Introduction
In recent years, the clinical applications of
positron emission tomography (PET) have undergone explosive growth
(1–6).
PET using F18-fluorodeoxyglucose (FDG) is a noninvasive whole-body
imaging technique used to evaluate various types of malignancies,
including breast cancer, and for tumor staging, tumor restaging,
the detection of recurrence and monitoring treatment responses
(1–6).
However, FDG uptake is not tumor-specific, and PET imaging also
provides quantitative information about numerous diseases,
including inflammation and infection (7). Several studies have reported cases of
neck and supraclavicular FDG uptake that were not associated with
any radiologically or clinically detectable pathology (8–10).
Atypical neck and supraclavicular FDG uptake is not an uncommon
finding and may be demonstrated to partly represent brown adipose
tissue (BAT) (10–12). BAT activation helps maintain normal
body temperatures in newborns (13–15).
Although the amount of BAT declines with age, islets of brown
adipocytes remain in the white adipose tissue of adult humans
(13–15). BAT has recently attracted attention,
since it consumes stored energy and may thereby be involved in
obesity and age-associated metabolic disease (15–17). Due
to the similarity of its biological property of hypermetabolism to
cancer cells, BAT may also show intense FDG uptake on FDG-PET
imaging (15–17). It has been reported that a high level
of BAT in adult humans is associated with cancer-induced cachexia
and may reflect an abnormality in a mechanism responsible for
substantial energy expenditure (15,18). BAT
has recently emerged as a topic of focus in the context of cancer
and tumor development (19–21). However, data on the association of BAT
and cancer are limited. In the present study, the association
between BAT activity detected by FDG-PET and clinicopathological
features in cases with primary breast cancer was examined.
Patients and methods
Patient information and data
analysis
The present study retrospectively investigated 156
female patients with primary breast cancer who underwent FDG-PET
preoperatively at the Department of General Surgical Science, Gunma
University (Gunma, Japan) between January 2010 and September 2014.
All the patients had undergone radical breast surgery. Patients
with previously diagnosed breast cancer or incomplete clinical
information were excluded. Additionally, male patients were
excluded. The mean age of the patients was 58.6±12.3 years, with an
age distribution between 32 and 86 years. Patients underwent their
FDG-PET/CT as part of the routine standard of care (5,6), and no
changes to the standard of care were made. The maximum standardized
uptake value (SUVmax) of primary tumors was calculated
in a routine clinical fashion. Written informed consent was
obtained from all patients for the use of their records and imaging
in future studies, and this was approved by the Clinical Ethics
Committee of Gumma University (Gumma, Japan).
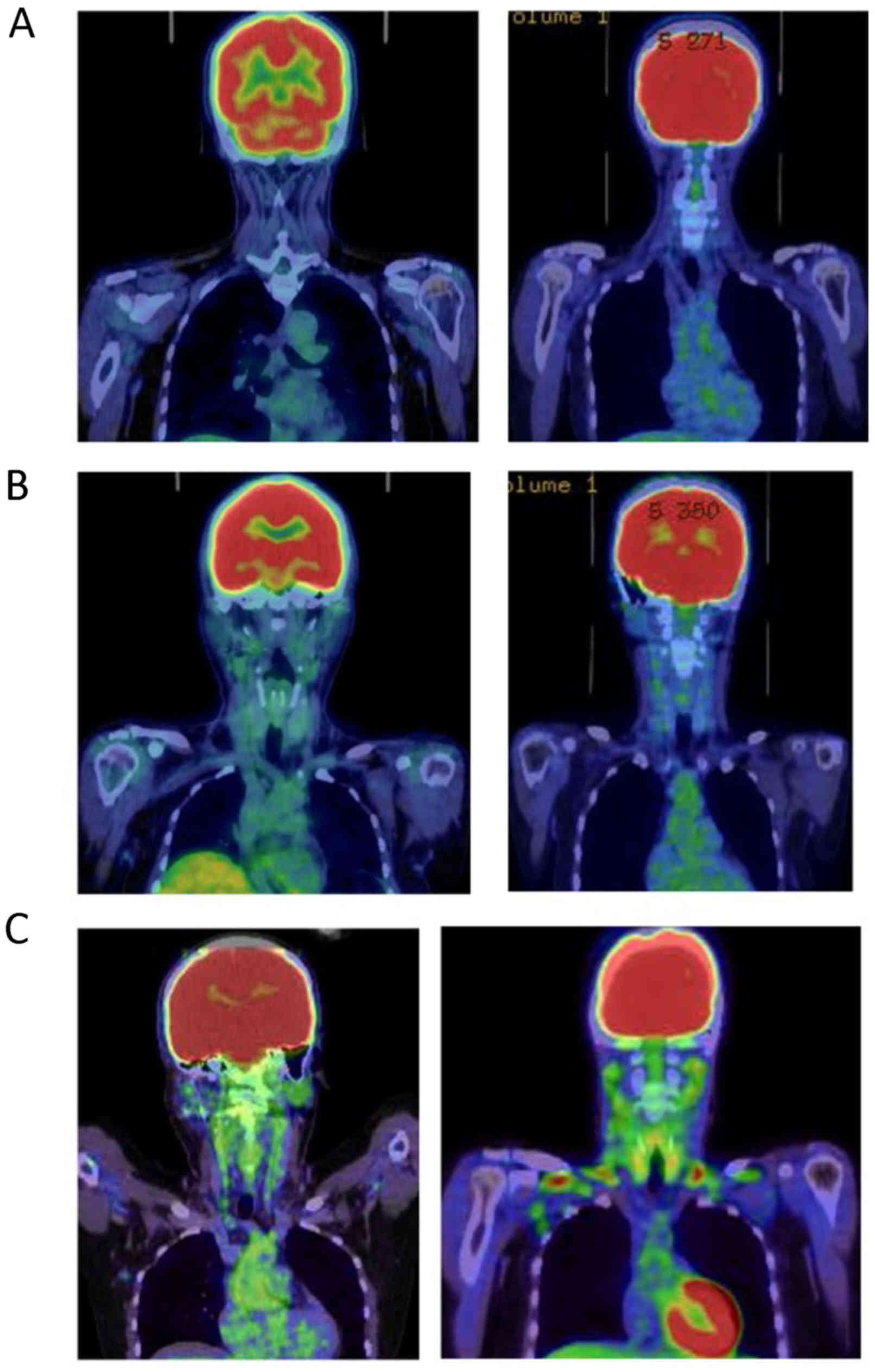
The distribution and intensity of BAT was reviewed
in all patients in the present study. Images were first assessed
visually for any atypical, non-pathological increased uptake in the
neck and/or supraclavicular region. Atypical uptake was defined as
lesions that exhibited an increased focal or linear FDG uptake
bilaterally in the neck and/or supraclavicular region. In patients
with atypical FDG uptake in the neck and/or supraclavicular region,
the intensity scores were graded as follows: 1, weak; 2, moderate;
and 3, intense (Fig. 1).
The details extracted from the database were age,
histological type, primary tumor size, nuclear grade, lymph node
metastasis, lymphatic or vascular invasion, estrogen (ER) or
progesterone (PgR) status, human epidermal growth factor receptor 2
(HER2) expression and SUVmax of the primary tumor, serum
tumor markers [carcinoembryonic antigen, (CEA)] and body mass index
(BMI). The ER and PgR statuses were assessed by ALLRED scores
(22,23). ALLRED scores are calculated by adding
two numbers, one reflecting the proportion of positive tumor cells
(0, none; 1, <1%; 2, 1–10%; 3, 11–33%; 4, 34–66%; and 5,
67–100%) and the other reflecting the intensity of immunoreactivity
(1, weak; 2, moderate; 3, strong). The maximum score is 8 and an
ALLRED score of ≥3 was defined as ER- and PgR-positive (22,23). The
overall median follow-up period was 21.8 months, and none of the
patients died of surgical complications.
Statistical analysis
The breast cancer cases were divided into 3 groups
on the basis of the intensity grade of the atypical FDG uptake in
the neck and/or supraclavicular region. All data were analyzed by
one-way analysis of variance with Fisher's adjustment. The breast
cancer cases were then divided into two groups on the basis of the
presence or absence of recurrence. A univariate statistical
analysis was conducted using Fisher's exact test or the
χ2 test with or without Yates' correction. To compare
the two groups, Student's t-test was performed. To test the
independence of the risk factors, the variables were entered into a
multivariate logistic regression model with a likelihood of
P<0.05. The relapse-free survival (RFS) was calculated using the
Kaplan-Meier method. The log-rank test was used to evaluate the
differences between the recurrence-free intervals. P<0.05 was
considered to indicate a statistically significant difference.
Results
Atypical neck and supraclavicular FDG
uptake is associated with HER2 and PgR expression in patients with
breast cancer
The 156 consecutive patients with breast cancer who
underwent FDG-PET preoperatively were analyzed. The patients with
breast cancer were divided into 3 groups based on the intensity of
atypical neck and/or supraclavicular FDG uptake. Among the 156
patients, 70 (44.9%) patients were grade 1, 65 (41.7%) were grade 2
and 21 (13.5%) were grade 3. Table I
summarizes not only the patient characteristics but also the
results of the analysis conducted to determine the association
between the atypical FDG uptake and clinicopathological variables.
The analysis revealed that HER2 expression (P<0.001) and PgR
expression (P=0.006) were statistically significant factors. The
tumor size and SUVmax of the primary tumor were
relatively increased in patients with high intense atypical FDG
uptake, compared with that in patients with weak or moderate
atypical FDG uptake; although, no statistically significant
difference was identified. Average age and BMI were not
statistically significant factors in the current study.
 | Table I.Patient characteristics and
clinicopathological features associated with atypical
supraclavicular FDG-uptake. |
Table I.
Patient characteristics and
clinicopathological features associated with atypical
supraclavicular FDG-uptake.
|
| FDG-uptake in
neck/supraclavicular region, n |
|
|---|
|
|
|
|
|---|
| Characteristic | Grade 1 (n=70) | Grade 2 (n=65) | Grade 3 (n=21) | P-value |
|---|
| Age,
yearsa | 58.2±13.0 | 58.8±11.4 | 59.1±11.0 | 0.940 |
| Postmenopausal | 49 | 19 | 15 | 0.519 |
| Tumor size,
mma | 22.5± 18.7 | 20.3±15.8 | 18.0±12.3 | 0.572 |
| Histological
type |
|
|
| 0.428 |
| Invasive
ductal carcinoma | 55 | 54 | 19 |
|
| Ductal
carcinoma in situ | 3 | 5 | 1 |
|
|
Others | 12 | 6 | 1 |
|
| SUVmax
of primary tumora | 4.0±5.4 | 3.5±3.4 | 2.3±1.7 | 0.219 |
| Lymph node
metastasis | 18 | 16 | 4 | 0.821 |
| ER-positive | 62 | 54 | 14 | 0.061 |
| PgR-positive | 60 | 50 | 11 | 0.006 |
| HER2-positive | 1 | 12 | 12 | <0.001 |
| Nuclear grade
3 | 22 | 23 | 7 | 0.888 |
| Lymphatic
invasion | 26 | 22 | 9 | 0.750 |
| Vascular
invasion | 11 | 12 | 1 | 0.314 |
| CEA, <3.0 | 15 | 11 | 5 | 0.717 |
| BMI,
kg/m2 a | 22.5±4.6 | 23.1±3.0 | 24.4±3.6 | 0.146 |
Atypical FDG uptake is associated with
short-term disease recurrence in breast cancer
The patients with breast cancer were divided into 2
groups based on the presence of recurrence. Among the 156 patients,
6 (3.8%) exhibited recurrent disease. Table II summarizes not only the patient
characteristics but also the results of the univariate analysis
conducted to determine the association between the
clinicopathological variables and recurrent disease. The univariate
analysis revealed that vascular invasion (P=0.047) and a low grade
of atypical FDG uptake (P=0.022) were statistically significant
factors. The multivariate analysis revealed that only a low grade
of atypical FDG uptake (P=0.038) was an independent risk factor of
short-term recurrence. Vascular invasion (P=0.079) lost its
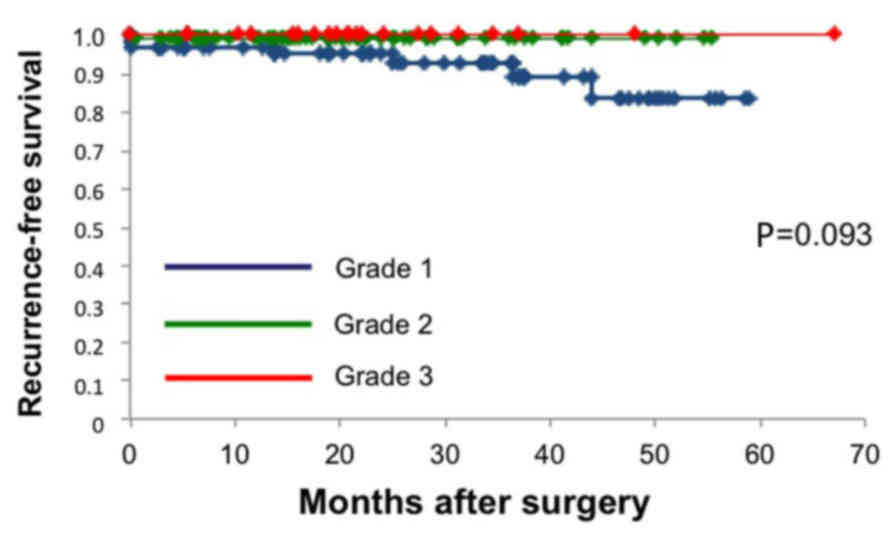
significance in the multivariate analysis. The RFS shown by the
Kaplan-Meier curves was relatively shorter for the patients with
grade 1 atypical FDG uptake, compared with those with grade 2 and
3, but no statistically significant differences were observed
(Fig. 2).
 | Table II.Patient characteristics and
clinicopathological features associated with recurrent disease. |
Table II.
Patient characteristics and
clinicopathological features associated with recurrent disease.
|
| Recurrence, n |
|
|---|
|
|
|
|
|---|
| Characteristic | Negative
(n=150) | Positive (n=6) | P-value |
|---|
| Atypical
FDG-uptake |
|
|
|
| Grade
1 | 64 | 6 | 0.022 |
| Grade
2 | 65 | 0 |
|
| Grade
3 | 21 | 0 |
|
| Age, years | 58.6±12.5 | 59.3±7.5 | 0.902 |
| Postmenopausal | 110 | 5 | 0.501 |
| Tumor size,
mma | 20.7±16.9 | 27.4±12.8 | 0.164 |
| Histological
type |
|
|
|
|
Invasive ductal carcinoma | 124 | 4 | 0.428 |
| Ductal
carcinoma in situ | 9 | 0 |
|
|
Others | 17 | 2 |
|
| SUVmax
of primary tumora | 3.5±4.4 | 4.3±2.3 | 0.331 |
| Lymph node
metastasis | 36 | 2 | 0.845 |
| ER-positive | 125 | 5 | 0.738 |
| PgR-positive | 116 | 5 | 0.505 |
| HER2-positive | 25 | 0 | 0.344 |
| Nuclear grade
3 | 49 | 3 | 0.371 |
| lymphatic
invasion | 53 | 4 | 0.130 |
| vascular
invasion | 21 | 3 | 0.047 |
| CEA (3.0<) | 29 | 2 | 0.907 |
| Adjuvant hormone
therapy | 116 | 5 | 0.595 |
| Adjuvant
chemotherapy | 46 | 3 | 0.321 |
| Adjuvant
HER2-targeted therapy | 21 | 0 | 0.414 |
| BMI
(kg/m2) | 24.4±3.6 | 25.1±5.2 | 0.188 |
Discussion
FDG-PET has been widely used for staging and
identifying recurrence in various types of cancer (1–6). Neck and
supraclavicular FDG uptake is not a frequent phenomenon in the
general PET population (8–12). Previous studies have reported that FDG
uptake in neck and supraclavicular lesions represents activated BAT
(10–12). Other studies reported such increased
FDG uptake in BAT at a frequency of 2.3–4% of patients (8–10,24,25).
However, a high prevalence of activated BAT has been reported in
patients with lymphoma (17%) or breast cancer (16.7–80%) among
limited numbers of patients (9,15,21,26). In
the present study, atypical FDG uptake, which may represent active
BAT, was detected in 86 of 156 patients (55.1%), and intense FDG
uptake was identified in 21 cases (13.5%). In patients with
cancer-induced cachexia, the prevalence of BAT was increased
compared with in age-matched controls (15,18). These
results indicated an evident association between BAT and cancer
status.
In the present study, the presence of atypical FDG
uptake in neck and/or supraclavicular lesions was an independent
risk factor for short-term disease recurrence, and all the patients
with recurrent disease had atypical FDG uptake, which may reflect
activated BAT. These results indicated that the presence of BAT may
be considered an indicator of a lower level of biological
aggressiveness and may be a prognostic factor in breast cancer. The
mechanisms of the potential association between BAT and breast
cancer have not been fully evaluated. There are two alternate
hypotheses: BAT may be actively involved in the progression of
breast cancer; and BAT may be passively involved in the progression
of breast cancer, and the activation of BAT may be secondary to the
breast cancer (21,27,28).
Notably, despite the presence of HER2 expression and a lower level
of PgR expression, which are negative prognostic factors, the
patient group in the present study with BAT included no patients
with recurrent disease. It may appear that a paradoxical phenomenon
was observed in the present study. However, breast cancer
progression is a complex process, and it is regulated by multiple
factors in addition to HER2 or PgR expression (29). These results indicated that disease
recurrence may also occur via other mechanisms associated with BAT.
Furthermore, HER2-positive breast cancer in the setting of
HER2-targeted therapy is no longer associated with poor prognosis
(30). In the current study,
HER2-targeted therapy was performed in 82.2% patients with HER2
positive breast cancer with grade 3 atypical FDG uptake. Additional
study is necessary to substantiate the effect of BAT on breast
cancer progression.
In the past several years, interest in subsets of
obesity-associated tumors, including breast cancer, has increased
in the field of cancer research (31–33).
Increased fatty acid synthesis, which contributes to energy
homeostasis and tumor growth, is a common feature of human tumors
(32). The nuclear receptor
peroxisome proliferation activated receptor γ (PPARγ), a
transcriptional master regulator of lipid metabolism, inhibits the
growth of several common cancers (33). An acetylation-defective PPARγ mutant
induces a brown phenotype in white adipocytes (33). The survival of breast cancer cells,
particularly those with HER2 overexpression, is highly dependent on
the lipid metabolism induced by PPARγ (34,35).
Another study reported that cancer cell proliferation was inhibited
by PPARγ mediated by lipid metabolism (31). Furthermore, in patients with
cancer-induced cachexia, PPARγ serves important roles in the
inflammation and inhibition of adipocyte differentiation (36). These findings, in combination with the
present results, indicate the possibility that PPARγ may perform
important roles in breast cancer progression and HER2 expression.
Additional study is required to explore the mechanisms of breast
cancer progression associated with PPARγ and BAT.
In the current study, PgR expression was associated
with the presence of BAT. Previous studies have proposed that BAT
metabolism is sex-dependent (9,37,38). Testosterone was shown to inhibit the
thermogenic response of BAT, and FDG uptake in BAT was revealed to
be high in non-menopausal female patients (9,37,38), which indicated that female sex
hormones promote FDG uptake in BAT.
The present study had certain limitations, the
primary one being that it was a retrospective analysis and the
number of cases was relatively small. Furthermore, there are other
known predictors or plausible factors associated with BAT
activation (18,19) that were not evaluated in the present
study, including environmental temperature, psychological factors
and catecholamine levels. FDG uptake in BAT has recently been
reported to occur more commonly in weight-loss patients (18,19).
However, other studies also reported no significant difference
between the BMI of patients with BAT (18,19), which
is consistent with our results. Additional investigation with a
large number of patients is required to evaluate the clinical
significance of BAT activation as detected by FDG-PET. However, the
clinical implications of the data obtained in the present study are
important, and to the best of our knowledge, this is the first
study describing the clinical features and risk of recurrence of
breast cancer associated with BAT.
In conclusion, the presence of atypical FDG uptake
in neck and/or supraclavicular lesion, which may represent active
BAT, was associated with HER2 and PgR expressions, and was an
independent prognostic factor. These results may represent an
evident association between BAT and cancer status. Additional study
is required to explore the mechanisms of breast cancer progression,
particularly the possibility of an association between PPARγ and
BAT.
Acknowledgements
The authors would like to thank Y. Saitoh, T. Yano,
Y. Matsui, A. Ishida and A. Ishiubo for their secretarial
assistance. The present study was supported by Grants-in-Aid from
the Japanese Ministry of Education, Culture, Sports, Science and
Technology (grant no. 17K10534).
References
|
1
|
Kresnik E, Gallowitsch HJ, Mikosch P,
Stettner H, Igerc I, Gomez I, Kumnig G and Lind P:
Fluorine-18-fluorodeoxyglucose positron emission tomography in the
preoperative assessment of thyroid nodules in an endemic goiter
area. Surgery. 133:294–299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Y: Clinical significance of thyroid
uptake on F18-fluorodeoxyglucose positron emission tomography. Ann
Nucl Med. 23:17–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fletcher JW, Djulbegovic B, Soares H,
Siegel BA, Lowe VJ, Lyman GH, Coleman RE, Wahl R, Paschold JC,
Avril N, et al: Recommendations on the use of F18-FDG PET in
oncology. J Nucl Med. 49:480–508. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Connor OJ, McDermott S, Slattery J,
Sahani D and Blake MA: The use of PET-CT in the assessment of
patients with colorectal carcinoma. Int J Surg Oncol.
2011:8465122011.PubMed/NCBI
|
|
5
|
Fujii T, Yajima R, Yamaguchi S, Tsutsumi
S, Asao T and Kuwano H: Is it possible to predict malignancy in
cases with focal thyroid incidentaloma identified by
18F-Fluorodeoxyglucose positron emission tomography? Am Surg.
78:141–143. 2012.PubMed/NCBI
|
|
6
|
Fujii T, Suto T, Kigure W, Morita H, Katoh
T, Yajima R, Tsutsumi S, Asao T and Kuwano H: Clinicopathological
features of second primary colorectal cancer incidentally
identified by 18F-FDG-PET. Hepatogastroenterology. 62:599–601.
2015.PubMed/NCBI
|
|
7
|
Cook GJ, Fogelman I and Maisey MN: Normal
physiological and benign pathological variants of
18-fluoro-2-deoxyglucose positron-emission tomography scanning:
Potential for error in interpretation. Semin Nucl Med. 26:308–314.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coohade C, Osman M, Pannu HK and Wahl RL:
Uptake in supraclavicular area fat (‘USA-fat’): Description on
18F-FDG PET/CT. J Nucl Med. 44:170–176. 2003.PubMed/NCBI
|
|
9
|
Rousseau C, Bourbouloux E, Campion L,
Fleury N, Bridji Bm Chatal JF, Resche I and Campone M: Brown fat in
breast cancer patients: Analysis of serial (18)F-FDG PET/CT scans.
Eur J Nucl Med Mol Imaging. 33:785–791. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cohade C, Mourtzikos KA and Wahl RL:
“USA-fat”: Prevalence is related to ambient outdoor
temperature-evaluation with 18F-FDG PET/CT. J Nucl Med.
44:1267–1270. 2003.PubMed/NCBI
|
|
11
|
Pace L, Nicolai E, D'Amico D, Ibello F,
Della Morte AM, Salvatore B, Pizzuti LM, Salvatore M and Soricalli
A: Determinants of physiologic 18F-FDG uptake in brown adipose
tissue in sequential PET/CT examinations. Mol Imaging Biol.
13:1029–1035. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cronin CG, Prakash P, Daniels GH, Boland
GW, Kalra MK, Halpern EF, Palmer EL and Blake MA: Brown fat at
PET/CT: Correlation with patient characteristics. Radiology.
263:836–842. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cannon B and Nedergaard J: Brown fat
tissue: Function and physiological significance. Physiol Rev.
84:277–359. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lean ME: Brown adipose tissue in humans.
Proc Nutr Soc. 48:pp. 243–256. 1989; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang YC, Chen TB, Hsu CC, Li SH, Wang PW,
Lee BF, Kuo CY and Chiu NT: The relationship between brown adopse
tissue activity and neoplastic status: An (18)F-FDG PET/CT study in
the tropics. Lipids Health Dis. 10:2382011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ricquier D: Biology of brown adipose
tissue: View from the chair. Int J Obes (Lond). 34 Suppl 1:S3–S6.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lecoultre V and Ravussin E: Brown adipose
tissue and aging. Curr Opin Clin Nutr Metab Care. 14:1–6. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shellock FG, Riedinger MS and Fishbein MC:
Brown adopose tissue in cancer patients: Possible cause of
cancer-induced cachexia. J Cancer Res Clin Oncol. 111:82–85. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berstein LM: Cancer and heterogeneity of
obesity: Apotential contribution of brown fat. Future Oncol.
8:1537–1548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sinha G: Homing in on the fat and caner
connection. J Natl Cancer Inst. 104:966–967. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao Q, Hersl J, La H, Smith M, Jenkins J,
Goloubeva O, Dilsizian V, Tkaczuk K, Chen W and Jones L: A pilot
study of FDG PET/CT detects a link between brown adipose tissue and
breast cancer. BMC Cancer. 14:1262014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
23
|
Shousha S: Oestrogen receptor status of
breast carcinoma: Allred/H score conversion table. Histopathology.
53:346–347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Truong MT, Erasmus JJ, Munden RF, Marom
EM, Sabloff BS, Gladish GW, Podoloff DA and Macapinlac HA: Focal
FDG uptake in mediastinal brown fat mimicking malignancy: A
potential pitfall resolved on PET/CT. AJR. 183:1127–1132. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hany TF, Gharehpapagh E, Kamel EM, Buck A,
Himms-Hagen J and Von Schulthess GK: Brown adipose tissue: A factor
to consider in symmetrical tracer uptake in the neck and upper
chest region. Eur J Nucl Med Mol Imaging. 29:1393–1398. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dobert N, Menzel C, Hamscho N, Wordehoff
N, Kranert WT and Grünwald F: Atypical thoracic and supraclavicular
FDG-uptake in paitents with Hodgkin's and non-Hodgkin's lymphoma. Q
J Nucl Med Mol Imaging. 48:33–38. 2004.PubMed/NCBI
|
|
27
|
Cinti S, Cancello R, Zingaretti MC, Ceresi
E, De Matteis R, Giordano A, Himms-Hagen J and Ricquier D:
CL316,243 and cold stress induce heterogeneous expression of UCP1
mRNA and protein in rodent brown adiposytes. J Histochem Cytochem.
50:21–31. 2001. View Article : Google Scholar
|
|
28
|
Lim S, Honek J, Xue Y, Seki T, Cao Z,
Andersson P, Yang X, Hosaka K and Cao Y: Cold-induced activation of
brown adipose tissue and adipose angiogenesis in mice. Nat Protoc.
7:606–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kurozumi S, Matsumoto H, Hayashi Y, Tozuka
K, Inoue K, Horiguchi J, Takeyoshi I, Oyama T and Kurosumi M: Power
of PgR expression as a prognostic factor for
ER-positive/HER2-negative breast cancer patients at intermediate
risk classified by the Ki67 labeling index. BMC Cancer. 17:3542017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Figueroa-Magalhães MC, Jelovac D, Connolly
RM and Wolff AC: Treatment of HER2-positive breast cancer. Breast.
23:128–136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park J, Morley TS, Kim M, Clegg DJ and
Scherer PE: Obesity and cancer-mechanisms underlying tumour
progression and recurrence. Nat Rev Endocrinol. 10:455–465. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Howe LR, Subbaramaiah K, Hudis CA and
Dannenberg AJ: Molecular pathways: Adipose inflammation as a
mediator of obesity-associated cancer. Clin Cancer Res.
19:6074–6083. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qiang L, Wang L, Kon N, Zhao W, Lee S,
Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR and Accili D: Brown
remodeling of white adipose tissue by SirT1-dependent deacetylation
of Pparγ. Cell. 150:620–632. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kourtidis A, Srinivasaiah R, Carkner RD,
Brosnan MJ and Conklin DS: Peroxisome proliferator-activated
receptor-gamma protects ERBB2-positive breast cancer cells from
palmitate toxity. Breast Cancer Res. 11:R162009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tian L, Wang C, Hagen FK, Gormley M, Addya
S, Soccio R, Casimiro MC, Zhou J, Powell MJ, Xu P, et al:
Acetylation-defective mutants of Pparγ are associated with
decreased lipid synthesis in breast cancer cells. Oncotarget.
5:7303–7315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Batista ML Jr, Peres SB, McDonald ME,
Alcantara PS, Olivan M, Otoch JP, Farmer SR and Seelaender M:
Adipose tissue inflammation and cancer cachexia: Possible role of
nuclear transcription factors. Cytokine. 57:9–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rodriguez-Cuenca S, Monjo M, Roca P and
Palou A: Opposite actions of testosterone and progesterone on UCP1
mRNA expression in cultured brown adipocytes. Cell Mol Life Sci.
59:1714–1723. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bartness TJ and Wade GN: Effects of
interscapular brown adipose tissue denervation on body weight and
energy metabolism in ovariectomized and estradiol-treated rats.
Behav Neurosci. 98:674–685. 1984. View Article : Google Scholar : PubMed/NCBI
|