Introduction
Glioma is the most common type of malignant tumor in
the central nervous system, and the annual incidence reaches
~7.2/100,000 (1). Owing to invasive
growth and intraoperative protection of the functional areas of the
brain, it is difficult to remove the tumor completely by surgery,
and therefore postoperative chemo-radiotherapy becomes an important
strategy for glioma (2). However,
glioma cells are not sensitive to clinical radiotherapy and
chemotherapy due to multidrug resistance, resulting in recurrence
even if surgery and postoperative chemo-radiotherapy are combined,
with a mean survival time of <14 months (3). Increasing evidence has indicated that
high expression of ATP-binding cassette transporter is one of the
important factors involved in tolerance to chemo-radiotherapy in
glioma (4–6). Furthermore, drug-resistance of glioma
cells may be abolished by decreasing the expression and function of
the ATP-binding cassette transporter family members in the cell
membranes (7). Therefore, this may be
a potential clinical strategy for the treatment of glioma (8). In the present study, the traditional
Chinese medicine Salvia miltiorrhiza ligustrazine (SML) was
revealed to inhibit the expression of multidrug resistance 1
(MDR1), multidrug resistance-associated protein 1 (MRP1) and lung
resistance protein (LRP) and promote the antitumor effect of
doxorubicin (DOX) in the DOX-resistant glioma SHG44/DOX cell line.
Data from the present study confirmed the novel function of this
drug in tumor treatment. Therefore, SML may be a potential adjuvant
agent for glioma chemo-radiotherapy.
Materials and methods
Reagents
Fetal bovine serum (FBS), RPMI-1640 medium and
penicillin/streptomycin were purchased from HyClone (GE Healthcare
Life Sciences, Logan, UT, USA). LRP, phosphorylated-glycoprotein
(p-gp), MRP1, GAPDH, Ki-67 nuclear antigen and proliferating cell
nuclear antigen (PCNA) primary antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Horseradish
peroxidase-conjugated secondary antibodies were purchased from
OriGene Technologies, Inc. (Beijing, China). DOX and SML were
obtained from the Department of Neurosurgery at The Second
Affiliated Hospital of Chongqing Medical University (Chongqing,
China).
Cell culture
The human glioma SHG44 cell line was purchased from
Shanghai Life Academy of Sciences Cell Library (Shanghai, China).
The SHG44 cells were maintained in RPMI-1640 supplemented with 100
U/ml penicillin, 100 mg/ml streptomycin and 10% FBS at 37°C. To
establish the DOX-resistant SHG44 (SHG44/DOX) cell line,
concentrations of DOX treatment were increased gradually between
0.01 and 1 µg/ml. Finally, the SHG44/DOX cells were able to grow
continually in medium with 0.1 µg/ml DOX.
Semi-quantitative reverse
transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from cells using RNAiso Plus
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
concentrations of these RNA samples were measured using a
spectrophotometer, and the RNA samples were reverse-transcribed
into cDNA using the Primescript RT reagent kit according to the
manufacturer's protocol (Takara Biotechnology Co., Ltd., Dalian,
China). The primer sequences and product sizes were as follows: LRP
(221 bp) forward, 5′-GTCTTCGGGCCTGAGCTGGTGTCG-3′ and reverse,
5′-CTTGGCCGTCTCTTGGGGGTCCTT-3′; MRP1 (326 bp) forward,
5′-CCGTGTACTCCAACGCTGC-3′ and reverse, 5′-CTGGACCGCTGACGCCGTGAC-3′;
MDR1 (310 bp) forward, 5′-CATGCTCAGACAGGATGTGAGT-3′ and reverse,
5′-AGTAGCGATCTTCCCAGAACCT-3′ and GAPDH (598 bp) forward,
5′-CCACCCATGGCAAATTCCATGGCA-3′ and reverse,
5′-TCTAGACGGCAGGTCAGGTCCAC-3′. The amplification conditions were as
follows: 95°C for 5 min, 95°C for 30 sec, 54.5°C for 30 sec and
72°C for 40 sec. The PCR program was administered for 30 cycles,
followed by a 5 min extension at 72°C. The experiment was repeated
five times. Following gel electrophoresis in 2% agarose gel,
ethidium bromide was used to observe the bands using Quantity One
4.6 computer software at 590 nm (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The relative levels of target genes were
normalized to GAPDH expression.
Western blot analysis
The cells were lysed using radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Beijing,
China) containing 1% phenylmethylsulfonyl fluoride. The BCA method
was used to determine protein concentrations. An equal amount (25
µg) of each sample was separated by 6–10% SDS-PAGE and transferred
to polyvinylidene fluoride (PVDF) membranes. Following blocking
with 5% goat serum at 37°C for 30 min (Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing China) and incubating with
primary antibodies against p-gp (dilution, 1:300; cat. no.
sc71557), MRP1 (dilution, 1:300; cat. no. sc7773), LRP (dilution,
1:300; cat. no. sc135975) and GAPDH (dilution, 1:1,000; cat. no.
sc47724) overnight at 4°C, the PVDF membranes were washed three
times with PBS and Tween-20 (PBST) buffer and incubated with
horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse
secondary antibody (dilution, 1:5,000; cat. nos. TA130003 and
TA130023) for 1 h at 37°C. The membranes were then washed three
times with PBST buffer and the amount of protein in each band was
observed using enhanced chemiluminescence reagents (Beyotime
Institute of Biotechnology) and quantified using Quantity One 4.6
computer software. GAPDH was used as a loading control. The
experiment was repeated five times.
Cell viability
The cells were seeded onto 96-well plates at a
density of 2,000 cells/well. Following treatment with 1, 0.1 µg/ml
DOX for 1, 2, 3 and 4 days; 2, 25, 50, 75 and 100 µg/ml SML for 1,
2, 3 and 4 days; 3, DOX alone or SML plus DOX for 1, 2, 3 and 4
days, the cells were washed with PBS. RPMI-1640 medium (100 µl) and
10 µl cell counting kit-8 solution (Beyotime Institute of
Biotechnology) were added to each well for another 2 h at 37°C. The
optical density value was read at 450 nm using an ultraviolet
spectrophotometer.
Cell apoptosis
The 2×105 cells were plated on a 6-well
plate. The SHG44/DOX cells were incubated at 37°C with 0.1 µg/ml
DOX or 0.1 µg/ml DOX plus 50 µg/ml SML for 96 h, and the early
apoptotic rate was analyzed by flow cytometry using the annexin
V-phycoerythrin/7-aminoactinomycin D apoptosis reagent kit (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) according to a previous
study (9).
DOX uptake
The SHG44/DOX cells were exposed to 0.1 µg/ml DOX or
0.1 µg/ml DOX plus 50 µg/ml SML for 2 h at 37°C. Subsequent to cell
lysis and supernatant collection, the intracellular concentrations
of DOX were tested using an ultraviolet spectrophotometer
(absorbance, 490 nm). In addition, the protein concentrations were
analyzed to standardize the uptake of DOX.
Xenograft tumor model
The male nude mice (n=9, 4 weeks old, weight 15 g,
maintained under specific pathogen free conditions at 20–26°C,
atmosphere 20–50 Pa, 12 h light/dark cycle, fed optionally) used in
the present study were provided by the experimental Animal Center
of Chongqing Medical University (Chongqing, China). All animal
studies were approved by the Ethics Committee of Chongqing Medical
University. The SHG44/DOX cells were resuspended in RPMI-1640
medium at a density of 2×106 cells per 80 µl and
injected subcutaneously. The 5 mg/kg DOX or 5 mg/kg DOX and 5 mg/kg
SML was injected into tumor tissues every 7 days through the tail
vein. Tumor volumes were recorded according to the formula
described previously (10) at 7, 14,
21 and 28 days post-inoculation.
Immunohistochemistry (IHC)
The mice were sacrificed at 28 days, and the
xenograft tumors were dissected at 4 µm and embedded in paraffin.
IHC was performed according to previous methods (9) in order to evaluate the expression of
Ki-67 (dilution, 1:100; cat. no. sc7844) and PCNA (dilution, 1:100;
cat. no. sc25280) in the tumor tissues. Briefly, sections (4 mm)
were deparaffinized in xylene and rehydrated in ethanol with a
descending concentration (90, 80, 70, 50% for 10 min,
respectively). Subsequently, these sections were treated with 3%
hydrogen peroxide, the antigen was retrieved in citrate buffer, and
nonspecific binding was blocked using 5% goat serum for 40 min at
37°C. Following washing with PBS, the sections were incubated
overnight at 4°C with the Ki-67 (1:100) and PCNA (1:100), followed
by incubation with the HRP goat anti-rabbit secondary antibody
(1:2,000) at 37°C for 35 min. Following staining with
3,3′-diaminobenzidine and counterstaining with hematoxylin (room
temperature for 5 min), the sections were observed using a light
microscope (DM6000 B; Leica Microsystems GmbH, Wetzlar, Germany).
The proliferation index (Ki-67 and PCNA index) was defined as the
percentage of positive cells from five randomly selected fields
under magnification, ×400. Negative controls (samples incubated
with PBS instead of primary antibody) were processed along with the
samples. There was no apparent immunoreactivity observed in
negative controls.
Statistical analysis
One-way analysis of variance (SML toxicity, drug
combination experiments in vitro and in vivo) and
Tukey's post-hoc test or a paired t-test (western blot, RT-PCR, DOX
toxicity) were performed using SPSS 20.0 (IBM SPSS, Armonk, NJ,
USA). All data are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Establishment and identification of
SHG44/DOX cells
The glioma SHG44 cells were exposed to DOX with
incremental concentrations, finally resulting in stable growth in
0.1 µg/ml DOX. RT-PCR analysis revealed that the mRNA levels of
drug resistance genes (MDR1, MRP1 and LRP) in SHG44/DOX cells were
significantly increased compared with SHG44 cells (Fig. 1A). Western blot analysis demonstrated
that p-gp, MRP1 and LRP protein were also significantly elevated in
SHG44/DOX cells compared with SHG44 cells (Fig. 1B). The IC50 values of DOX
in SHG44 and SHG44/DOX cells were 1.64±0.27 and 17.58±0.31 mg/ml,
respectively. Following treatment with 0.1 µg/ml DOX for 1, 2, 3
and 4 days, the viability of SHG44/DOX cells was higher compared
with SHG44 cells (Fig. 1C). These
results indicated that the SHG44/DOX cells successfully exhibited
the DOX-resistant phenotype.
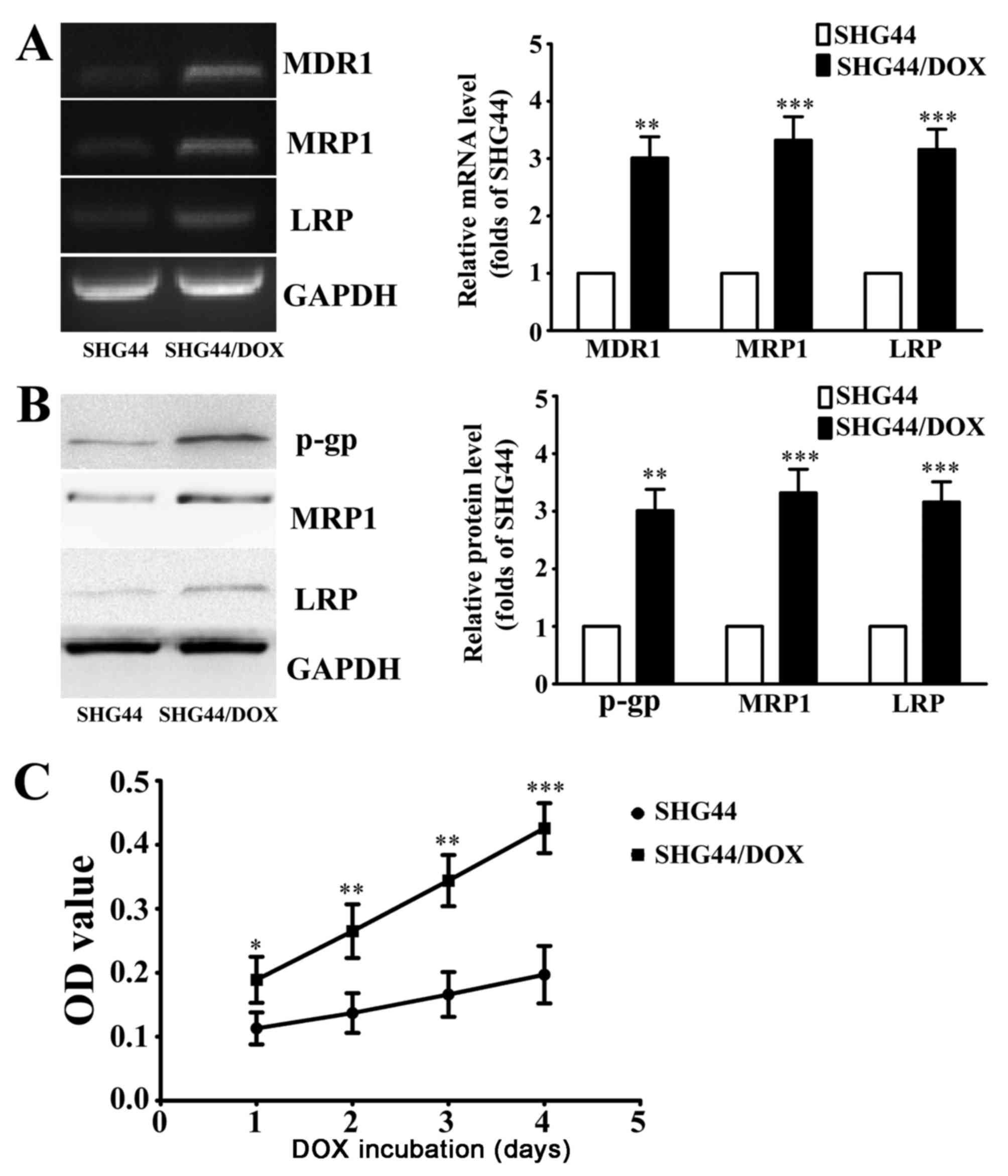 | Figure 1.Characteristics of the DOX-resistant
glioma SHG44/DOX cell line. (A) Reverse transcription-polymerase
chain reaction analysis of mRNA levels of MDR1, MRP1 and LRP in
SHG44 and SHG44/DOX cells (n=5). (B) Western blot analysis was used
to determine the expression of three resistance proteins in SHG44
and SHG44/DOX cells (n=5). (C) Following incubation with 0.1 µg/ml
DOX for 1, 2, 3, 4 days, cell viability was detected using cell
counting kit-8 solution (n=9). *P<0.05, **P<0.01,
***P<0.001 vs. SHG44 cells. DOX, doxorubicin; MDR1, multidrug
resistance 1; MRP1, multidrug resistance-associated protein 1; LRP,
lung resistance protein; p-gp, phosphorylated-glycoprotein; OD,
optical density; SHG44/DOX, DOX-resistant SHG44 cell line. |
Cytotoxicity of SML in SHG44/DOX
cells
In order to avoid the toxicity of SML, the non-toxic
concentrations of this traditional Chinese medicine were selected.
Following treatment with a concentration of 50 µg/ml or below for
1, 2, 3 and 4 days, SML did not inhibit growth of SHG44/DOX cells.
However, the traditional Chinese medicine was able to reduce
proliferation of SHG44/DOX cells at a concentration of 75 µg/ml and
above (Fig. 2). Therefore, 50 µg/ml
SML was used as a non-toxic concentration for subsequent study.
SML inhibits DOX-resistance of
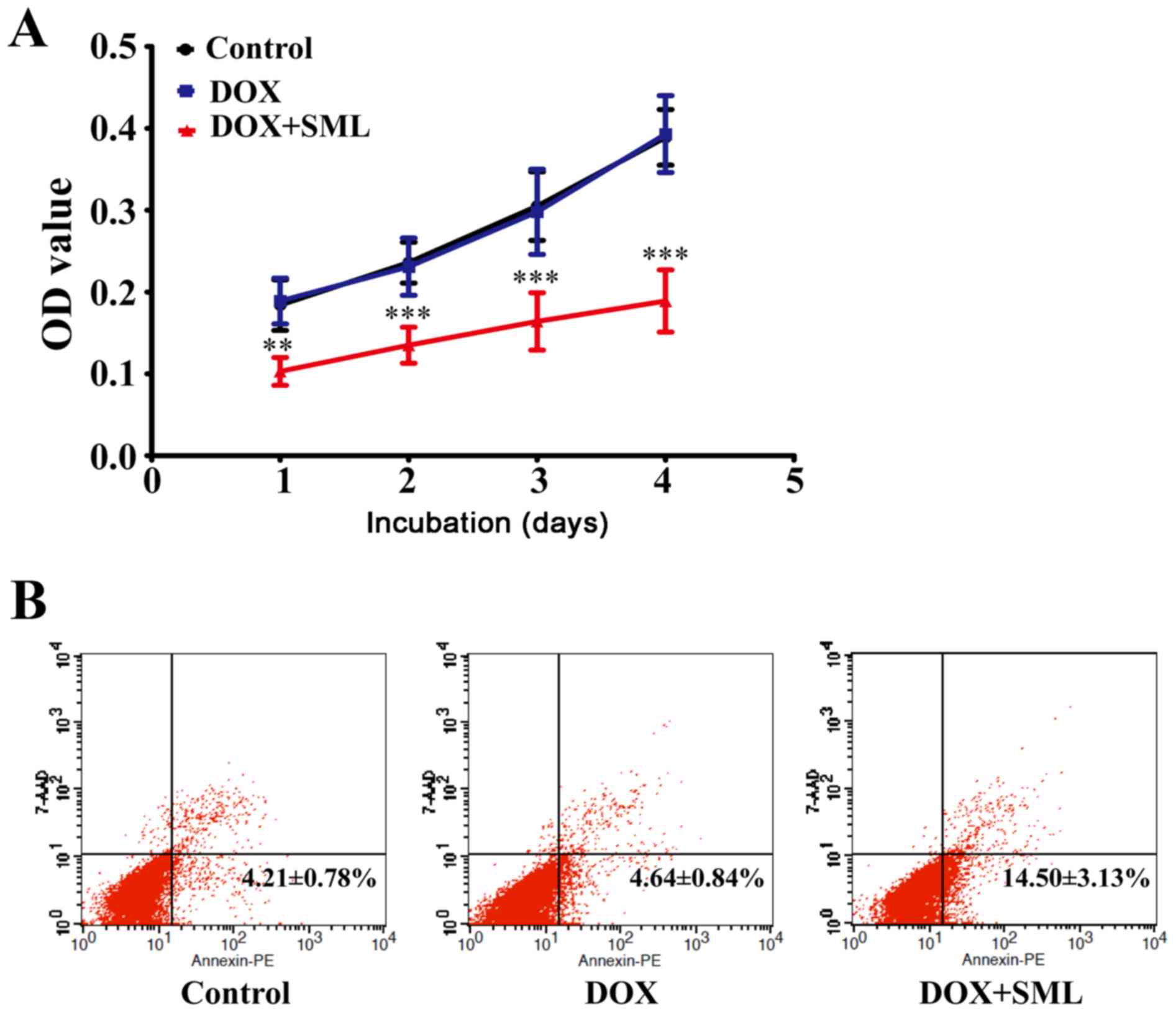
SHG44/DOX cells
Although little cytotoxicity was observed in the 0.1
µg/ml DOX alone treatment group, 50 µg/ml SML plus 0.1 µg/ml DOX
was able to suppress growth of SHG44/DOX cells after 1, 2, 3 and 4
days (Fig. 3A). Flow cytometry
demonstrated that the rates of early apoptosis were lower in the
control and 0.1 µg/ml DOX alone groups compared with the 50 µg/ml
SML plus 0.1 µg/ml DOX group after 96 h (P<0.05; Fig. 3B).
DOX-resistance of SHG44/DOX is
attenuated by SML in vivo
Subcutaneous injections of SHG44/DOX cells were
administered in nude mice to develop xenograft tumors. As shown in
Fig. 4A, 5 mg/kg DOX was able to
slightly inhibit SHG44/DOX growth in vivo compared with the
control group after 21 days. However, the tumor suppression was
stronger in the 5 mg/kg DOX plus 5 mg/kg SML group compared with
the 5 mg/kg DOX alone group. Furthermore, the Ki-67 and PCNA
proliferation indexes were significantly lower in the 5 mg/kg DOX
plus 5 mg/kg SML group compared with the 5 mg/kg DOX alone group
(Fig. 4B). The present data affirmed
that SML reduced DOX-resistance in SHG44/DOX cells and improved the
antitumor function of DOX.
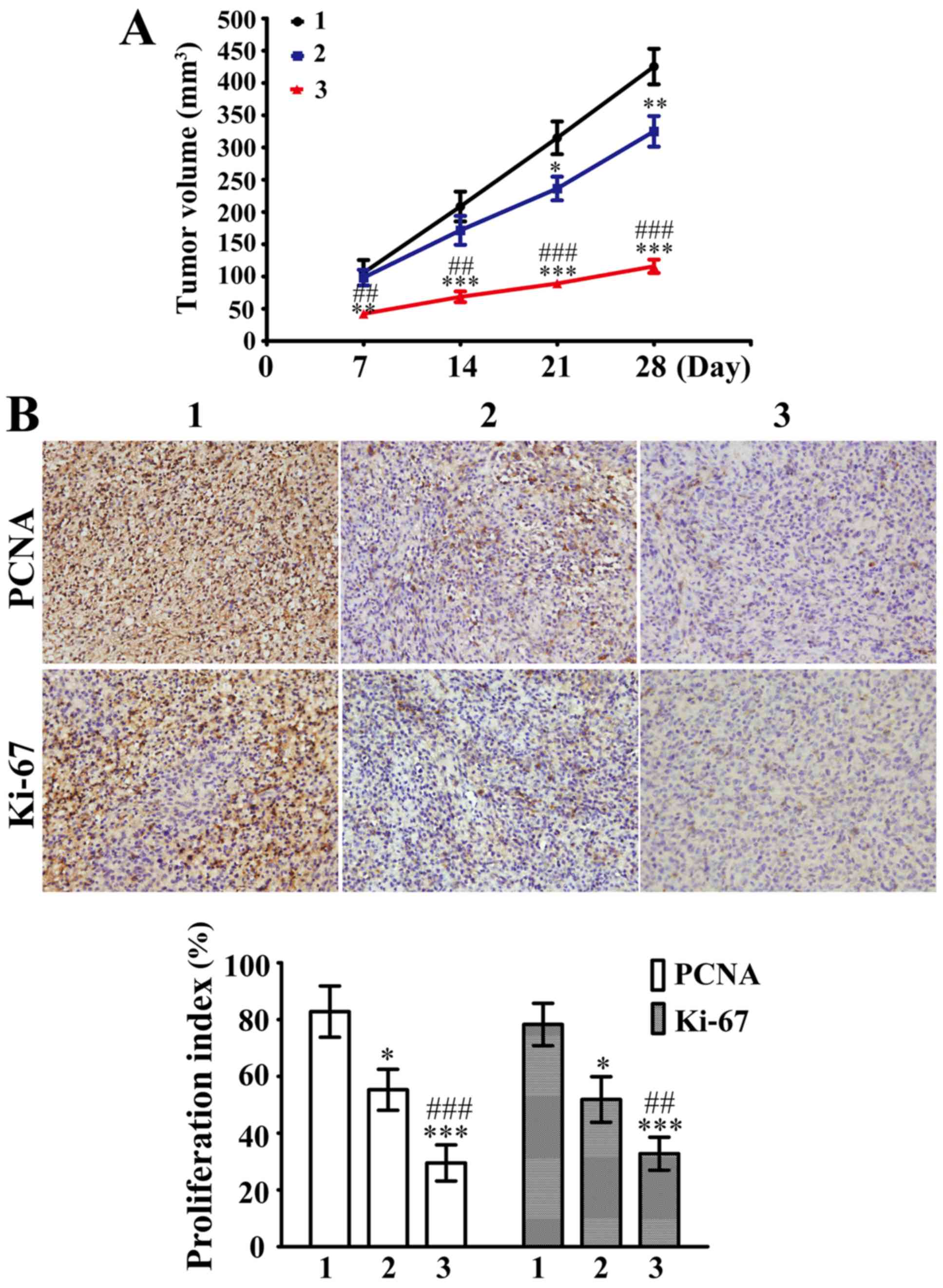 | Figure 4.SML inhibited DOX-resistance in
DOX-resistant SHG44 cells in vivo. (A) The xenograft tumors
were divided into 1, control; 2, DOX alone; and 3, DOX plus SML
groups. The tumor volumes were calculated at day 7, 14, 21 and 28
(n=3). (B) Immunohistochemical analysis was performed to analyze
the percentage of cells positive for PCNA and Ki-67 in these tumor
samples 28 days post-inoculation. Magnification, ×400. *P<0.05,
**P<0.01, ***P<0.001 vs. control group;
##P<0.01, ###P<0.001 vs. DOX alone
group. SML, Salvia miltiorrhiza ligustrazine; DOX,
doxorubicin; PCNA, proliferating cell nuclear antigen. |
SML downregulates multidrug resistance
genes and facilitates DOX uptake in SHG44/DOX cells
Following treatment with 0.1 µg/ml DOX for 2 h, the
intracellular concentration of DOX was determined using
spectrophotometry. It was demonstrated that concentration of DOX
was higher in SHG44 cells compared with SHG44/DOX cells (Fig. 5A). Notably, treatment with 50 µg/ml
SML significantly increased the intracellular concentrations of DOX
in SHG44/DOX cells (Fig. 5A). RT-qPCR
and western blot analysis demonstrated that the mRNA and protein
levels of MDR1, MRP1 and LRP were significantly downregulated by 50
µg/ml SML in SHG44/DOX cells (Fig. 5B and
C).
 | Figure 5.Effects of multidrug resistance genes
and DOX uptake in SHG44/DOX cells. (A) Following exposure to DOX
for 2 h, the spectrophotometer was used to investigate
intracellular concentrations of DOX in SHG44, SHG44/DOX and
SHG44/DOX cells that were treated simultaneously with SML
***P<0.001 vs. DOX treated-SHG44 cells; ###P<0.001
vs. SHG44/DOX incubated with DOX alone; n=5. 1, SHG44 cells treated
with DOX alone; 2, SHG44/DOX cells treated with DOX alone; 3,
SHG44/DOX cells treated with DOX + SML. (B) Reverse
transcription-polymerase chain reaction analysis to observe the
effects of SML treatment on the levels of MDR1, MRP1 and LRP mRNA
in SHG44/DOX cells. **P<0.01, ***P<0.001 vs. the DOX alone
group; n=5. (C) Western blot analysis to analyze the effects of SML
treatment on the protein expression of p-gp, MRP1 and LRP in
SHG44/DOX cells. ***P<0.001 vs. DOX alone group; (n=5). SML,
Salvia miltiorrhiza ligustrazine; DOX, doxorubicin; MDR1,
multidrug resistance 1; MRP1, multidrug resistance-associated
protein 1; LRP, lung resistance protein; p-gp, phosphorylated
glycoprotein; SHG44/DOX, DOX-resistant SHG44. |
Discussion
SML improves circulatory disturbance by
vasodilation, and is also able to reduce blood viscosity by
promoting fibrinolysis (11,12). Additionally, ligustrazine is able to
increase the surface charge of erythrocytes and platelet to inhibit
blood viscosity (13). Therefore, SML
has played an important role in the treatment of cardiovascular and
cerebrovascular diseases in China, having been widely used for the
treatment of a number of conditions, including cerebral infarction,
coronary disease, diabetic nephropathy and pulmonary heart disease
(14–17). In previous years, with continuous
research and clinical observation of the pharmacological effects of
SML, SML has been indicated to have anticancer characteristics
(18). Lin et al (19) demonstrated that SML was able to
inhibit glucose metabolism of gastric cancer cells, prevent
proliferation and promote apoptosis of cancer cells by increasing
levels of p53 mRNA. This contributed to the inactivation of AKT
signaling pathways and cell arrest in the G2/M phase. Gong et
al (20) reported that SML
blocked the growth of prostate cancer by inhibiting tumor
angiogenesis in mice. Wang et al (21) confirmed that ligustrazine affected the
translocation of nuclear factor-κB p65 from the nucleus to
cytoplasm, and subsequently was able to alter its transcriptional
activity and the expression of target genes. This led to the
reduction in growth of osteosarcoma cells in vitro and in
vivo. In addition, a previous study revealed that ligustrazine
suppressed cyclooxygenase 2 and reduced the ability of lung cancer
cells to migrate and invade (22).
These data suggested that SML may be a potent agent for clinical
therapy of tumors.
Multidrug resistance was indicated to be an
important factor that hindered the complete elimination of glioma
cells during radiotherapy and chemotherapy, resulting in poor
prognosis for patients with glioma (23). The mechanisms of resistance may be
associated with the expression of ATP-binding cassette transporter
(ABC transporter) on the glioma cell membranes, which represents a
group of transporters, including 49 genes and 7 different
functional subfamilies (24). A
number of studies have reported that the expression of LRP, MRP1
and p-gp in these subfamilies was upregulated in glioma cells
(4,25). These drug resistance genes caused
failure in chemotherapy treatment of glioma by a number of
mechanisms, including efflux of drugs, increasing intracellular
alkaline, changing membrane lipid structure and intracellular ionic
environment, and accelerating glutathione to form the GST-X pump
(26,27). These data suggested that
downregulation of these genes may be helpful for increasing the
intracellular concentrations of chemotherapy drugs and inducing
antitumor effects. Therefore, these genes may be potential targets
for gene therapy in glioma.
To date, a number of studies have shown that SML
inhibits the proliferation of glioma (28,29).
However, to the best of our knowledge, there was no study reporting
whether SML reduced resistance to chemotherapy. In the present
study, a DOX-resistant glioma SHG44/DOX cell line was established,
and it was revealed that the SHG44/DOX cells were more sensitive to
DOX following exposure to SML. The combination of SML with DOX
reversed the drug resistance of SHG44/DOX by inhibiting
proliferation and tumor growth, and promoting early apoptosis. In
studies on breast and colon cancer, the potential mechanisms of
reversal of chemotherapeutic resistance were associated with
inhibiting the levels of ABC transporter in tumor cells (30,31). In
the present study, it was also demonstrated that SML increased the
intercellular concentration of DOX by reducing the expression of
drug resistance genes in SHG44/DOX cells, which increased the
antitumor ability of DOX.
In summary, the present study provides the
experimental basis for clinical application of SML to reverse
glioma chemotherapy resistance and improve the efficacy of
chemotherapy drugs on the disease. The antitumor effects of SML may
be a novel strategy for brain tumor chemotherapy, which may enable
the development of Chinese medicine in cancer therapy.
Acknowledgements
The present study was supported by the Scientific
Research Project of Health Bureau, Chongqing Municipal Health
Bureau Traditional Chinese Medicine Scientific Research Project
(grant no. ZY201402092).
References
|
1
|
Sturm D, Bender S, Jones DT, Lichter P,
Grill J, Becher O, Hawkins C, Majewski J, Jones C, Costello JF, et
al: Paediatric and adult glioblastoma: Multiform (epi)genomic
culprits emerge. Nat Rev Cancer. 14:92–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Auffinger B, Spencer D, Pytel P, Ahmed AU
and Lesniak MS: The role of glioma stem cells in chemotherapy
resistance and glioblastoma multiforme recurrence. Expert Rev
Neurother. 15:741–752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Faria GP, de Oliveira JA, de Oliveira
JG, Romano Sde O, Neto VM and Maia RC: Differences in the
expression pattern of P-glycoprotein and MRP1 in low-grade and
high-grade gliomas. Cancer Invest. 26:883–889. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin Y, Bin ZQ, Qiang H, Liang C, Hua C,
Jun D, Dong WA and Qing L: ABCG2 is related with the grade of
glioma and resistance to mitoxantone, a chemotherapeutic drug for
glioma. J Cancer Res Clin Oncol. 135:1369–1376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bleau AM, Huse JT and Holland EC: The
ABCG2 resistance network of glioblastoma. Cell Cycle. 8:2936–2944.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang R, Saito R, Shibahara I, Sugiyama S,
Kanamori M, Sonoda Y and Tominaga T: Temozolomide reverses
doxorubicin resistance by inhibiting P-glycoprotein in malignant
glioma cells. J Neurooncol. 126:235–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh MS and Lamprecht A: P-glycoprotein
inhibition of drug resistant cell lines by nanoparticles. Drug Dev
Ind Pharm. 42:325–331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang N, Chen S, Deng J, Huang Q, Liao P,
Wang F and Cheng Y: Over-expression of S100A9 in human glioma and
in-vitro inhibition by aspirin. Eur J Cancer Prev. 22:585–595.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen S, Zhao H, Deng J, Liao P, Xu Z and
Cheng Y: Comparative proteomics of glioma stem cells and
differentiated tumor cells identifies S100A9 as a potential
therapeutic target. J Cell Biochem. 114:2795–2808. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng Y, Ng ES, Kwan YW, Lau CB, Cheung DW,
Koon JC, Zhang Z, Zuo Z, Leung PC, Fung KP and Lam FF: Cerebral
vasodilator properties of Danshen and Gegen: A study of their
combined efficacy and mechanisms of actions. Phytomedicine.
21:391–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Poppel PC, Breedveld P, Abbink EJ,
Roelofs H, van Heerde W, Smits P, Lin W, Tan AH, Russel FG, Donders
R, et al: Salvia miltiorrhiza root water-extract (Danshen)
has no beneficial effect on cardiovascular risk factors. A
Randomized Double-Blind Cross-Over Trial. PLoS One.
10:e01286952015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai X, Chen Z, Pan X, Xia L, Chen P, Yang
Y, Hu H, Zhang J, Li K, Ge J, et al: Inhibition of angiogenesis,
fibrosis and thrombosis by tetramethylpyrazine: Mechanisms
contributing to the SDF-1/CXCR4 axis. PLoS One. 9:e881762014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin TH and Hsieh CL: Pharmacological
effects of Salvia miltiorrhiza (Danshen) on cerebral
infarction. Chin Med. 5:222010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo J, Song W, Yang G, Xu H and Chen K:
Compound danshen (Salvia miltiorrhiza) dripping pill for
coronary heart disease: An overview of systematic reviews. Am J
Chin Med. 43:25–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SH, Kim YS, Lee SJ and Lee BC: The
protective effect of Salvia miltiorrhiza in an animal model
of early experimentally induced diabetic nephropathy. J
Ethnopharmacol. 137:1409–1414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Huang Y, Zhao C, Qin X, Zhu Q, Chen
S and Qu J: Salvia miltiorrhiza injection on pulmonary heart
disease: A systematic review and meta-analysis. Am J Chin Med.
42:1315–1331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen X, Guo J, Bao J, Lu J and Wang Y: The
anticancer properties of Salvia miltiorrhiza Bunge
(Danshen): A systematic review. Med Res Rev. 34:768–794. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin LL, Hsia CR, Hsu CL, Huang HC and Juan
HF: Integrating transcriptomics and proteomics to show that
tanshinone IIA suppresses cell growth by blocking glucose
metabolism in gastric cancer cells. BMC Genomics. 16:412015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong Y, Li Y, Lu Y, Li L, Abdolmaleky H,
Blackburn GL and Zhou JR: Bioactive tanshinones in Salvia
miltiorrhiza inhibit the growth of prostate cancer cells in
vitro and in mice. Int J Cancer. 129:1042–1052. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Fu Q and Zhao W:
Tetramethylpyrazine inhibits osteosarcoma cell proliferation via
downregulation of NF-κB in vitro in vivo. Mol Med Rep.
8:984–988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng CY, Xiao W, Zhu MX, Pan XJ, Yang ZH
and Zhou SY: Inhibition of cyclooxygenase-2 by tetramethylpyrazine
and its effects on A549 cell invasion and metastasis. Int J Oncol.
40:2029–2037. 2012.PubMed/NCBI
|
|
23
|
Tivnan A, Zakaria Z, O'Leary C, Kögel D,
Pokorny JL, Sarkaria JN and Prehn JH: Inhibition of multidrug
resistance protein 1 (MRP1) improves chemotherapy drug response in
primary and recurrent glioblastoma multiforme. Front Neurosci.
9:2182015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Jonge-Peeters SD, Kuipers F, de Vries
EG and Vellenga E: ABC transporter expression in hematopoietic stem
cells and the role in AML drug resistance. Crit Rev Oncol Hematol.
62:214–226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Andersson U, Malmer B, Bergenheim AT,
Brännström T and Henriksson R: Heterogeneity in the expression of
markers for drug resistance in brain tumors. Clin Neuropathol.
23:21–27. 2004.PubMed/NCBI
|
|
26
|
Agarwal S, Sane R, Gallardo JL, Ohlfest JR
and Elmquist WF: Distribution of gefitinib to the brain is limited
by P-glycoprotein (ABCB1) and breast cancer resistance protein
(ABCG2)-mediated active efflux. J Pharmacol Exp Ther. 334:147–155.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deeley RG and Cole SP: Substrate
recognition and transport by multidrug resistance protein 1
(ABCC1). FEBS Lett. 580:1103–1111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu K, Chen Z, Pan X, Yang Y, Tian S, Zhang
J, Ge J, Ambati B and Zhuang J: Tetramethylpyrazine-mediated
suppression of C6 gliomas involves inhibition of chemokine receptor
CXCR4 expression. Oncol Rep. 28:955–960. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu L, Li C, Li D, Wang Y, Zhou C, Shao W,
Peng J, You Y, Zhang X and Shen X: Cryptotanshinone inhibits human
glioma cell proliferation by suppressing STAT3 signaling. Mol Cell
Biochem. 381:273–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai J, Chen S, Zhang W, Zheng X, Hu S,
Pang C, Lu J, Xing J and Dong Y: Salvianolic acid A reverses
paclitaxel resistance in human breast cancer MCF-7 cells via
targeting the expression of transgelin 2 and attenuating PI3K/Akt
pathway. Phytomedicine. 21:1725–1732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu T, To KK, Wang L, Zhang L, Lu L, Shen
J, Chan RL, Li M, Yeung JH and Cho CH: Reversal of P-glycoprotein
(P-gp) mediated multidrug resistance in colon cancer cells by
cryptotanshinone and dihydrotanshinone of Salvia
miltiorrhiza. Phytomedicine. 21:1264–1272. 2014. View Article : Google Scholar : PubMed/NCBI
|