Introduction
Lung cancer is the most common incident cancer and
the leading cause of cancer death (1,2). In 2015,
and approximately 733,300 new lung cancer cases are diagnosed and
610,200 patients died of lung cancer in China1. Non-small cell lung
cancer (NSCLC) accounts for approximately 87% of lung cancer cases
(3). Although the mainstays of
treatment for lung cancer (such as surgery, radiotherapy,
chemotherapy and targeted therapy) have made considerable progress,
NSCLC remains an aggressive lung cancer associated with a poor
prognosis. Long-term survival of lung cancer is less than 10%
(4–6).
Thus, finding new biomarkers for predicting progression and
prognosis of NSCLC is warranted and urgently needed to improve
clinical management of patients with NSCLC.
Carbonic anhydrase IV is one of twelve active human
isozymes and one of four expressed on the extracellular surfaces of
certain endothelial and epithelial cells, which catalyzes the
reversible hydration of CO2 to HCO3- and H+
(7,8).
Carbonic anhydrase IV (CA IV) was found in human normal tissues
like kidney and lung, with the remarkable diversity in tissue
distribution, subcellular location, and biological function
(9,10). Several CAs are reportedly involved in
NSCLC tumorigenesis, progression, regulation of cell proliferation,
target therapy and prognosis, excepted CA IV. For example, CA IX,
CAXII, and CA I are upregulated in NSCLC tumor tissues and appear
to act as oncogenes or prognostic factors (11–14). CA-RP
VIII and CA IX expression in NSCLC are related to NSCLC cell
invasion and proliferation (15,16). CA IX
serves as a target for anticancer therapy (17). A new study found that CA IV is
frequently silenced in colorectal cancer and the silencing of CA IV
is regulated by promoter hyper-methylation (18). Moreover, CA IV is a novel tumor
suppressor in CRC which was found to be associated with the
inhibition of the Wnt/β-catenin signalling pathway. The normal lung
expression of CA IV was found to be developmentally regulated in
the luminal side of the alveolar capillary endothelium cells. We
hypothesize that, like many other CAs, CA IV might be associated
with NSCLC. Our preliminary studies using high-throughput
microarrays and quantitative real-time RT-PCR (RT-qPCR) revealed
that the expression of CA IV was downregulated in NSCLC tissues
(1). However, the mechanism involved
in the influences of CA IV on biological functions in NSCLC is very
complicated and the exact clinical roles of CA IV are still
remained unclear, making further validation is necessary.
In the present study, the relative expression of CA
IV is estimated by RT-qPCR in 114 resected NSCLC tissues compared
to levels in their paired NT. The relationship between expression
levels of CA IV, clinical pathological features and overall
survivals in NSCLC patients is investigated. We overexpress CA IV
mRNA basing on NSCLC A549 and NCI-H1299 cell lines by
lentivirus-mediated technology.
Materials and methods
Patient samples
The 114 NSCLC tissues and corresponding adjacent
normal tissues (NT) were obtained from patients who underwent
surgery at the First Affiliated Hospital of Wenzhou Medical
University, China, from August 2013 to October 2015. This study was
approved by the Ethical Committee of the First Affiliated Hospital
of Wenzhou Medical University, and all patients signed informed
consent for the collection and use of their tissues for this study.
The clinical pathological features of patients (Table I) were assessed according to the World
Health Organization classification (19) and the TNM staging system. The NSCLC
and matched NT samples were snap-frozen in liquid nitrogen
immediately after resection. Patients were regularly followed up by
telephone or other means, the longest follow-up time was 31 months.
To investigate the association of CA IV expression with prognosis,
the survival data was divided into high and low groups, a value
superior or equal to 2 was defined as CA IV overexpression, based
on the 2−ΔΔCq method (20).
 | Table I.Relationship between clinical
pathological features and CAIV mRNA expression levels in 114 cases
of patients with NSCLC. |
Table I.
Relationship between clinical
pathological features and CAIV mRNA expression levels in 114 cases
of patients with NSCLC.
| Parameter | Cases | 2−ΔΔCq of
CA IV mRNA Median (range) | Kruskal-Wallis H or
Mann-Whitney test | P-value |
|---|
| Gender | 114 |
| 235.12 | 0.643 |
| Male | 56 | 0.445
(0.027–0.945) |
|
|
|
Female | 58 | 0.556
(0.025–0.887) |
|
|
| TMN stage | 114 |
| 147.231a | 0.575 |
| Ia | 24 | 0.532
(0.554–0.942) |
|
|
| Ib | 56 | 0.511
(0.499–0.856) |
|
|
| IIa | 14 | 0.359
(0.215–0.656) |
|
|
| IIb | 4 | 0.224
(0.092–0.535) |
|
|
| IIIa | 16 | 0.167
(0.077–0.339) |
|
|
| Histological
degree | 114 |
| 5.345a | 0.712 |
| Poor | 22 | 0.575
(0.132–0.945) |
|
|
|
Poor-moderate | 14 | 0.414
(0.121–0.945) |
|
|
|
Moderate | 34 | 0.323
(0.082–0.876) |
|
|
|
Moderate-high | 18 | 0.323
(0.097–0.819) |
|
|
| High | 26 | 0.281
(0.095–0.778) |
|
|
| Lymph node
metastasis | 114 |
| 1.712 | 0.015 |
| Yes | 28 | 0.054
(0.025–0.164) |
|
|
| No | 86 | 0.431
(0.287–0.943) |
|
|
| Smoking | 114 |
| 243.12 | 0.312 |
| Yes | 40 | 0.431
(0.026–0.931) |
|
|
| No | 74 | 0.421
(0.021–0.923) |
|
|
| histological
subtype |
|
| 5.320 | 0.724 |
| LAD | 68 | 0.412
(0.031–0.921) |
|
|
| SCC | 40 | 0.429
(0.026–0.927) |
|
|
| Big cell
cancer | 16 | 0.412
(0.021–0.908) |
|
|
Quantitative PCR
Approximately 100 mg tissues from liquid nitrogen
were cut off with a high-pressure sterile surgical scissors and
then placed in a 4 ml centrifuge tube treated with 0.1% DEPC water.
Take 1 ml of TRIzol reagent (Invitrogen, Carlsbad, CA, USA) into
the centrifuge tube, then extracted the total RNA follow the
reagent instructions. cDNA was reverse-transcribed from total RNA
samples using an RT Reagent kit (Takara Bio, Dalian, China), based
on the manufacturer's instructions. The measurement of CA IV and
β-actin mRNA was performed by RT-qPCR with SYBR Premix Ex Taq in
ABI 7000 instrument. CA IV forward primer:
5′-CTGGTGCTACGAGGTTCAA-3′ and reverse primer:
5′-GCCTTGGTGGTGACGAT-3′; β-actin sense primer:
5′-CCTGGCACCCAGCACAAT-3′, antisense primer:
5′-GCTGATCCACATCTGCTGGAA-3′. 2 µg of total RNA were transcribed
into cDNA and PCR reaction was performed in a final volume of 20
µl, containing 10 µl of SYBR Premix (2x), 2 µl of cDNA template, 1
µl of each primer (10 mM), and 6 µl of double-distilled water. The
quantitative real-time PCR reaction consisted of an initial
denaturation step of 10 min at 95°C, 40 cycles of 5 sec at 95°C, 30
sec at 60°C, and a final extension step of 5 min at 72°C. Each
sample was performed in triplicate and the median was used to
calculate the relative concentrations with β-actin as an internal
control gene (ΔCt=Ct median CA IV-Ct median β-actin), and
2−ΔΔCq in expression was calculated (20).
Cell culture
Six human NSCLC cell lines (SPCA-1, NCI-H1975,
LTEP-a2, NCI-H1299, NCI-H441 and A549) and normal human bronchial
epithelial BEAS-2B were all purchased from the Cell Bank of the
Chinese Academy of Sciences and maintained with complete medium
(containing 10% fetal bovine serum and 90% RPMI1640) at 37°C, 5%
CO2, complete medium was changed at least once every two
days.
Lentivirus-mediated overexpression
vector transfection
A549 and NCI-H1299 cells were transfected
overexpression vector targeting CA IV as well as a negative control
(Genechem, Co., Ltd., Shanghai, China). Transfection was
accomplished by seeding 2×105 cells into a six-well
plate, and after 24 h, the medium was aspirated and incubated with
transfection complex, according to the manufacturer's protocols.
The A549 and NCI-H1299 cells were infected with lentivirus for 72 h
and the overexpression efficiency was detected by RT-qPCR.
Cell proliferation assay
Cell viability was evaluated by Cell Counting Kit-8
(Corning Inc, Acton, MA, USA) abiding by the manufacturer's
protocols. Briefly, 3,000 cells of Stable transduced A549 and
NCI-H1299 were suspended and seeded into a 96-well plate with
supplemented medium (10% fetal bovine serum) and cell growth was
monitored every 24 h for 7 days. The next day, the CA IV
overexpression cells were incubated with CCK-8 for 1 h, and the
absorbance was measured at 450 nm using a multifunctional
microplate reader (Tecan, Männedorf, Switzerland) in the 1, 3, 5,
and 7 d. This experiment was done in quadruplicate cells.
Statistical methods
Statistical analysis was performed by SPSS software
v19 (SPSS Inc., Chicago, IL, USA). Statistical differences in CA IV
mRNA expression levels among different groups were calculated using
the non-parametric Kruskal-Wallis H test and Mann-Whitney U test
for the skewed distribution or the one-way ANOVA and Dunnett's test
for the normal distribution after the Kolmogorov-Smirnov test. The
Kaplan-Meier method was used to calculate the overall survival
rate, and the prognostic significance was evaluated by the log-rank
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
The expression level of CA IV mRNA in
NSCLC and adjacent tissues and its relationship with clinical
data
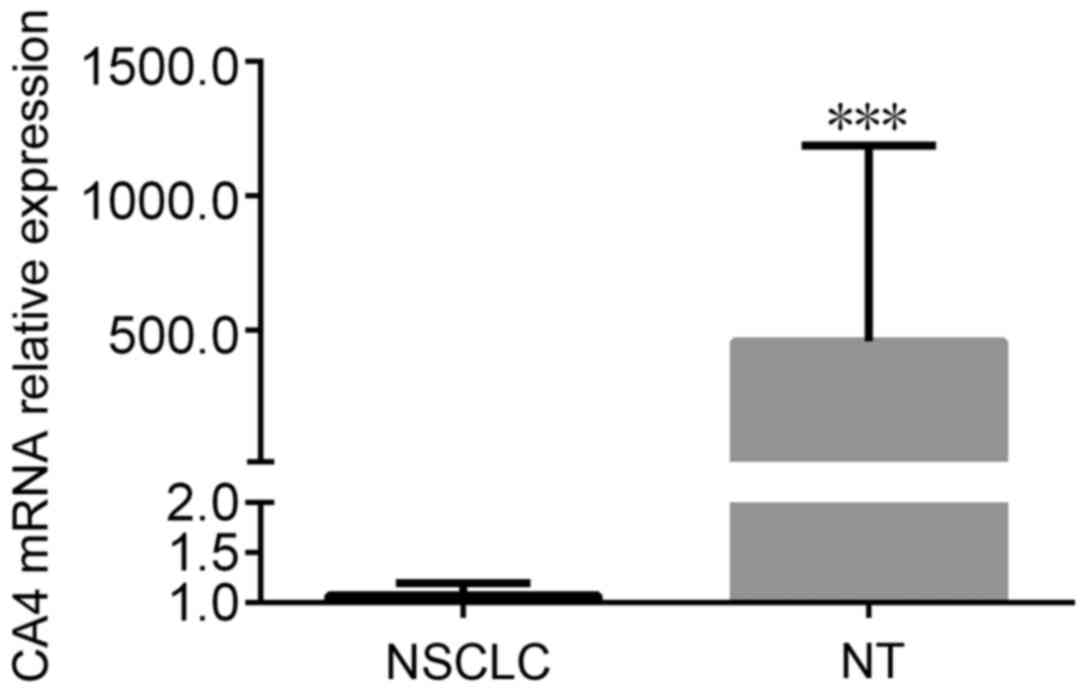
Overall, CA IV mRNA expression level of NSCLC is
0.543 (0.032–0.956) and markedly lower than its adjacent normal
tissues (Mann-Whitney U=0.000, P<0.001) (Fig. 1). According to Table I, we observed that the CA IV level of
NSCLC with lymph node metastasis group was significantly lower than
that of NSCLC without lymph node metastasis group (Mann-Whitney
U=1.712, P=0.015). CA IV expression levels among five TMN stages
were not different (Kruskal-Wallis H test=147.231, P=0.575). The CA
IV mRNA expression was also not relative to the histology
differentiation (Kruskal-Wallis H test=5.345, P=0.712), smoking
habits (Mann-Whitney U=243.12, P=0.312), gender (Mann-Whitney
U=235.12, P=0.643), and histological subtype (Kruskal-Wallis H
test=5.320, P=0.724).
The relation of CA IV mRNA expression
to the prognosis of NSCLC
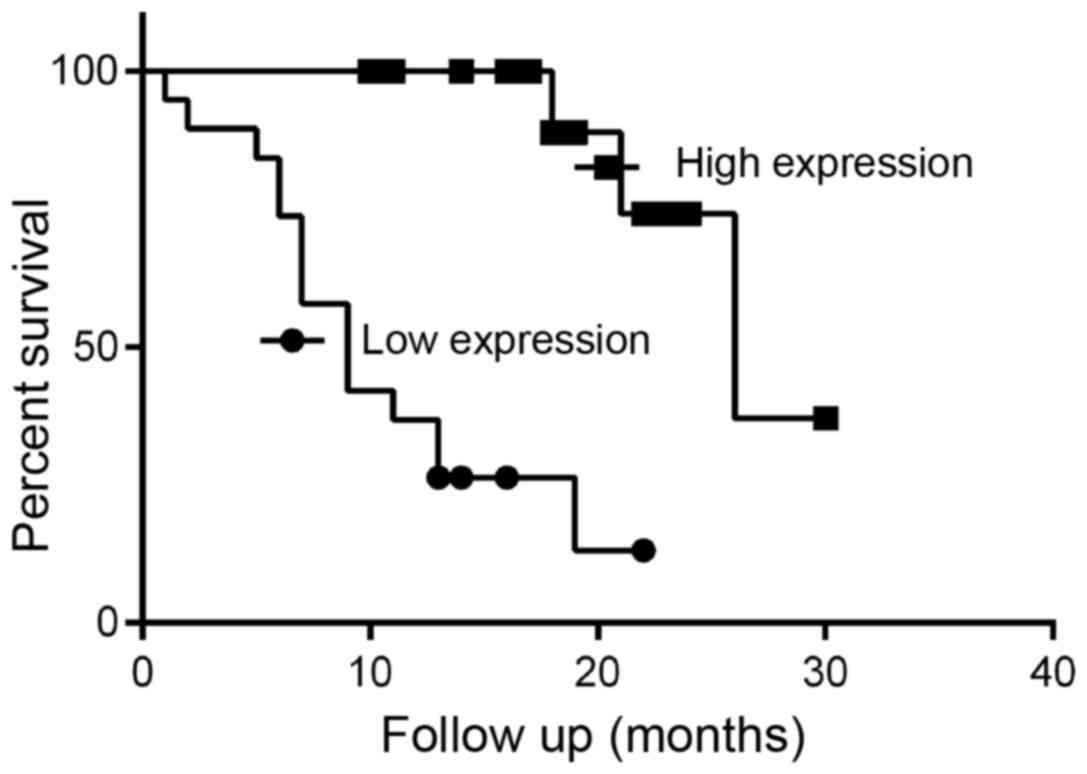
The overall survival time of low CA IV expression
group (median 9 months) was marginally lower than that of the high
expression (median 26 months) (χ2=18.36, P<0.001)
(Fig. 2). Reduced CA IV expression
was associated with shorter overall survival in patients with
NSCLC. This finding suggests that reduced CA IV mRNA
expression was a predictive factor of poor survival in NSCLC.
The expression level of CA IV mRNA
from six NSCLC cell lines
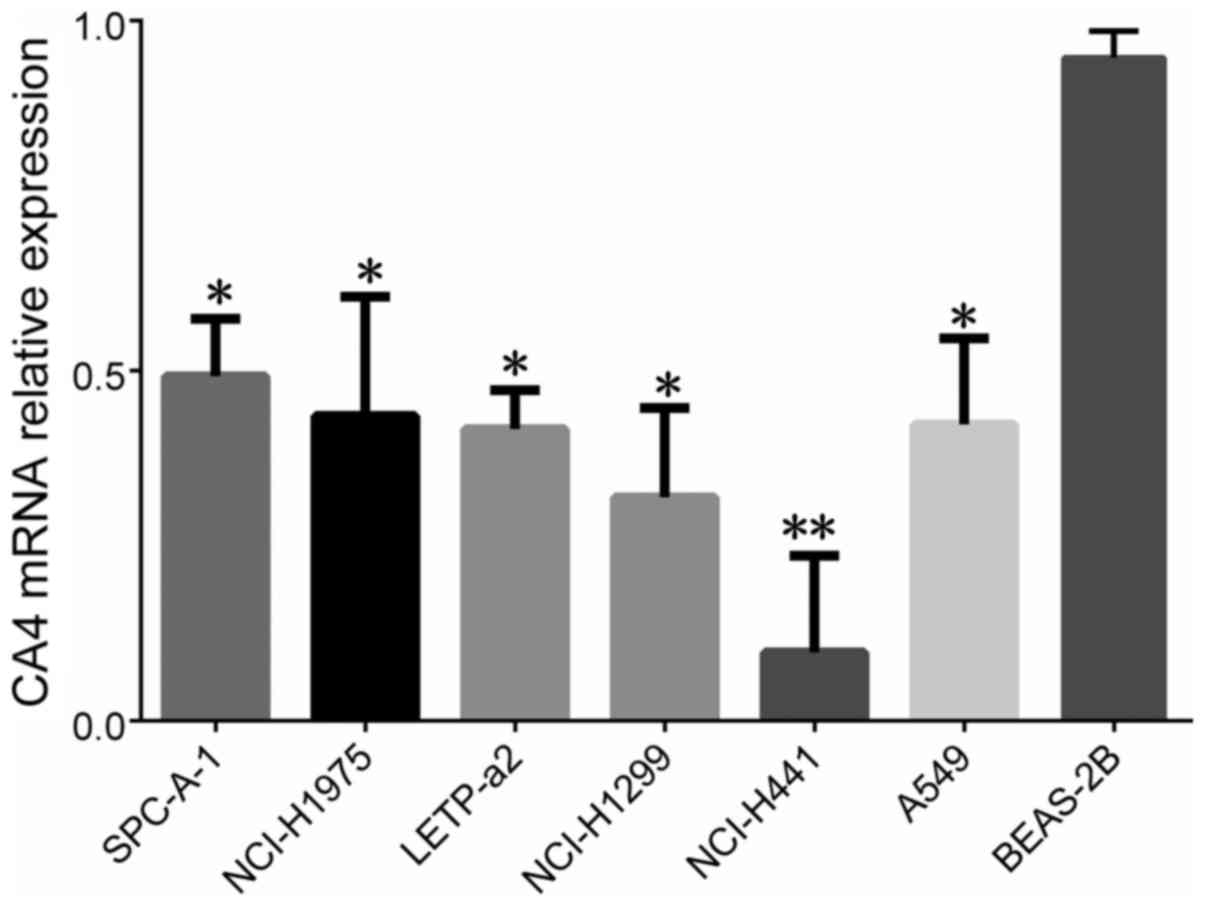
We detected the expression levels of CA IV from six
NSCLC cell lines (including SPCA-1, NCI-H1975, LTEP-a2, NCI-H1299,
NCI-H441 and A549) by RT-qPCR. It was shown that the expression
levels of CA IV from SPCA-1 (P<0.05), NCI-H1975 (P<0.05),
LTEP-a2 (P<0.05), NCI-H1299 (P<0.05), A549 (P<0.05) and
NCI-H441 (P<0.01) were lower, compared to normal human bronchial
epithelial BEAS-2B cell line (Fig.
3).
CA IV can regulate ability of cell
proliferation
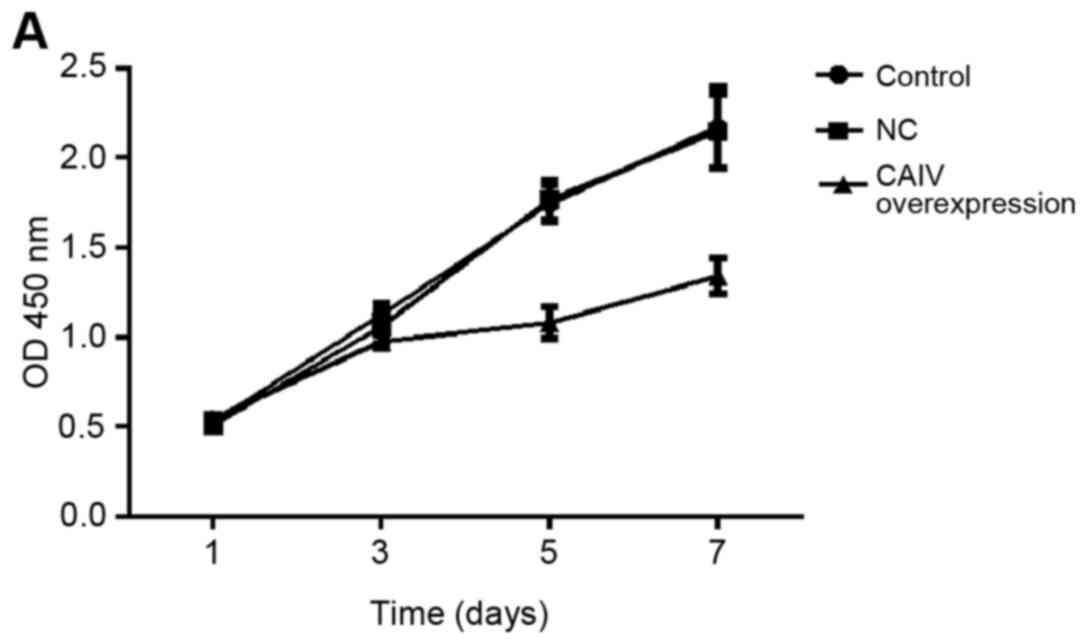
Growth curves of untreatedA549 and NCI-H1299 cells
(control), cell stably transduced with non-targeting negative
control (NC) and a vector for overexpression of CA IV (CA IV
overexpression) were gradually increased with the change of time
(Fig. 4A and B). Compared with the
1d, the OD450 nm of the 3, 5, and 7 d in control group were
significantly increased (P<0.05, P<0.01 and P<0.001), the
same results were also found in the NC group and CA IV
overexpression group, respectively. Compared with corresponding
days of control group or NC group, the OD450 nm of 1, 3 d in CA IV
overexpression group were no statistically significant difference
(P>0.05), while that of the 5 d (P<0.05) and the 7 d
(P<0.01) were significantly reduced, it indicates that cell
proliferation ability of A549 and NCI-H1299 lines was significantly
suppressed by CA IV overexpression (Fig.
4).
Discussion
In the present study, we first demonstrated that CA
IV mRNA expression was significantly decreased in NSCLC tissues
compared with their adjacent normal tissues via RT-qPCR. Compared
to normal human bronchial epithelial BEAS-2B cell line, it was
shown that the expression levels of CA IV were also reduced in all
six NSCLC cell lines. These results suggest an aberrant
downregulation of CA IV in NSCLC and hint CA IV gene may be a
potent tumor suppressor molecule. Notably, other members of the
carbonicanhydrase family were hypoxia-inducible molecules in
various types of solid cancers with a more aggressive phenotype and
upregulated by hypoxia-inducible factor 1 (HIF-1) (21–23). In
order to further study the mechanism of CA IV, we established CA IV
overexpression of A549 and NCI-H1299 cell lines by
lentivirus-mediated technology. After CA IV was overexpressed, cell
proliferation ability of A549 and NCI-H1299 remarkably decreased.
Based on these observations, we conclude that low expression of CA
IV promotes cell proliferation and CA IV acts as a tumor suppressor
gene. To the best of our knowledge, this is the first identified
that CA IV expression associated with tumor suppressor potential in
NSCLC. Zhang shown that CA4 was silenced in all nine colon cancer
cell lines and 92.6% of colon cancer. The re-expression of CA4
inhibited cell proliferation, induced apoptosis and cell cycle
arrest in the G1 phase. CA4 inhibited the activity of the Wnt
signalling pathway and mediated the degradation of β-catenin. CA4
interacted with Wilms' tumour 1-associating protein (WTAP) and
induced WTAP protein degradation through polyubiquitination.
Moreover, CA4 promoted the transcriptional activity of Wilms'
tumour 1 (WT1), an antagonist of the Wnt pathway, which resulted in
the induction of transducin β-like protein 1 (TBL1) and the
degradation of β-catenin (18), we
will carry out an in-depth study about the mechanism of CA IV in
the NSCLC.
The diagnostic and prognostic value of carbonic
anhydrases were proved in many solid tumors. It is generally
acknowledged that overexpression of CAs can predict poor survival
of patients, containing those with NSCLC (14,24).
However, clinical properties and prognostic significance of CA IV
in NSCLC remain unclear. In our study, the CA IV expression in
NSCLC with lymph node metastasis group was significantly lower than
that of NSCLC without lymph node metastasis group, while it was not
relative to TMN stages, histology differentiation, gender, and
smoking. Survival analysis showed that survival time of low
expression CA IV group was significantly shorter than high
expression CA IV group in NSCLC patients. Low CA IV expression is
associated with cell proliferation and lymph node metastasis, which
means that low CA IV expression accelerates tumor growth and
aggressiveness, resulting in a poor prognosis. Those findings
suggest that reduced CA IV expression is a predictive factor of
poor survival in NSCLC.
To summarize, our studies ascertain for the first
time that the expression of CA IV is downregulated in NSCLC and
associated with promoting cell proliferation and lymph node
metastasis. The low expression of CA IV may serve as an indicator
of poor prognosis in NSCLC.
Acknowledgements
The present study was financially supported by the
National Natural Science Foundation of China (8140736, 81672088),
the Zhejiang Provincial Natural Science Foundation (LQ16H160020),
and the Wenzhou Municipal Science and Technology Bureau, China
(Y20150097).
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stinchcombe TE and Socinski MA: Current
treatments for advanced stage non-small cell lung cancer. Proc Am
Thorac Soc. 6:233–241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mahalingam D, Mita A, Mita MM, Nawrocki ST
and Giles FJ: Targeted therapy for advanced non-small cell lung
cancers: Historical perspective, current practices, and future
development. Curr Probl Cancer. 33:73–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogawa E, Takenaka K, Katakura H, Adachi M,
Otake Y, Toda Y, Kotani H, Manabe T, Wada H and Tanaka F:
Perimembrane Aurora-A expression is a significant prognostic factor
in correlation with proliferative activity in non-small-cell lung
cancer (NSCLC). Ann Surg Oncol. 15:547–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Waheed A and Sly WS: Membrane associated
carbonic anhydrase IV (CA IV): A personal and historical
perspective. Subcell Biochem. 75:157–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tashian RE: The carbonic anhydrases:
Widening perspectives on their evolution, expression and function.
BioEssays: News and reviews in molecular, cellular and
developmental biology. 10:186–192. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu XL and Sly WS: Carbonic anhydrase IV
from human lung. Purification, characterization and comparison with
membrane carbonic anhydrase from human kidney. J Biol Chem.
265:8795–8801. 1990.PubMed/NCBI
|
|
10
|
Carter ND, Fryer A, Grant AG, Hume R,
Strange RG and Wistrand PJ: Membrane specific carbonic anhydrase
(CAIV) expression in human tissues. Biochim Biophys Acta.
1026:113–116. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang DB, Lu XK, Zhang X, Li ZG and Li CX:
Carbonic anhydrase 1 is a promising biomarker for early detection
of non-small cell lung cancer. Tumour Biol. 37:553–559. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ilie M, Hofman V, Zangari J, Chiche J,
Mouroux J, Mazure NM, Pouysségur J, Brest P and Hofman P: Response
of CAIX and CAXII to in vitro re-oxygenation and clinical
significance of the combined expression in NSCLC patients. Lung
cancer. 82:16–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malentacchi F, Simi L, Nannelli C,
Andreani M, Janni A, Pastorekova S and Orlando C: Alternative
splicing variants of carbonic anhydrase IX in human non-small cell
lung cancer. Lung cancer. 64:271–276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giatromanolaki A, Koukourakis MI, Sivridis
E, Pastorek J, Wykoff CC, Gatter KC and Harris AL: Expression of
hypoxia-inducible carbonic anhydrase-9 relates to angiogenic
pathways and independently to poor outcome in non-small cell lung
cancer. Cancer Res. 61:7992–7998. 2001.PubMed/NCBI
|
|
15
|
Kim SJ, Rabbani ZN, Dewhirst MW,
Vujaskovic Z, Vollmer RT, Schreiber EG, Oosterwijk E and Kelley MJ:
Expression of HIF-1alpha, CA IX, VEGF, and MMP-9 in surgically
resected non-small cell lung cancer. Lung cancer. 49:325–335. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu SH, Takeuchi T, Fujita J, Ishida T,
Akisawa Y, Nishimori I, Kohsaki T, Onishi S, Sonobe H and Ohtsuki
Y: Effect of carbonic anhydrase-related protein VIII expression on
lung adenocarcinoma cell growth. Lung cancer. 44:273–280. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong BC, Zhang H, Qin L, Chen H, Fang C,
Lu A and Yang Z: Carbonic anhydrase IX-directed immunoliposomes for
targeted drug delivery to human lung cancer cells in vitro. Drug
Des Devel Ther. 8:993–1001. 2014.PubMed/NCBI
|
|
18
|
Zhang J, Tsoi H, Li X, Wang H, Gao J, Wang
K, Go MY, Ng SC, Chan FK, Sung JJ and Yu J: Carbonic anhydrase IV
inhibits colon cancer development by inhibiting the Wnt signalling
pathway through targeting the WTAP-WT1-TBL1 axis. Gut.
65:1482–1493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brambilla E, Travis WD, Colby TV, Corrin B
and Shimosato Y: The new World Health Organization classification
of lung tumours. Eur Respir J. 18:1059–1068. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren S, Peng Z, Mao JH, Yu Y, Yin C, Gao X,
Cui Z, Zhang J, Yi K, Xu W, et al: RNA-seq analysis of prostate
cancer in the Chinese population identifies recurrent gene fusions,
cancer-associated long noncoding RNAs and aberrant alternative
splicings. Cell Res. 22:806–821. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Malentacchi F, Vinci S, Melina AD, Kuncova
J, Villari D, Nesi G, Selli C, Orlando C, Pazzagli M and Pinzani P:
Urinary carbonic anhydrase IX splicing messenger RNA variants in
urogenital cancers. Urol Oncol. 34:292.e9–292.e16. 2016. View Article : Google Scholar
|
|
22
|
Ambrosio MR, Di Serio C, Danza G, Rocca
BJ, Ginori A, Prudovsky I, Marchionni N, Del Vecchio MT and
Tarantini F: Carbonic anhydrase IX is a marker of hypoxia and
correlates with higher Gleason scores and ISUP grading in prostate
cancer. Diagn Pathol. 11:452016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jamali S, Klier M, Ames S, Barros LF,
McKenna R, Deitmer JW and Becker HM: Hypoxia-induced carbonic
anhydrase IX facilitates lactate flux in human breast cancer cells
by non-catalytic function. Sci Rep. 5:136052015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Kuijk SJ, Yaromina A, Houben R,
Niemans R, Lambin P and Dubois LJ: Prognostic significance of
carbonic anhydrase IX expression in cancer patients: A
meta-analysis. Front Oncol. 6:692016. View Article : Google Scholar : PubMed/NCBI
|