Introduction
The incidence of cervical cancer is relative high
among all malignant tumors in women. Accurate staging and early
diagnosis play a key role in treatment and prognosis of cervical
cancer. Cytology examination and related specialized examination
are still the main methods used in the diagnosis of cervical
cancer. However, these methods cannot be used to accurately
determine the scope of tumor invasion (1). Ultrasonography plays a certain role in
diagnosis and staging of cervical cancer. However, the function of
ultrasonography is limited for patients with small lesions or
without obvious morphological changes in cervix (2). Contrast-enhanced ultrasonography can be
used to dynamically observe the characteristics of microcirculation
blood perfusion in tissues, so this method can be used to
effectively detect malignant tumors (3). Recent studies have shown that cell
adhesion molecules play important roles in the onset and
development of cancers. As a main type of cell adhesion molecule
(4), E-cadherin is highly accumulated
in epithelial cell of mature tissue. E-cadherin can participate in
the transmission and exchange of information between cells and
regulate the formation and development of embryonic tissues,
E-cadherin can also maintain the polarity and integrity of the
morphology of epithelial cells, and regulate adhesion aggregation
between epithelial cells and the differentiation of epithelial
cells (5). Lymph node metastasis can
significantly affect the prognosis of cervical cancer. Therefore,
the determination of lymph node metastasis has significant effect
on the staged operation of patients with cervical cancer. In this
study, patients with cervical cancer were examined by
contrast-enhanced ultrasonography. Expression of E-cadherin in
cervical cancer tissues of patients with different metastatic
conditions was measured by enzyme-linked immunosorbent assay
(ELISA). In addition, the correlation between the parameters of
contrast-enhanced ultrasonography in evaluating cervical cancer
metastasis and expression of E-cadherin were also explored.
Materials and methods
General information
In total 120 patients with cervical cancer were
selected from January 2015 to December 2016. i) Inclusion criteria:
cervical cancer patients aged 45 to 65 years. ii) Exclusion
criteria: patients with hepatitis virus and human immunodeficiency
virus infection; patients with autoimmune diseases; patients with
other types of malignant tumor; patients with severe cardiovascular
and cerebrovascular diseases; patients with psychiatric disorders.
iii) Causes of loss of contact and response measures: expected
causes: patients quit the test, patients transferred to another
hospital; response measures: add new participants at the ratio of
1:1 according to the inclusion and exclusion criteria. iv) Medical
ethics issues: patients or their family members signed written
informed consent, the safety of patients was ensured according to
the relevant principles of clinical guidelines, and privacy
(medical record) of patients were protected. v) Double-blind
clinical experiments: researchers were divided into four groups,
group 1 was responsible for the selection and grouping of patients,
group 2 was responsible for the treatment, group 3 was responsible
for the observation and data collection, and group 4 was
responsible for statistical analysis and manuscript writing. The
grouping is confidential to the patients, and the four groups of
researchers were confidential to each other. This study was
approved by the Ethics Committee of Affiliated Hospital of Hebei
University. Signed written informed consents were obtained from the
patients and/or guardians.
Research methods
Grouping
According to the results of postoperative
pathological examination, 120 patients with cervical cancer were
divided into distant metastasis group (group A), lymph node
metastasis without distant metastasis group (group B) and no
metastasis group (group C).
Contrast-enhanced ultrasonography
Contrast-enhanced ultrasonography was performed
using a real-time three-dimensional color Doppler Voluson E8
Ultrasonic Diagnostic Instrument (GE Healthcare, Cleveland, OH,
USA). The probe frequency is 3.5 MHz and the mechanical index is
0.11. Routine abdominal or vaginal ultrasound examination was
performed for all the patients to determine tumor size, location,
shape and number. The transabdominal acoustic contrast agent
SonoVue (Bracco, Courcouronnes, France) was used. Saline (5 ml) was
injected into the bottle containing SonoVue and mixed. Then SonoVue
suspension (2.0 ml) was injected through elbow vein, and 5 ml of
saline was then used. The built-in timer of the ultrasonic
instrument was started. Changes in echo intensity of lesions and
the process of contrast agent perfusion were observed, and the
dynamic images were stored and quantitatively analyzed.
Quantitative analysis of ultrasound images
The built-in acoustic quantitative time-strength
curve analysis software was used to draw the curve. Freehand method
was used to cover the whole area of solid tumors. With the normal
uterine muscle as a control, automatic marking method was used to
obtain relevant quantitative parameters including baseline
intensity, peak intensity, arrival time, time to peak, enhances
intensity and perfusion time were calculated. Enhanced intensity
(peak intensity-baseline intensity, perfusion time) time to
peak-arrival time. The ROC curve was drawn and the area below the
curve was calculated. The sensitivity and specificity of different
truncation points were calculated to estimate the ideal value of
enhanced intensity in determining lymph node metastasis.
ELISA to detect the expression of E-cadherin in
cervical cancer tissue
Human E-cadherin assay kit used in this study is
from ImmunoWay Biotechnology Co. (Plano, TX, USA). The specific
steps are as follows: i) blood was extracted from patients and
transferred to coagulation tubes, followed by centrifugation (2,650
× g) for 10 min to collect the supernatant, which was transferred
to EP tubes and stored at −80°C; ii) The standard was diluted
according to the instructions of kit and three repeat wells were
set for each concentration; iii) Standard well, sample well and
blank control well were set, and 100 µl sample was added into each
well, the plate was covered and incubated at 37°C for 2.5 h; iv)
300 µl of washing solution was added into each well, and washing
was performed 4 times; v) 100 µl of biotin-labeled antibody was
added to each well and incubated for 1 h at room temperature; vi)
step 4 was repeated; vii) 100 µl of enzyme conjugate working
solution was added into each well, the plate was covered and
incubated at room temperature for 45 min; viii) Step 4 was
repeated; ix) 100 µl of color development reagent was added into
each well and incubated for 10–20 min at room temperature in the
dark. x) 100 µl of stop solution was added into each well and the
absorbance at 450 nm was measured using a microplate reader.
Statistical analysis
All data were analyzed by SPSS 20.0 statistical
analysis software (IBM, Armonk, NY, USA). Data were expressed as
mean ± standard deviation, and single-factor analysis of variance
was performed, the comparison among multiple groups, and LSD t-test
was performed for the comparisons between two groups, P<0.05 was
considered to be statistically significant.
Results
Clinicopathological parameters of
cervical cancer patients
In this study, 120 patients with cervical cancer,
including 93 patients with squamous cell carcinoma and 27 patients
with adenocarcinoma were involved. Distant metastasis was found in
28 cases, lymph node metastasis without distant metastasis was
found in 43 cases, and 49 cases showed no metastasis. Results of
pathological staging showed that the number of patients with stage
I, II and III were 31, 38 and 51, respectively (Table I).
 | Table I.Clinicopathological parameters of
cervical cancer patients. |
Table I.
Clinicopathological parameters of
cervical cancer patients.
| Clinicopathological
parameter | Cases (n) |
|---|
| Types of tissue |
|
| Squamous
cell carcinoma | 93 |
|
Adenocarcinoma | 27 |
| Metastasis |
|
| Distant
metastasis | 28 |
| Lymph
node metastasis | 43 |
| No
metastasis | 49 |
| Pathological
staging |
|
| Stage
I | 31 |
| Stage
II | 38 |
| Stage
III | 51 |
Comparison of contrast-enhanced
ultrasonography parameters among three groups
Comparison of parameters of contrast-enhanced
ultrasonography showed that, the baseline intensity of group A was
11.9±2.2 dB, which was significantly lower than that of group B and
C. Baseline intensity of group B was significantly lower than that
of group C (13.0±2.4 vs. 15.3±3.6 dB), significant differences were
found among the three groups (P<0.05). The P-value of the
comparison of enhanced intensity among three groups was 0.052,
which is close to 0.05. No significant differences in peak
intensity, arrival time, time to peak and perfusion time were found
among groups (P>0.05) (Table
II).
 | Table II.Comparison of contrast-enhanced
ultrasonography parameters among three groups. |
Table II.
Comparison of contrast-enhanced
ultrasonography parameters among three groups.
| Items | Group A (n=28) | Group B (n=43) | Group C (n=49) | F-value | P-value |
|---|
| Baseline intensity
(dB) | 11.9±2.2 | 13.0±2.4a | 15.3±3.6a,b | 15.367 | <0.05 |
| Peak intensity
(dB) | 95.8±9.3 | 93.8±8.1 | 91.3±9.5 |
0.935 | >0.05 |
| Enhanced intensity
(dB) | 85.9±6.1 | 84.3±6.6 | 78.9±6.2 |
0.881 | >0.05 |
| Arrival time
(sec) | 17.5±3.6 | 17.0±3.4 | 16.4±4.4 |
0.827 | >0.05 |
| Time to peak
(sec) | 27.3±5.0 | 27.0±5.3 | 26.5±5.7 |
0.904 | >0.05 |
| Perfusion time
(sec) | 10.2±2.4 | 10.8±2.0 | 11.2±1.8 |
0.752 | >0.05 |
Comparison of sensitivity, specificity
and area under ROC curve between different cut points of enhanced
intensity
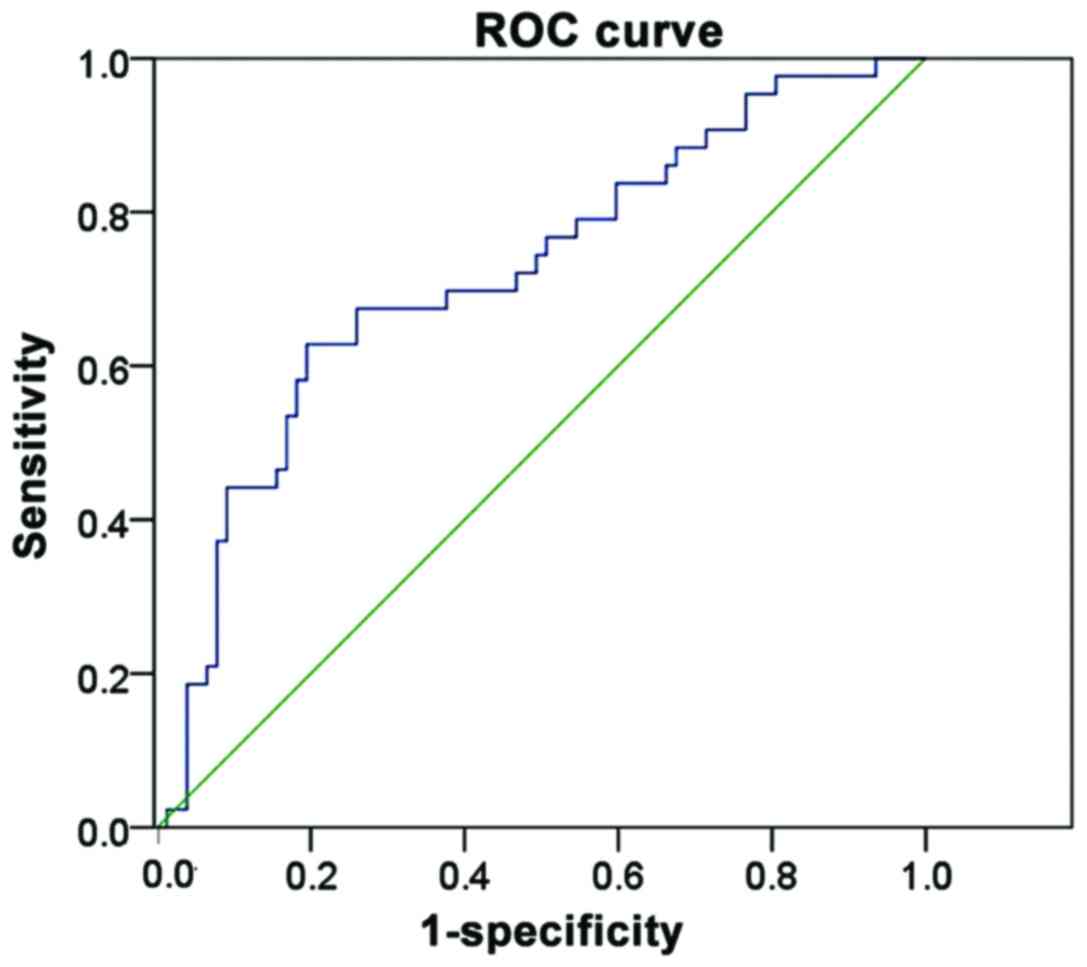
In this study, ROC curve was drawn and the area
under the curve was calculated to evaluate the sensitivity and
specificity of different cut points of enhanced intensity in
evaluating tumor metastasis of cervical cancer patients. Results
showed that the sensitivity and specificity of the use of enhanced
intensity ~83.7 dB in evaluating tumor metastasis of patients with
cervical cancer were 82.42 and 79.32%, respectively, and the area
under ROC curve was 0.809 (Table
III and Fig. 1).
 | Table III.Comparison of sensitivity, specificity
and area under ROC curve between different cut points of enhanced
intensity. |
Table III.
Comparison of sensitivity, specificity
and area under ROC curve between different cut points of enhanced
intensity.
| Cut points of
enhanced intensity (dB) | Sensitivity (%) | Specificity (%) | Area under the
curve |
|---|
| 73.1 | 100 | 37.82 |
0.788 |
| 83.7 | 82.42 | 79.32 |
0.809 |
| 85.9 | 62.81 | 80.52 |
0.787 |
| 86.0 | 60.53 | 80.51 |
0.776 |
| 86.2 | 58.11 | 81.83 |
0.738 |
| 86.8 | 47.60 | 100 |
0.725 |
Expression of E-cadherin in cervical
cancer tissues of patients in three groups
The expression of E-cadherin in cervical cancer
tissues was detected by ELISA. Results showed that the expression
level of E-cadherin protein in group A was 0.030±0.003 ng/ml, which
was significantly lower than that of group B and C (P<0.05), and
the expression level of E-cadherin in group B was significantly
lower than that in group C (0.037±0.007 vs. 0.045±0.012 ng/ml),
significant differences were found among groups (P<0.05)
(Table IV).
 | Table IV.Comparison of expression levels of
E-cadherin in cervical cancer tissues of patients in three
groups. |
Table IV.
Comparison of expression levels of
E-cadherin in cervical cancer tissues of patients in three
groups.
| Groups | n | Level of E-cadherin
(ng/ml) |
|---|
| Group A | 28 | 0.030±0.003 |
| Group B | 43 |
0.037±0.007a |
| Group C | 49 |
0.045±0.012a,b |
| F-value | – | 23.148 |
| P-value | – | <0.05 |
Correlations between the parameters of
contrast-enhanced ultrasonography in evaluating cervical cancer
metastasis and expression of E-cadherin
Correlations between the parameters of
contrast-enhanced ultrasonography in evaluating cervical cancer
metastasis and expression of E-cadherin were analyzed by Pearsons
correlation analysis. Results showed that the baseline intensity of
contrast-enhanced ultrasonography was positively correlated with
the expression level of E-cadherin (P<0.05), while enhanced
intensity of contrast-enhanced ultrasonography was negatively
correlated with the expression level of E-cadherin (P<0.05)
(Table V).
 | Table V.Correlations between the parameters of
contrast-enhanced ultrasonography in evaluating cervical cancer
metastasis and expression levels of E-cadherin. |
Table V.
Correlations between the parameters of
contrast-enhanced ultrasonography in evaluating cervical cancer
metastasis and expression levels of E-cadherin.
|
| Expression levels of
E-cadherin |
|---|
|
|
|
|---|
| Parameters of
contrast-enhanced ultrasonography | rs | P-value |
|---|
| Baseline
intensity | 0.657 | 0.007 |
| Peak intensity | −0.164 | 0.235 |
| Enhanced
intensity | −0.526 | 0.001 |
| Arrival time | −0.102 | 0.742 |
| Time to peak | −0.023 | 0.303 |
| Perfusion time | 0.171 | 0.869 |
Correlations between the parameters of
contrast-enhanced ultrasonography in evaluating cervical cancer
metastasis and clinicopathological parameters of patients
Correlations between the parameters of
contrast-enhanced ultrasonography in evaluating cervical cancer
metastasis and clinicopathological parameters of patients were
performed by Pearsons correlation analysis. Results showed that
baseline intensity and enhanced intensity were closely correlated
with the tumor metastasis and pathological stages of patients. No
significant correlation was found between parameters of
contrast-enhanced ultrasonography and tissue types (P>0.05)
(Table VI).
 | Table VI.Correlations between the parameters of
contrast-enhanced ultrasonography in evaluating cervical cancer
metastasis and clinicopathological parameters of patients. |
Table VI.
Correlations between the parameters of
contrast-enhanced ultrasonography in evaluating cervical cancer
metastasis and clinicopathological parameters of patients.
|
| Tissue type | Tumor metastasis | Pathological
stage |
|---|
|
|
|
|
|
|---|
| Parameters of
contrast-enhanced ultrasonography | rs | P-value | rs | P-value | rs | P-value |
|---|
| Baseline
intensity | 0.733 | 0.125 | 0.782 | 0.005 | 0.679 | 0.012 |
| Peak intensity | 0.421 | 0.336 | 0.347 | 0.213 | 0.412 | 0.116 |
| Enhanced
intensity | 0.706 | 0.148 | 0.623 | 0.011 | 0.653 | 0.028 |
| Arrival time | 0.112 | 0.657 | 0.135 | 0.654 | 0.388 | 0.235 |
| Time to peak | 0.234 | 0.326 | 0.149 | 0.661 | 0.109 | 0.719 |
| Perfusion time | 0.128 | 0.817 | 0.112 | 0.732 | 0.364 | 0.349 |
Discussion
The incidence of cervical cancer is relatively high
among all malignant tumors in women. Biological characteristics of
and staging of the tumors usually determine the selection of
treatment strategies, take cervical cancer as an example, although
studies have shown that abnormal expression of some cytokines can
be used as indicators for the diagnosis of cervical cancer
(6), the tumor size and scope of
infiltration are still the key factors that determine the treatment
and prognosis of cervical cancer. Cytology examination and related
specialized examination are still the main methods used for the
diagnosis of cervical cancer, so the scope of infiltration cannot
be determined, let alone the metastasis, causing difficulties in
diagnosis (7,8). Ultrasound provides new insight for the
staging and diagnosis of cervical cancer, and the accuracy of
diagnosis was increased significantly with the use of ultrasound
(9).
Insufficient blood supply is common for normal lymph
nodes, while blood supply can be significantly improved with the
existing of lymph node metastasis, so the conditions of blood
supply of lymph nodes detected by ultrasound can be used to
determine the lymph node metastasis (10). Intestinal gas interference and
abdominal fat attenuation and other adverse conditions can affect
the examination of abdominal, pelvic lymph nodes. In addition,
color Doppler ultrasound is affected by the resolution of the
instrument, operator's experience, sampling angle and parameter
adjustment and other factors, so this technique is not sensitive to
low-speed blood flow and cannot be used to fully understand the
internal blood supply to lymph nodes. Therefore, conditions of
blood supply to abdominal, pelvic lymph nodes detected by
conventional ultrasound examination cannot be used to accurately
determine the lymph node metastasis (11). Contrast-enhanced ultrasonography is a
new imaging technique that can be used to dynamically and clearly
observe microvascular, especially tumor blood vessels, by injecting
contrast agents. Specific diagnosis of lesions can be made based on
the patterns of contrast agent perfusion, such as speed and
intensity. In this study, the baseline intensity of group A was
11.9±2.2 dB, which was significantly lower than that of group B and
C. Baseline intensity of group B was significantly lower than that
of group C (13.0±2.4 vs. 15.3±3.6 dB), significant differences were
found among three groups (P<0.05). The P-value of the comparison
of enhanced intensity among three groups was 0.052, which is close
to 0.05. The data suggest that differences in the parameters of
contrast-enhanced ultrasonography can be used to determine the
status of lymph node metastasis. The quality of imaging can be
affected by patient's body mass index, ultrasonic attenuation and
the depth of lesions (12), the
specificity and sensitivity of different cut points of enhanced
intensity in determining tumor metastasis were compared. The
initial intensity with contrast agent perfusion was the baseline
intensity. Baseline intensity can be affected by scope of
liquefactive necrosis and degree of fibrosis, so baseline intensity
can be used to differentiation degrees and biological
characteristics of tumors (13). The
maximum intensity is the peak intensity. Peak intensity includes
baseline intensity. So, the difference between peak intensity and
the baseline strength is the enhanced intensity. Enhanced intensity
is a normalized value and the differences of enhanced intensity
between individuals were not big. So enhanced intensity can be used
to more clearly understand the conditions of blood perfusion and
more accurately determine metastasis of cervical cancer (14). Studies have confirmed that microvessel
density can significantly affect the enhanced intensity of
contrast-enhanced ultrasonography. As an accurate tumor
angiogenesis index, microvessel density can significantly affect
the onset and development (15).
Microvessel density can also significantly affect lymph node
metastasis, biological behavior and the degree of differentiation
of tumors. One of the important causes of tumor lymph node
metastasis is intravascular angiogenesis (16). In this study, the ROC curve was drawn
and the area under the curve was calculated to evaluate the
sensitivity and specificity of different cut-off points of enhanced
intensity in predicting tumor lymph node metastasis. Results showed
that the sensitivity and specificity of the use of enhanced
intensity ~83.7 dB in evaluating tumor metastasis of patients with
cervical cancer were 82.42 and 79.32%, respectively. In addition,
Pearsons correlation analysis showed that baseline intensity and
enhanced intensity were closely correlated with the tumor
metastasis and pathological stages of patients.
E-cadherin is highly accumulated in epithelial cells
of mature tissue and early stage embryo tissue. As a kind of
transmembrane glycoprotein, E-cadherin can mediate adhesion between
epithelial cells. As one of the tumor suppressor proteins, loss of
E-cadherin expression can lead to the development and distant
metastasis of tumors (17).
E-cadherin inactivation is critical in the development of cervical
cancer. Inactivation of E-cadherin can induce the occurrence of EMT
and the activation of Wnt signaling pathway. Decreased E-cadherin
expression can cause migration and invasion of tumor cells
(18). Compared with wild-type cell
line, the migration ability of E-cadherin mutant cell lines was
significantly increased, indicating that cell migration can be
affected by the inactivation of E-cadherin (19). In this study, the expression of
E-cadherin in cervical cancer tissues was detected by ELISA. The
results showed that the expression of E-cadherin protein in
patients with distant metastasis was significantly lower than that
in patients only with lymph node metastasis and patients without
metastasis. Expression level of E-cadherin in patients only with
lymph node metastasis was significantly higher than that in
patients without metastasis, significant differences in the
expression level of E-cadherin protein were found among three
groups. This finding is consistent with previous studies (20), indicating that adhesion between
cervical cancer cells can be reduced by the reduced expression of
E-cadherin, leading to the failure of tissue in inhibiting
metastasis, further causing the diffuse growth and distant
metastasis of cervical cancer cells. So, the observation of the
changes in expression level of E-cadherin can play a role in the
diagnosis of cervical cancer.
In conclusion, quantitative analysis of the results
of contrast-enhanced ultrasonography can play a certain role in the
determination of cervical cancer metastasis. The combined use of
contrast-enhanced ultrasonography and E-cadherin expression can
significantly improve the diagnosis and treatment of cervical
cancer.
References
|
1
|
Park JW, Park DM, Choi BK, Kwon BS, Seong
JK, Green JE, Kim DY and Kim HK: Establishment and characterization
of metastatic gastric cancer cell lines from murine gastric
adenocarcinoma lacking Smad4, p53, and E-cadherin. Mol Carcinog.
54:1521–1527. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang X, Qian Y, Wu H, Xie X, Zhou Q, Wang
Y, Kuang W, Shen L, Li K, Su J, et al: Aberrant expression of
osteopontin and E-cadherin indicates radiation resistance and poor
prognosis for patients with cervical carcinoma. J Histochem
Cytochem. 63:88–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y, Chen XG and Liang CZ: Expressions
of E-cadherin and N-cadherin in prostate cancer and their
implications. Zhonghua Nan Ke Xue. 20:781–786. 2014.(In Chinese).
PubMed/NCBI
|
|
4
|
Slowinska-Klencka D, Sporny S,
Stasikowska-Kanicka O, Popowicz B and Klencki M: E-cadherin
expression is more associated with histopathological type of
thyroid cancer than with the metastatic potential of tumors. Folia
Histochem Cytobiol. 50:519–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dellaportas D, Koureas A, Contis J,
Lykoudis PM, Vraka I, Psychogios D, Kondi-Pafiti A and Voros DK:
Contrast-enhanced color Doppler ultrasonography for preoperative
evaluation of sentinel lymph node in breast cancer patients. Breast
Care (Basel). 10:331–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Biedka M, Makarewicz R, Marszałek A, Sir
J, Kardymowicz H and Goralewska A: Labeling of microvessel density,
lymphatic vessel density and potential role of proangiogenic and
lymphangiogenic factors as a predictive/prognostic factors after
radiotherapy in patients with cervical cancer. Eur J Gynaecol
Oncol. 33:399–405. 2012.PubMed/NCBI
|
|
7
|
Shiyan L, Pintong H, Zongmin W, Fuguang H,
Zhiqiang Z, Yan Y and Cosgrove D: The relationship between enhanced
intensity and microvessel density of gastric carcinoma using double
contrast-enhanced ultrasonography. Ultrasound Med Biol.
35:1086–1091. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Ye Z, Sun H and Bai R: Grading of
uterine cervical cancer by using the ADC difference value and its
correlation with microvascular density and vascular endothelial
growth factor. Eur Radiol. 23:757–765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakamura K, Joja I, Nagasaka T, Haruma T
and Hiramatsu Y: Maximum standardized lymph node uptake value could
be an important predictor of recurrence and survival in patients
with cervical cancer. Eur J Obstet Gynecol Reprod Biol. 173:77–82.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamrava M: Potential role of ultrasound
imaging in interstitial image based cervical cancer brachytherapy.
J Contemp Brachytherapy. 6:223–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zaridah S: A review of cervical cancer
research in Malaysia. Med J Malaysia. 69 Suppl A:33–41.
2014.PubMed/NCBI
|
|
12
|
Benckert C, Thelen A, Cramer T, Weichert
W, Gaebelein G, Gessner R and Jonas S: Impact of microvessel
density on lymph node metastasis and survival after curative
resection of pancreatic cancer. Surg Today. 42:169–176. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang J, Shang X, Zhang H, Ma W, Xu Y,
Zhou Q, Gao Y, Yu S and Qi Y: Correlation between maximum intensity
and microvessel density for differentiation of malignant from
benign thyroid nodules on contrast-enhanced sonography. J
Ultrasound Med. 33:1257–1263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang MX, Mulvana H, Gauthier T, Lim AK,
Cosgrove DO, Eckersley RJ and Stride E: Quantitative
contrast-enhanced ultrasound imaging: A review of sources of
variability. Interface Focus. 1:520–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li B, Shi H, Wang F, Hong D, Lv W, Xie X
and Cheng X: Expression of E-, P- and N-cadherin and its clinical
significance in cervical squamous cell carcinoma and precancerous
lesions. PLoS One. 11:e01559102016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng J, Qi S, Wang P, Li W, Song L, Liu C
and Li F: Meta-analysis of downregulated E-cadherin as a poor
prognostic biomarker for cervical cancer. Future Oncol. 24:102–104.
2015.
|
|
17
|
Wagih HM, El-Ageery SM and Alghaithy AA: A
study of RUNX3, E-cadherin and β-catenin in CagA-positive
Helicobacter pylori associated chronic gastritis in Saudi
patients. Eur Rev Med Pharmacol Sci. 19:1416–1429. 2015.PubMed/NCBI
|
|
18
|
Myong NH: Loss of E-cadherin and
acquisition of vimentin in epithelial-mesenchymal transition are
noble indicators of uterine cervix cancer progression. Korean J
Pathol. 46:341–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng Y, Zhou Y, Jiang W, Yang X, Zhu J,
Feng D, Wei Y, Li M, Yao F, Hu W, et al: Significance of
E-cadherin, β-catenin, and vimentin expression as postoperative
prognosis indicators in cervical squamous cell carcinoma. Hum
Pathol. 43:1213–1220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Do TV, Kubba LA, Du H, Sturgis CD and
Woodruff TK: Transforming growth factor-beta1, transforming growth
factor-beta2, and transforming growth factor-beta3 enhance ovarian
cancer metastatic potential by inducing a Smad3-dependent
epithelial-to-mesenchymal transition. Mol Cancer Res. 6:695–705.
2008. View Article : Google Scholar : PubMed/NCBI
|