and development (19). The YAP
gene locus is amplified in human malignancies, including glioma,
medulloblastoma (20), oral squamous
cell carcinoma and hepatocellular carcinoma (HCC) (21). Consistently, upregulated YAP
expression and nuclear localization have been observed in multiple
types of human malignant tumors, including liver, colon, ovarian,
lung and prostate cancer (22).
Furthermore, YAP has been associated with tumor development and the
prognosis of patients with cancer (21). YAP shuttles between the cytoplasm and
the nucleus, where it induces the expression of pro-proliferative
and anti-apoptotic genes via interactions with transcription
factors, particularly TEA domain (TEAD) family members (23). When the upstream Hippo kinase receives
an extracellular growth inhibition signal, YAP is phosphorylated
and inactivated, which results in the inhibition of transcriptional
activity through the cytoplasmic retention of YAP and subsequent
ubiquitin-mediated proteasomal degradation, therefore gene
expression is downregulated (23). By
contrast, when the kinase receives a growth promoting signal,
hypo-phosphorylated YAP translocates into the nucleus and induces
target gene expression (24) to
regulate tissue homeostasis, organ size, regeneration and
tumorigenesis.
A Sirt1-YAP signaling pathway in which YAP is
regulated by Sirt1-mediated deacetylation has been identified in
cancer cells; a cycle of acetylation/deacetylation of nuclear YAP
exists downstream of the Hippo signaling pathway (25). The deacetylation of YAP2 by Sirt1
promotes the YAP2/TEAD4 association, resulting in YAP2/TEAD4
transcriptional activation and cell growth in HCC cells, and Sirt1
promotes cisplatin (CDDP)-induced YAP2 nuclear accumulation and
inhibits CDDP-induced apoptosis (26,27).
Until now, to the best of our knowledge, few studies
have been published regarding the mechanisms by which obesity
affects gastric cancer growth. Our group previously developed a
model of murine gastric cancer using C57BL/6j high fat dietary
obese mice and flank-implanted murine gastric cancer cells. The
results demonstrated that diet-induced obese mice exhibited
metabolic changes and larger subcutaneous tumors than lean mice
(28). In addition, histological
analyses demonstrated that obesity not only enhanced cellular
growth, but also reduced cellular apoptosis (28). The aim of the present study was to use
this in vivo mouse model to investigate the molecular
mechanisms underlying the association of obesity with gastric
cancer growth.
Materials and methods
Cell culture
The gastric cancer cell line used in this study,
murine forestomach carcinoma cell line (MFC), was purchased from
The Cell Bank Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China) (29). MFC
cells were maintained in RPMI-1640 medium (Cellgro; Corning
Incorporated, Corning, NY, USA) containing 10% fetal bovine serum
(Valley Biomedical Products & Services, Inc., Winchester, VA,
USA), 100 U/ml penicillin and 100 µg/ml streptomycin. Cells were
maintained in a 37°C humidified incubator with 5%
CO2.
Diet induced obesity model and in vivo
gastric cancer model
Three- to five-week-old male C57BL/6j mice (n=36)
were obtained from Shanghai Laboratory Animal Center (Chinese
Academy of Sciences, Shanghai, China) and bred under standard
conditions, controlled at a temperature of 20–25°C and 40–50%
humidity with a 12 h light/dark cycle, and the body weight of these
animals ranged between 15–18 g. Mice were randomly divided into two
groups, then were weaned onto a high-fat diet (35.5% fat, 36.3%
carbohydrate, 20.0% protein, n=24) and normal diet (5.4% fat, 51.0%
carbohydrate, 22.9% protein, n=12) (30), respectively for 12 weeks, and then
mice were divided into three groups (lean, obese and non-obese) as
previously described (31). The mice
in different groups were maintained on their previous diet until
the end of the experiment. The mice were allowed access to their
specific diet and water ad libitum.
To detect the impact of obesity on tumor growth,
each of 12 obese, 12 lean and 12 non-obese mice was injected
subcutaneously with 2.0×106 MFC cells into the right
flank and monitored daily to check for the presence of palpable
tumors. Then all mice were maintained on normal or high fat diet
for another 2 weeks. Tumor growth was observed in 100% of the obese
animals, in 75% (9/12) of the non-obese mice and in 83.3% (10/12)
of the lean mice. In all experiments, body weight and food intake
were checked twice per week. At the end of experiment, following
overnight fasting, the animals were sacrificed, and tumor tissues
were extracted, immediately frozen in liquid nitrogen, and stored
at −80°C until RNA and protein extraction. The tumor volumes
measured range between 8 and 150 mm3.
All experiments were approved by the Xi'an Jiaotong
University Institutional Animal Care and Use Committee following
‘Principles of laboratory animal care’ (NIH publication no. 85–23,
revised 1985). All surgery was performed under sodium pentobarbital
anesthesia and all efforts were made to minimize suffering.
In vitro study of the effect of
obesity on Sirt1/YAP
Following overnight fasting and anesthesia, the
blood of these mice was obtained via cardiac puncturing. Sera
samples were separated by centrifugation for 10 min at 2,000 × g
and 4°C and stored at −80°C until measurements were performed.
Biological behaviors of MFC cells induced by sera of mice were
analyzed as previously reported (31). The expression of Sirt1 and YAP was
examined in cultured cells with exposure to RPMI-1640 or 5% sera of
obese mice or lean animals to detect whether endocrine mechanism of
obesity may be responsible for the growth of MFC cells.
Immunohistochemical analysis
Tumors were obtained from the in vivo
xenograft model and fixed in 10% neutral buffered formalin for 24 h
at room temperature, then were embedded in paraffin. The paraffin
blocks were cut on a microtome into 5 mm-thick sections. The tumor
sections were dewaxed and dehydrated with descending alcohol series
(10 min for 100% alcohol, then 5 min for 95, 90 and 80% alcohol).
Rehydration, antigen retrieval in citrate buffer, endogenous
peroxidase activity was blocked for 10 min using 3.0% hydrogen
peroxide at room temperature, then the sections were blocked with
10% goat plasma (cat. no. SAP-9100; ZSGB-BIO, Beijing, China) for
30 min at room temperature, then separately incubated with the
primary antibodies directed against Sirt1 and YAP (both rabbit
anti-mouse) at 4°C overnight. The primary antibodies were detected
using biotinylated secondary goat anti-rabbit antibodies (cat. no.
SAP-9100; ZSGB-BIO, Beijing, China) for 30 min at room temperature
following the manufacturer's recommendations. The staining of the
sections was performed using the horseradish
peroxidase-streptavidin conjugates for Sirt1 and YAP (SP method).
The primary antibodies used and their experimental conditions are
summarized in Table I. The scoring
system for Sirt1 and YAP expression was performed as previously
described (31). Briefly, staining
intensity was expressed as four grades: 0, none; 1, weak; 2,
moderate; and 3, strong. The percentage of positive MFC cells was
expressed as: 0, <5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; and 4,
>75%. The staining intensity and average percentage of positive
MFC cells were assayed for 10 independent high power fields (×400)
with the help of Olympus microscope (type BHS, Japan). The total
score was calculated by multiplying the staining intensity and the
percentage of positive MFC cells. All histological analyses were
carried out by three independent observers.
 | Table I.List of antibodies and dilutions used
in the present study. |
Table I.
List of antibodies and dilutions used
in the present study.
| Protein | Experiment | Final
concentration | Catalog number | Supplier |
|---|
| Sirt1 | WB | 1:200 | sc-15404 | Santa Cruz
Biotechnology, Inc., Dallas, TX, USA |
|
| IHC | 1:50 | sc-15404 | Santa Cruz
Biotechnology, Inc. |
| YAP | WB | 1:200 | ab52771 | Abcam, Cambridge,
UK |
|
| IHC | 1:50 | ab52771 | Abcam |
| GAPDH | WB | 1:500 | sc-47724 | Santa Cruz
Biotechnology, Inc. |
RNA expression studies
Total RNA was extracted from the target cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
To verify the stability of these mRNA, the products were separated
on 0.8% agarose gel with 0.5 µg/ml ethidium bromide electrophoresis
and visualized under ultraviolet light by a gel imaging analysis
system (vJS-2000; Pei & Qing Science & Technology, Inc.,
Shanghai, China). Expression of Sirt1 and YAP mRNA was quantified
by reverse transcription-polymerase chain reaction (RT-PCR). RNA
was reverse transcribed to complementary DNA using the High
Capacity 1st Strand Synthesis kit (Takara Bio, Inc., Otsu, Japan).
The PCR reaction was performed using an iCycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) with the following
thermocycling conditions: A pre-heating step at 95°C for 10 min
followed by 30 repeats of 94.0°C for 30 sec and 55.0°C for 30 sec,
then 72°C for 1 min. The products were separated on 2% agarose gel
with 0.5 µg/ml ethidium bromide electrophoresis and visualized
under ultraviolet light by a gel imaging analysis system (vJS-2000;
Pei & Qing Science & Technology, Inc., Shanghai, China).
The primer sequences were generated using National Center for
Biotechnology Information Primer-BLAST and are presented in
Table II. Transcript levels were
normalized to GAPDH. For validation, each experiment was carried
out in triplicate.
 | Table II.List of primer sequences. |
Table II.
List of primer sequences.
| Gene | Primer | 5′→3′
Sequences | PCR size (bp) |
|---|
| GAPDH | Forward |
CGTAGACAAAATGGTGAAGG | 296 |
|
| Reverse |
GACTCCACGACATACTCAGC |
|
| Sirt1 | Forward |
TTGTGAAGCTGTTCGTGGAG | 412 |
|
| Reverse |
GCGTGGAGGTTTTTCAGTA |
|
| YAP | Forward |
CCCTGATGATGTACCACTGCC | 623 |
|
| Reverse |
CCACTGTTAAGAAAGGGATCGG |
Protein extraction and western blot
analysis
High quality nuclear protein for western blot
analysis was extracted from the cultured cells by exposure to
RPMI-1640 or 5% sera of obese mice or lean animals for 24 h with a
Nuclear Protein Extraction kit (cat. no. P0028; Beyotime Institute
of Biotechnology, Haimen, China) according to the manufacturer's
protocol. Protein concentration was measured using a BCA kit
(Pierce; Thermo Fisher Scientific, Inc.). A total of 20 µg protein
was loaded per lane, then were run on a 10% SDS-PAGE and were
transferred to nitrocellulose membranes (EMD Millipore, Billerica,
MA, USA) using a Bio-Rad Mini PROTEAN 3 System, according to the
manufacturer's protocol. The nitrocellulose membranes were then
blocked in TBST with 5% non-fat dry milk at 37°C for 2 h.
Subsequently, the membranes were incubated with a 1:200 dilution of
the primary antibodies for Sirt1 and YAP, and a 1:500 dilution of
anti-GAPDH at 4°C overnight (Table
I). Anti-rabbit (cat. no. ZB-2301; ZSGB-BIO, Beijing, China) or
anti-mouse IgG (cat. no. sc-516102; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) antibodies with a 1:5,000 dilution were used as
the secondary antibodies, and incubated with the membrane for 1 h
at room temperature. The membranes were then developed with
enhanced chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.)
by an enhanced chemiluminescence detection system (Amersham
Biosciences, Piscataway, NJ, USA). For validation, each experiment
was carried out in triplicate.
Statistical analysis
Values were expressed as the mean ± standard
deviation. Statistical differences were estimated by one-way
analysis of variance followed by a Dunnett's test or Spearman rank
test using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Obese mice exhibit a larger tumor size
and increased visfatin levels compared with non-obese and lean
mice
Mice were segregated by weight for further analysis
as previously described (31). Obese,
non-obese and lean mice were selected at the time of
transplantation. The obese mice were significantly larger compared
with the non-obese and lean mice in body weight (P<0.0.5;
Table III). Tumor growth was
observed in 100% of the obese animals, in 75% (9/12) of the
non-obese mice and in 83.3% (10/12) of the lean mice. At the end of
the experiment, the obese mice possessed significantly larger
tumors compared with the non-obese and lean mice (both P<0.05;
Table III), but there was no
difference between lean mice and non-obese mice (P>0.05), and
they exhibited a positive correlation with the final body weight of
the mice (r=0.75; P<0.05; n=31).
 | Table III.The weight of mice and subcutaneous
tumors. |
Table III.
The weight of mice and subcutaneous
tumors.
| Parameters | Obese (n=12) | Non-obese
(n=9) | Lean (n=10) |
|---|
| Body weight
(g) |
35.4±2.8a |
28.7±2.3b | 28.5±1.2 |
| Tumor weight
(mg) |
134.2±17.3a |
83.4±15.3b | 77.2±14.9 |
Diet-induced obese mice have been demonstrated to
exhibit metabolic changes, including insulin resistance, glucose
intolerance, hyperglycemia and hyperinsulinemia, and altered
adipokine levels (31). The serum
visfatin levels from this previous study were as follows: Obese
(n=12), 44.3±3.6 ng/ml (P<0.01 vs. non-obese and P<0.01 vs.
lean); non-obese (n=9), 38.9±2.7 ng/ml (P>0.05 vs. lean); lean
(n=10), 39.6±3.4 ng/ml (31). This
indicated that obesity may promote murine gastric cancer growth via
endocrine mechanisms.
Since no significant differences were observed in
tumor and body weight (P>0.05; Table
III) or serum visfatin level and other metabolites (data not
shown) between the non-obese and lean groups, further analyses were
restricted to the obese and lean groups.
Expression of Sirt1 and YAP in tumors
is positively correlated
The mechanisms by which obesity affects gastric
cancer growth are complex. Obesity may promote gastric cancer
growth via the pro-survival Sirt1/YAP signaling pathway (27). Therefore, the expression of Sirt1 and
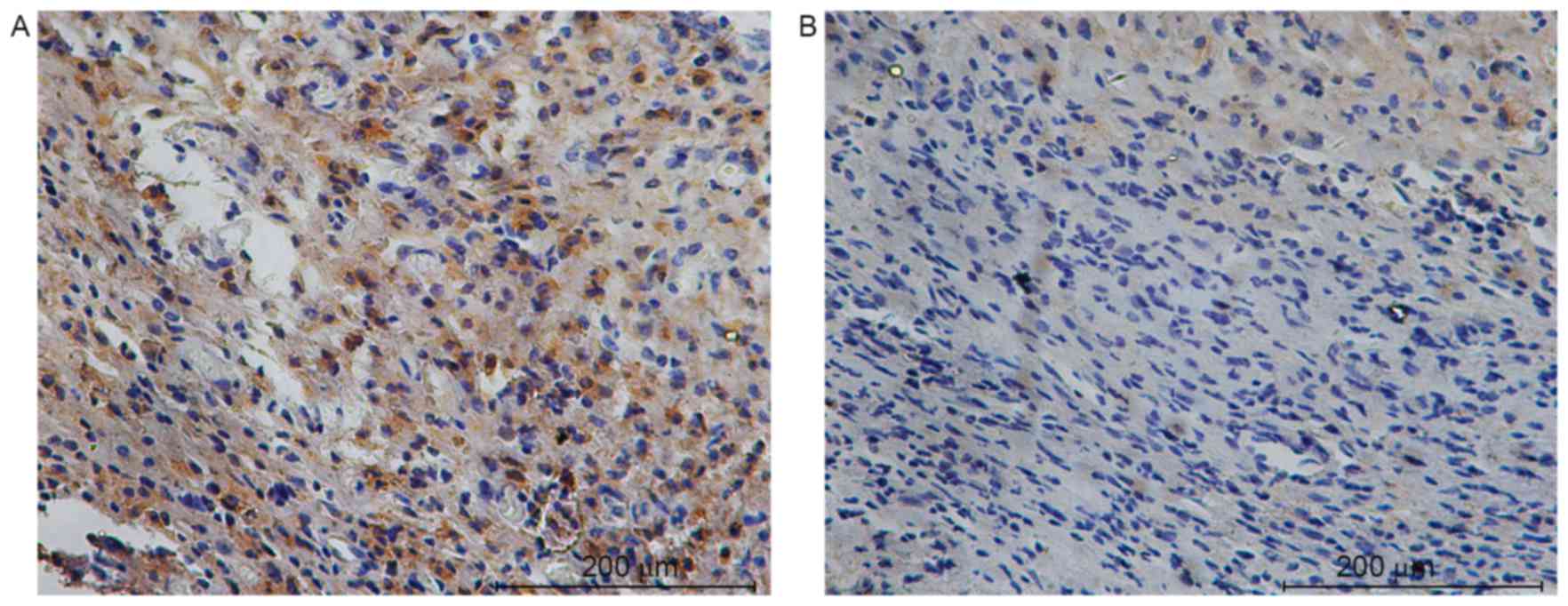
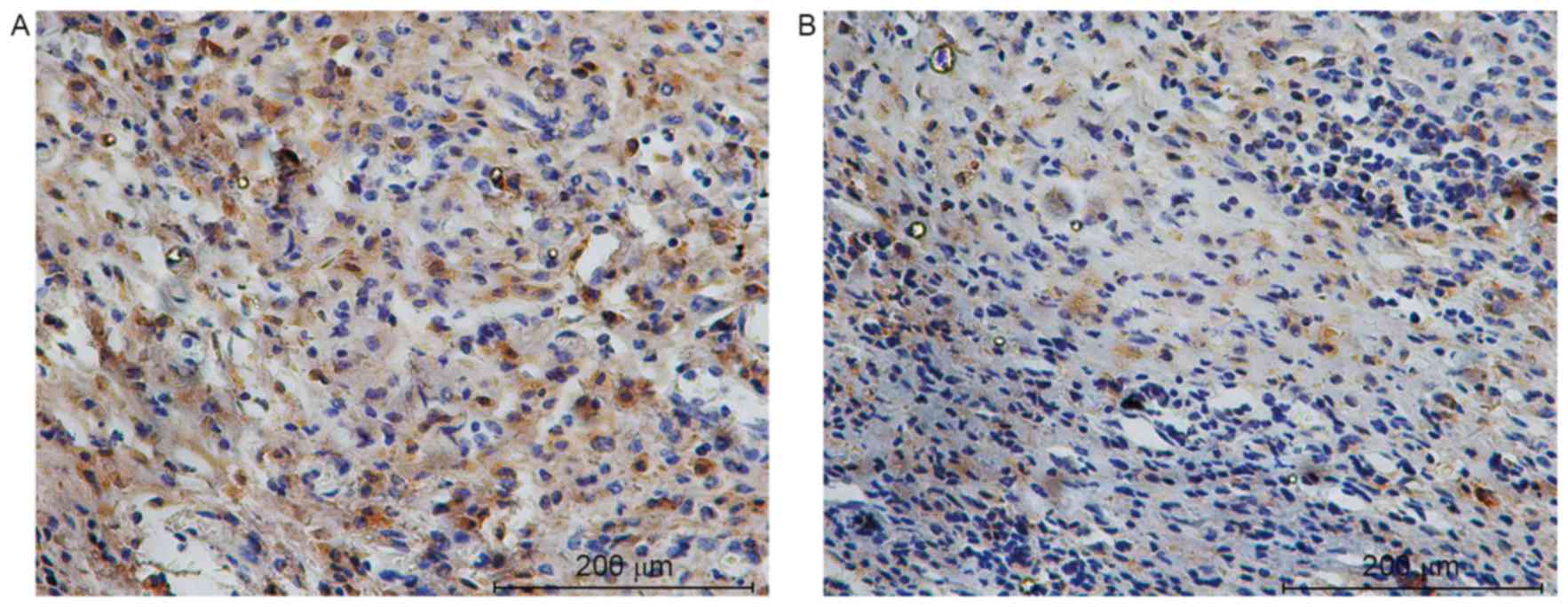
YAP was investigated in subcutaneous tumors by
immunohistochemistry. Sirt1 and YAP localized primarily in the cell
nucleus and their expression was significantly elevated in obese
mice compared with the control (7.75±2.05 vs. 0.58±0.52; t=11.743;
P<0.001 for Sirt1 and 9.08±1.68 vs. 1.25±0.62; t=15.176;
P<0.001 for YAP; Figs. 1 and
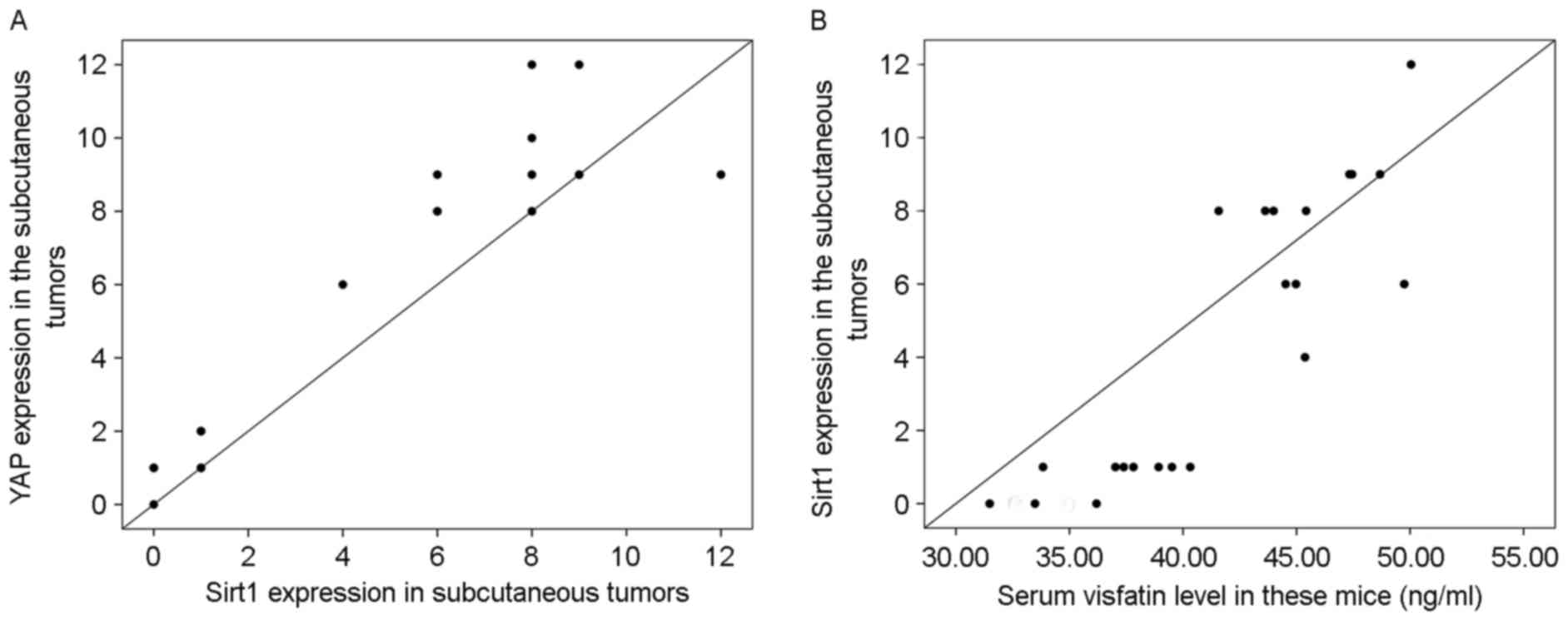
2). The level of Sirt1 protein was
positively correlated with that of YAP (r=0.94; P<0.001; n=22;
Fig. 3A) in the tumors. In addition,
the serum visfatin level was positively correlated with Sirt1
protein in the tumors (r=0.89; P<0.001; n=22; Fig. 3B).
Obesity promotes the growth of gastric
cancer via the Sirt1/YAP pathway
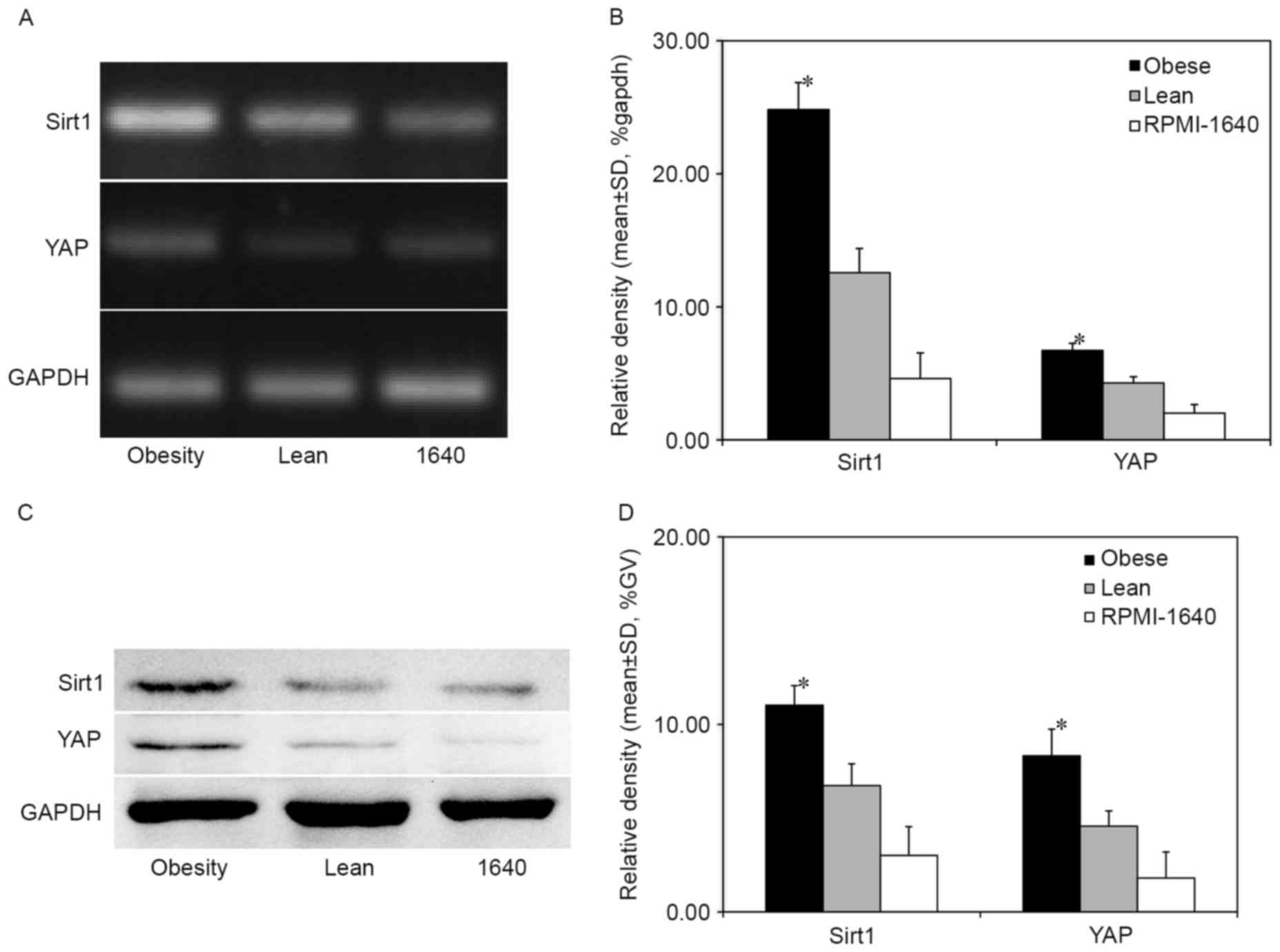
The expression of Sirt1 and YAP was investigated in
MFC cells cultured with 5% sera of obese or lean mice. Sirt1 and
YAP were significantly upregulated at the mRNA and nuclear protein
levels in cells treated with sera from obese mice compared with
those treated with sera from lean mice and the control RPMI-1640
group without serum (both P<0.05; Fig.
4). This result suggests that obesity could promote MFC cell
migration and proliferation, decrease MFC cellular apoptosis and
accelerate cell cycle progression (31) by promoting the Sirt1/YAP signaling
pathway.
Discussion
In the present study, a gastric cancer in
vivo model was used to investigate the association between
obesity and gastric cancer growth. The subcutaneous tumor weight
was significantly larger in obese C57BL/6j mice compared with
non-obese and lean animals. No significant differences in body
weight, tumor weight, the serum visfatin level and levels of other
metabolites were identified between the non-obese and lean mice,
indicating that obesity, but not the high-fat diet itself, promoted
murine gastric cancer growth.
Obesity upregulates the expression level of
pro-survival signals, which may shift the apoptotic balance of MFC
cells towards survival (5). One
target of this obesity-mediated effect is the Sirt1/YAP
pro-survival pathway. In the present study, immunohistochemical
analysis demonstrated that YAP expression in the MFC xenografts was
elevated in obese mice compared with the lean mice, and its
expression in cultured MFC cells was significantly increased at the
mRNA and protein levels in obese mice compared with the lean mice.
Although Sirt1 serves dual roles in the growth and progression of
tumors (11), in the present study,
Sirt1 was upregulated in tumors from obese mice, which may prompt
the growth of MFC cells in vitro. Therefore, obesity
accelerates murine gastric cancer growth. However, how specific
adipose tissues regulate MFC cell growth through the Sirt1/YAP
pro-survival signaling pathway remains unknown and requires further
investigation.
White adipose tissue is an energy store that
secretes a large number of adipokines (5). Adipokines are primarily secreted by
adipocytes and are small, biologically active factors that may
serve significant roles in stimulating tumor growth and progression
(32). Adipokines, including visfatin
are implicated in cell growth, proliferation and angiogenesis
(32). Visfatin acts as a NAD
biosynthetic enzyme similar to Nampt and catalyzes the transfer of
a phosphoribosyl group from 5-phosphoribosyl-1-pyrophosphate to
nicotinamide, forming nicotinamide mononucleotide (NMN) and
pyrophosphate (8). NMN is then
converted to NAD by nicotinamide mononucleotide adenylyltransferase
(Nmnat) (33,34) through the NAD+ salvage
pathway. Increased levels of visfatin in obesity may promote
gastric cancer growth and development by functioning as a NAD
biosynthetic enzyme to increase the NAD+ content, which
directly affects the ability of Sirt1, then activates YAP to affect
tumor growth and development. In the present study, the serum
visfatin level was significantly increased in obese animals
(44.3±3.6 ng/ml) compared with lean animals (39.6±3.4 ng/ml) and
was associated with the tumor weight (r=0.79; P<0.05; n=31).
This is consistent with previous suggestions that adipokines and
other growth factors secreted in the context of obesity may enhance
cancer cell survival and solid tumor growth (30). Furthermore, the serum visfatin level
was positively correlated with Sirt1 protein in the tumors in the
present study, which suggests that visfatin may serve a role in the
link between obesity and the Sirt1/YAP pro-survival pathway. YAP
acts as the target and terminal effector of the Hippo signaling
pathway, which regulates development and cell-contact inhibition
(19), and may link obesity with the
Hippo signaling pathway.
A previous study demonstrated that YAP is regulated
by Sirt1-mediated deacetylation in cancer cells: Sirt1 deacetylates
nuclear YAP protein, which upregulates or induces expression of
pro-proliferation and anti-apoptotic genes via interactions with
transcription factors, particularly TEAD (23). Cytoplasmic YAP is phosphorylated,
which inactivates it and primes it for ubiquitin-mediated
proteasomal degradation (26,27). Results from the present study
demonstrated that obesity enhances the expression of Sirt1 and YAP
protein. Future studies are required to verify the direct
regulation of YAP by Sirt1.
In conclusion, the present study demonstrated that
obesity potentiates transplanted tumor growth in mice, potentially
through regulation of the Sirt1/YAP signaling pathway. The
Sirt1/YAP signaling pathway may promote gastric cancer cell
migration, proliferation and survival, and increase cell cycling
through an endocrine mechanism. However, this requires future
study.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81472245).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Flegal KM, Carroll MD, Kit BK and Ogden
CL: Prevalence of obesity and trends in the distribution of body
mass index among US adults, 1999–2010. JAMA. 307:491–497. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Pergola G and Silvestris F: Obesity as
a major risk factor for cancer. J Obes. 2013:2915462013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Doherty MG, Freedman ND, Hollenbeck AR,
Schatzkin A and Abnet CC: A prospective cohort study of obesity and
risk of oesophageal and gastric adenocarcinoma in the NIH-AARP diet
and health study. Gut. 61:1261–1268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nieman KM, Romero IL, Van Houten B and
Lengyel E: Adipose tissue and adipocytes support tumorigenesis and
metastasis. Biochim Biophys Acta. 1831:1533–1541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CH, Woo YC, Wang Y, Yeung CY, Xu A and
Lam KS: Obesity, adipokines and cancer: An update. Clin Endocrinol
(Oxf). 83:147–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galli M, Van Gool F, Rongvaux A, Andris F
and Leo O: The nicotinamide phosphoribosyltransferase: A molecular
link between metabolism, inflammation, and cancer. Cancer Res.
70:8–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Revollo JR, Grimm AA and Imai S: The
regulation of nicotinamide adenine dinucleotide biosynthesis by
Nampt/PBEF/visfatin in mammals. Curr Opin Gastroenterol.
23:164–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bi TQ and Che XM: Nampt/PBEF/visfatin and
cancer. Cancer Biol Ther. 10:119–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bi TQ, Che XM, Liao XH, Zhang DJ, Long HL,
Li HJ and Zhao W: Overexpression of Nampt in gastric cancer and
chemopotentiating effects of the Nampt inhibitor FK866 in
combination with fluorouracil. Oncol Rep. 26:1251–1257.
2011.PubMed/NCBI
|
|
11
|
Bosch-Presegué L and Vaquero A: The dual
role of sirtuins in cancer. Genes Cancer. 2:648–662. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asaka R, Miyamoto T, Yamada Y, Ando H,
Mvunta DH, Kobara H and Shiozawa T: Sirtuin 1 promotes the growth
and cisplatin resistance of endometrial carcinoma cells: A novel
therapeutic target. Lab Invest. 95:1363–1373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin Z and Fang D: The roles of SIRT1 in
cancer. Genes Cancer. 4:97–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vellinga TT, Borovski T, de Boer VC,
Fatrai S, van Schelven S, Trumpi K, Verheem A, Snoeren N, Emmink
BL, Koster J, et al: SIRT1/PGC1α-dependent increase in oxidative
phosphorylation supports chemotherapy resistance of colon cancer.
Clin Cancer Res. 21:2870–2879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Derr RS, van Hoesel AQ, Benard A,
Goossens-Beumer IJ, Sajet A, Dekker-Ensink NG, de Kruijf EM,
Bastiaannet E, Smit VT, van de Velde CJ and Kuppen PJ: High nuclear
expression levels of histone-modifying enzymes LSD1, HDAC2 and
SIRT1 in tumor cells correlate with decreased survival and
increased relapse in breast cancer patients. BMC Cancer.
14:6042014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Wang X and Chen P: MiR-204 down
regulates SIRT1 and reverts SIRT1-induced epithelial-mesenchymal
transition, anoikis resistance and invasion in gastric cancer
cells. BMC Cancer. 13:2902013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen WY, Wang DH, Yen RC, Luo J, Gu W and
Baylin SB: Tumor suppressor HIC1 directly regulates SIRT1 to
modulate p53-dependent DNA-damage responses. Cell. 123:437–448.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim CS: Human SIRT1: A potential biomarker
for tumorigenesis? Cell Biol Int. 31:636–637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Santucci M, Vignudelli T, Ferrari S, Mor
M, Scalvini L, Bolognesi ML, Uliassi E and Costi MP: The hippo
pathway and YAP/TAZ-TEAD protein-protein interaction as targets for
regenerative medicine and cancer treatment. J Med Chem.
58:4857–4873. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Orr BA, Bai H, Odia Y, Jain D, Anders RA
and Eberhart CG: Yes-associated protein 1 is widely expressed in
human brain tumors and promotes glioblastoma growth. J Neuropathol
Exp Neurol. 70:568–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan SW, Lim CJ, Chen L, Chong YF, Huang
C, Song H and Hong W: The Hippo pathway in biological control and
cancer development. J Cell Physiol. 226:928–939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moroishi T, Hansen CG and Guan KL: The
emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 15:73–79.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mo JS, Park HW and Guan KL: The Hippo
signaling pathway in stem cell biology and cancer. EMBO Rep.
15:642–656. 2014.PubMed/NCBI
|
|
24
|
Johnson R and Halder G: The two faces of
Hippo: Targeting the Hippo pathway for regenerative medicine and
cancer treatment. Nat Rev Drug Discov. 13:63–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hata S, Hirayama J, Kajiho H, Nakagawa K,
Hata Y, Katada T, Furutani-Seiki M and Nishina H: A novel
acetylation cycle of transcription co-activator Yes-associated
protein that is downstream of Hippo pathway is triggered in
response to SN2 alkylating agents. J Biol Chem. 287:22089–22098.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Tang X, Weng W, Qiao Y, Lin J, Liu
W, Liu R, Ma L, Yu W, Yu Y, et al: The membrane protein melanoma
cell adhesion molecule (MCAM) is a novel tumor marker that
stimulates tumorigenesis in hepatocellular carcinoma. Oncogene.
34:5781–5795. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mao B, Hu F, Cheng J, Wang P, Xu M, Yuan
F, Meng S, Wang Y, Yuan Z and Bi W: SIRT1 regulates YAP2-mediated
cell proliferation and chemoresistance in hepatocellular carcinoma.
Oncogene. 33:1468–1474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li HJ, Che XM, Zhao W, He SC, Zhang ZL and
Chen R: Diet-induced obesity potentiates the growth of gastric
cancer in mice. Exp Ther Med. 4:615–620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qian SS, Gao J, Wang JX, Liu Y and Dong
HY: Establishment of a mouse forestomach carcinoma cell line (MFC)
with spontaneous hematogenous metastasis and preliminary study of
its biological characteristics. Zhonghua Zhong Liu Za Zhi.
9:261–264. 1987.(In Chinese). PubMed/NCBI
|
|
30
|
Yakar S, Nunez NP, Pennisi P, Brodt P, Sun
H, Fallavollita L, Zhao H, Scavo L, Novosyadlyy R, Kurshan N, et
al: Increased tumor growth in mice with diet-induced obesity:
Impact of ovarian hormones. Endocrinology. 147:5826–5834. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li HJ, Che XM, Zhao W, He SC, Zhang ZL,
Chen R, Fan L and Jia ZL: Diet-induced obesity promotes murine
gastric cancer growth through a nampt/sirt1/c-myc positive feedback
loop. Oncol Rep. 30:2153–2160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deng T, Lyon CJ, Bergin S, Caligiuri MA
and Hsueh WA: Obesity, Inflammation, and Cancer. Annu Rev Pathol.
11:421–449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garten A, Petzold S, Körner A, Imai S and
Kiess W: Nampt: Linking NAD biology, metabolism and cancer. Trends
Endocrinol Metab. 20:130–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pilz S, Mangge H, Obermayer-Pietsch B and
Marz W: Visfatin/pre-B-cell colony-enhancing factor: A protein with
various suggested functions. J Endocrinol Invest. 30:138–144. 2007.
View Article : Google Scholar : PubMed/NCBI
|