Introduction
A gastrointestinal stromal tumor (GIST) was the most
common mesenchymal tumor of the gastrointestinal tract in the
United States of America in 2007 (1).
The stomach is the most frequent anatomical location of these
tumors (2). Complete surgical
resection, which may avoid tumor rupture or injury to the
pseudocapsule, remains the sole method that may result in a
permanent cure for patients with GISTs (3). Laparoscopic wedge resection of a gastric
GIST represents a widely accepted surgical treatment (4). However, identifying the incisional
margin from a serosal laparoscopic view remains challenging. An
intraluminal endoscopic view enables the incisional margin to be
more precisely identified. Previous studies have reported a local
full-thickness resection technique that uses flexible endoscopy and
laparoscopy for gastric tumors (laparoscopic and endoscopic
cooperative surgery), and uses more precise cutting, which
decreases unnecessary and excessive resection that may deform the
stomach (4,5). Combining laparoscopic and endoscopic
approaches with non-exposure techniques when treating neoplasia is
useful for a full-thickness resection, as it may prevent stomach
contents from entering the clean abdominal cavity, and provides a
transabdominal retrieval route of tumor; however, this technique is
limited, as it is associated with the excessive resection of the
mucosa and difficulty in determining the resection line (4,5).
The novel technique discussed in the present study
for gastric GISTs, known as non-exposed endoscopic wall-inversion
surgery (NEWS), is minimally invasive and may aid in more precisely
determining the resection line with decreased risk of peritoneal
contamination or the exposure of the GIST to the peritoneal cavity
(6–9).
To the best of our knowledge, the present study describes the first
use of NEWS for a patient with a small gastric GIST in
Thailand.
Case report
A 61-year old female presented with jaundice and
fatigue in February 2016 at Phra Nakhon Si Ayutthaya Hospital
(Ayutthaya, Thailand). The clinical examination and blood test for
antibodies demonstrated that the patient was IgM positive and IgG
negative, and the patient was diagnosed with acute viral Hepatitis
A. in Phra Nakhon Si Ayutthaya Hospital (Ayutthaya province,
Thailand). The patient was treated with supportive care for 2 weeks
without fulminant hepatic failure. At 4 months the severity of the
symptoms had decreased, but the liver function tests revealed that
the transaminase concentration in the blood remained elevated. The
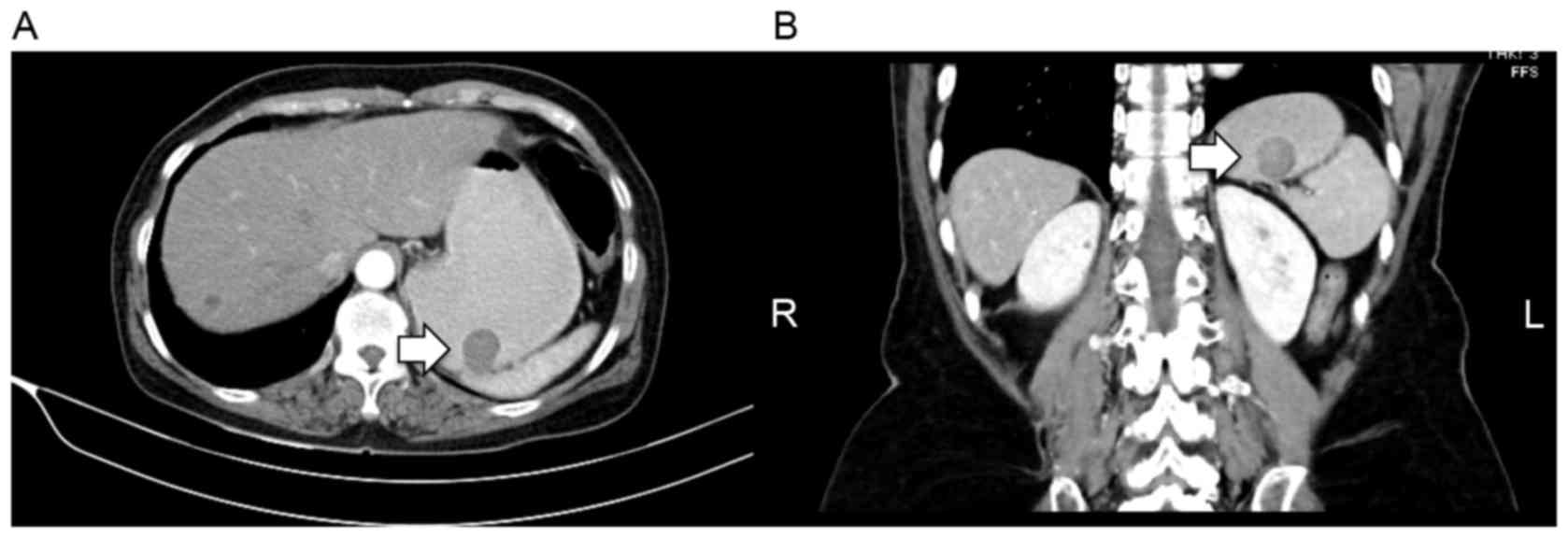
computed tomography (CT) scans demonstrated normal liver parenchyma
with multiple liver cysts. The CT scans, obtained as the patient
was assessed for hepatitis, revealed a gastric mass that protruded
into the lumen, with no evidence of lymph node or distant
metastasis (Fig. 1A and B). The
patient was referred to the Department of Surgery, Faculty of
Medicine, Thammasat University (Pathumthani, Thailand) where the
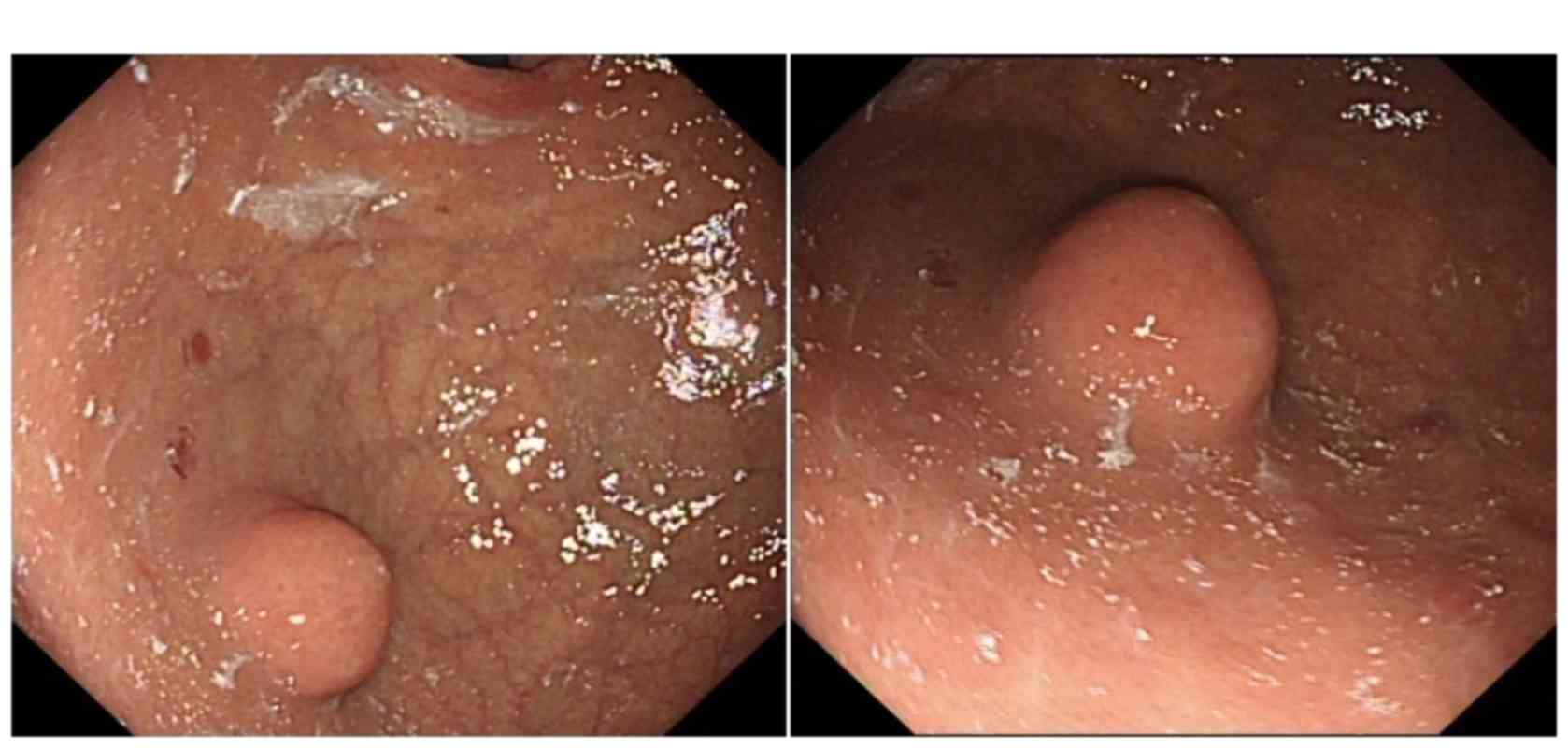
gastric mass was evaluated. Endoscopic examination revealed a
2.5×2.0-cm subepithelial tumor located in the posterior wall of the
upper gastric body (Fig. 2). The
patient was informed of multiple treatment options and consented to
undergo NEWS. NEWS was performed as follows: The patient was placed
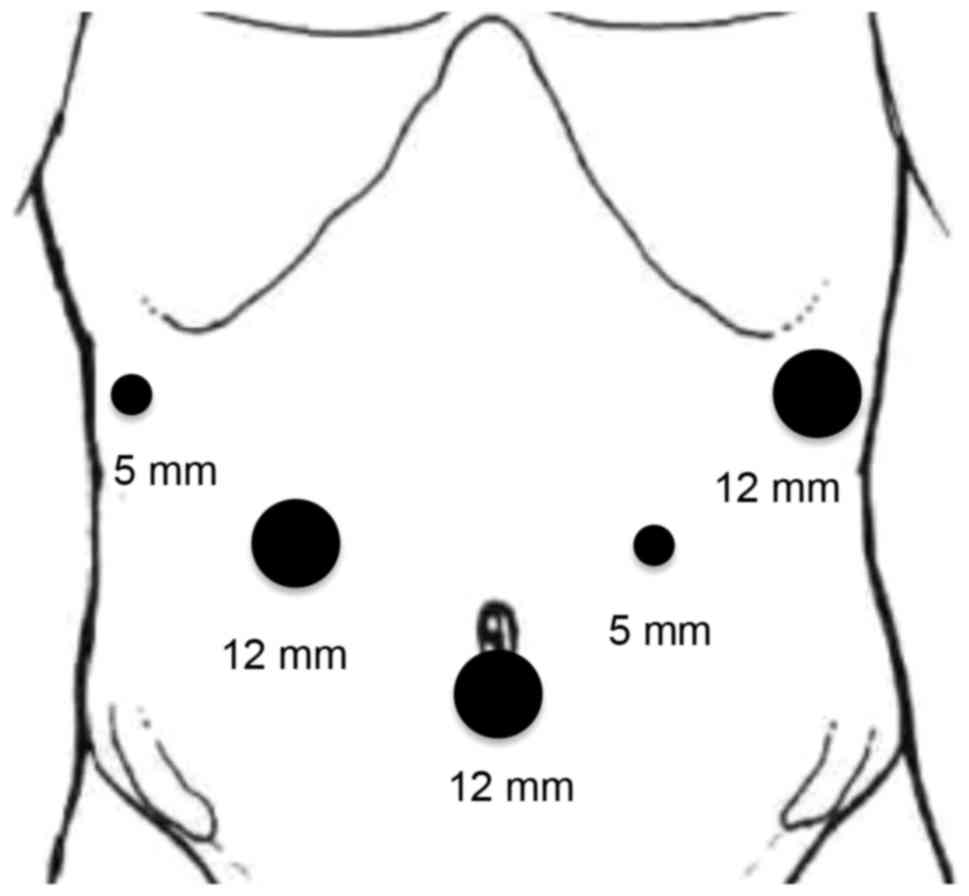
under general anesthesia in a supine position with legs apart. A
12-mm camera port was inserted into the umbilical portion of the
abdomen as the 1st trocar; pneumoperitoneal gas was also inserted
through this port. Subsequently, 5- and 12-mm trocars were placed
in the left and right upper quadrants, two in each quadrant, with
five trocars in total (Fig. 3). The
intestinal clip was attached to the jejunum behind the
duodenojejunal junction to prevent endoscopic intraluminal air from
passing into the small bowel; the presence of endoscopic
intraluminal air in the small bowel would render the laparoscopic
procedure more difficult. Flexible endoscopy was performed using a
flexible overtube (MD-48518; Sumius; Sumitomo Bakelite Co., Ltd.,
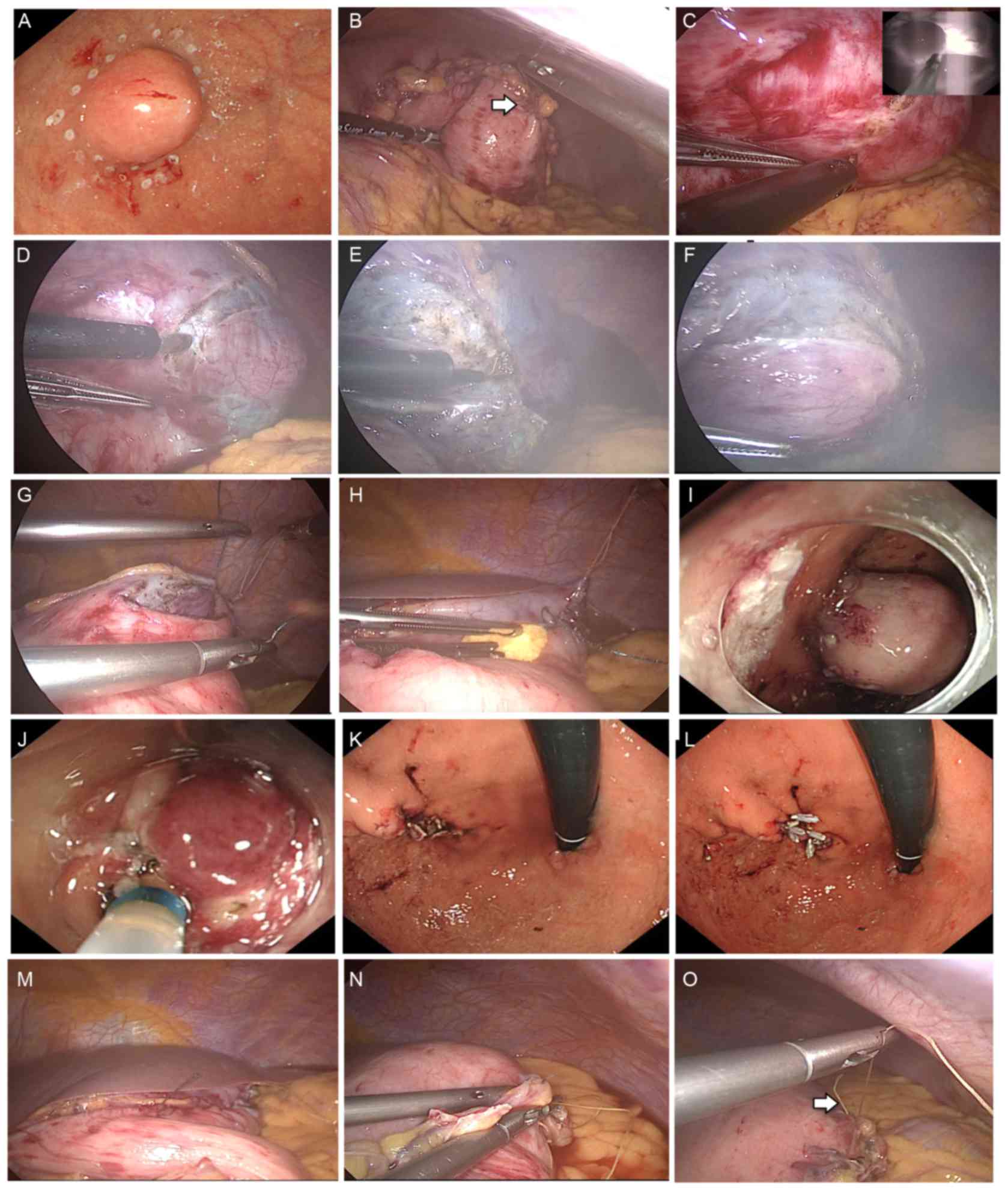
Tokyo, Japan). Subsequently, multiple mucosal markings were
generated around the subepithelial mass using a dual knife
(KD-650L; Olympus Corporation, Tokyo, Japan) (Fig. 4A). Next, the gastrocolic ligament was
dissected using a laparoscopic LigaSure™ (LF1637; Medtronic,
Dublin, Ireland). A 3-0 braided absorbable suture (Polysorb™;
SL-822; Medtronic, Watford, UK) was used to generate greater
curvature and lift for improved exposure to the gastric posterior
wall (Fig. 4B). Multiple serosal
markings were produced using laparoscopy from the outside of the
stomach, opposite the inner mucosal markings previously produced
using a Surgiwand™ II 5-mm cautery with a spatula tip (178099;
Medtronic), and guided by a dual knife pressed against the gastric
wall (Fig. 4C). The injection
solution was prepared with 200 ml Glyceol (glycerin 10%, fructose
5%, NaCl 0.9%) and 1 ml of indigo carmine dye. The solution was
endoscopically injected into the submucosal layer adjacent to the
lesion. The circumferential seromuscular incision was made down to
the submucosa strata, which had been stained with indigo carmine
dye (Fig. 4D-F). The seromuscular
incision was continuously sutured using a 3-0 V-Loc™ (VLOCL0804;
Medtronic) (Fig. 4G) to invert the
lesion into the gastric lumen, while an approximately lesion-sized
sponge was cut and inserted between the serosal layer of the
inverted lesion and the continuous serosal suture line (Fig. 4H). Following inversion into the
gastric lumen, images of the lesion were captured (Fig. 4I). The lesion was subsequently removed
via endoscopic circumferential mucosal incision using a dual knife,
a flexible endoscope (GIF-HQ290; Olympus Corporation) (Fig. 4J) and a VIO300D device (Erbe
Elektromedizin GmbH, Tübingen, Germany). The sponge created
traction and prevented the serosal suture from being cut during the
sequential endoscopic procedure. The detached lesion and sponge
were perorally removed using an endoscopic retrieval device (Roth
Net foreign body retriever; 00711052; US Endoscopy, Mentor, OH,
USA). The mucosal edges were closed using multiple endoscopic long
clips (HX-610-135L; Olympus Corporation) (Fig. 4K and L). An air leakage test was
performed through pooling, with normal saline, along the serosal
suture line using endoscopic and laparoscopic views (Fig. 4M). The pinhole of the hanging suture
was detected and the suture was applied to repair it (Fig. 4N and O). An air leakage test was
subsequently performed again. Following the evaluation, the
procedure was completed by removing the intestinal clip, port and
trocars, and suturing the abdominal incision.
The surgical duration between incision and the
closure of the abdominal wall was 219 min. No intraoperative or
immediate postoperative complications were detected. The estimated
blood loss was <10 ml. The extracted surgical specimen revealed
a clear margin, and gastric mucosa and serosa was present on the
tumor capsule (Fig. 5A and B). The
final pathological report diagnosed the patient with a GIST
(maximal dimension, 2.2 cm) with a 1/50 high-power field mitotic
fig. that was graded low mitotic rate according to the tumor node
metastasis histopathological staging (10), which indicated a decreased risk of
aggressive behavior (11) (Fig. 5C-F). Immunohistochemical staining
confirmed the presence of KIT proto-oncogene receptor tyrosine
kinase, the absence of S100 calcium-binding proteins and desmin,
and the diagnosis of a GIST (Fig.
5G-I). The staining protocol was as follows: Tissues were fixed
in 10% formalin for at least 8 h at room temperature prior to IHC
staining, and sliced into 3-µm thick sections. Hematoxylin and
eosin straining was performed using the Thermo Scientific™ Gemini
AS Automated Slide Stainer (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 40°C for 1 h 8 min. Primary antibodies against
CD 117 (c-kit, cat no. A4502; concentration, 10.6 g/l Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA), S100 (cat no. 790-2914;
concentration 10 µg/ml) and Desmin (cat no. 760-2513; concentration
5 µg/ml) (both from Ventana Medical Systems, Inc., Tuscon, AZ, USA)
were incubated with the tissues at 37°C for 32, 16 and 12 min,
respectively. The tissues were incubated with the secondary
antibody, an indirect, biotin-free system for detecting mouse IgG,
mouse IgM and rabbit primary antibodies (cat no. 760-500;
concentration, <50 µg/ml) at 37°C for 8 min, and visualized with
the ultraView Universal DAB Detection kit (Ventana Medical Systems,
Inc.) using Benchmark XT IHC/ISH staining module (Ventana Medical
Systems, Inc.), and examination with light microscopy at
magnifications ×10, ×100, ×400 and ×600. The CD117 antibody
indicated diffuse and strong cytoplasmic and membranous staining of
the spindle cell component, but the sample was negative for S100
and desmin, compatible with GIST (Fig.
5G-I). On the second postoperative day, the condition of the
patient was stable and the patient began an oral fluid diet. The
patient was discharged 5 days after the surgery. In a follow-up
visit 4 weeks following the surgery, the patient was healthy and
without complaint regarding oral intake, and the surgical scars had
healed without complications. From the surgical and pathological
results, the patient exhibited a good prognosis and low risk for
metastasis. The requirement for patient consent for publication of
this study was waived by the Human Research Ethics Committee of
Thammasat University.
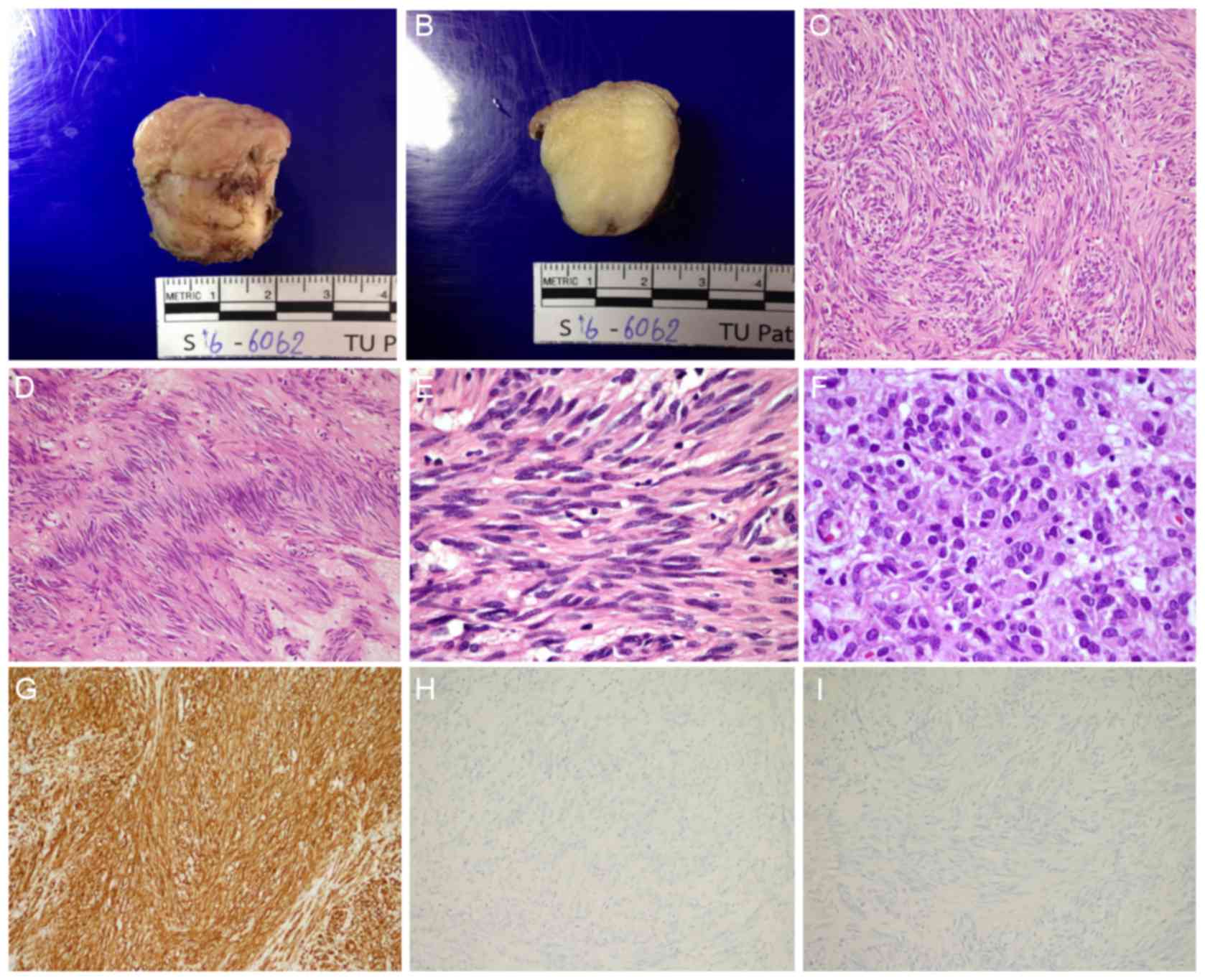 | Figure 5.Gross, histopathological and
immunohistochemical analysis of the resected specimen. (A) A
well-circumscribed, rubbery, white/tan, submucosal gastric nodule
with overlying white/tan mucosa. (B) Cut surfaces revealed a
homogeneous, fine, fibrillary, white appearance. (C) Hematoxylin
and eosin staining showed spindle cell components exhibited
intersecting and fascicular pattern (original magnification, ×100).
(D) Spindle cell components exhibited neurilemmoma-like nuclear
palisading (original magnification, ×100). (E) Spindle cell
components revealed fibrillary cytoplasm, perinuclear
vacuolization, bland elongated and wavy nuclei, fine nuclear
chromatin, and inconspicuous nucleoli (original magnification,
×600). (F) Epithelioid cell components revealed tumor cells with
distinct cell borders, eosinophilic cytoplasm, round-to-oval
nuclei, variation in nucleus size, fine chromatin and inconspicuous
nucleoli (original magnification, ×600). (G) KIT proto-oncogene
receptor tyrosine kinase demonstrated diffuse immunoreactivity
(original magnification, ×100). (H) S100 calcium-binding proteins
demonstrated a negative result (original magnification, ×100). (I)
Desmin revealed a negative result (original magnification,
×100). |
Discussion
Surgical resection with negative margins is a major
standard of care for GISTs (3).
Multiple previous studies have developed and described endoscopic
and laparoscopic approaches for treating patients with gastric
tumors that demonstrate advantages of using intraluminal and
intraperitoneal procedures during the same surgical period compared
with laparoscopic wedge resection (4–5). These
procedures increase the precision of the tumor resection margin and
minimize unnecessary cutting of the gastric wall. The major risk of
these techniques is peritoneal contamination, which may result in
infection or tumor cell seeding. NEWS is a novel technique
developed by Goto et al (6)
that consists of a minimally invasive procedure using laparoscopic
surgery and endoscopic intervention with a full-thickness resection
of the gastric wall and a decreased contamination risk. NEWS has
been used in patients with schwannoma, GISTs, granuloma, an ectopic
pancreas and neurinoma (7,12,13), and
has been combined with sentinel node basin dissection for early
stage gastric cancer (8–9).
The patient in the present study underwent NEWS for
the removal of a <3-cm diameter GIST that could be removed
perorally. For small subepithelial tumors, an endoscopic ultrasound
(EUS) was not performed since EUS may not differentiate GISTs from
other hypoechoic lesions from the fourth layer of the stomach and
EUS-fine-needle aspiration is associated with a poor diagnostic
yield. The NEWS procedure in this patient was successful, resulting
in complete removal of the tumor, with full-thickness resection of
all layers of the gastric wall without injury to tumor capsule
without complications. The pathological report revealed that the
gastric GIST and surrounding tumor capsule were completely removed
without rupture. The surgical duration (219 min) was decreased
compared with that of the longest surgery described by Goto et
al (13) (range, 171–270 min;
mean, 213.5 min), and may further decrease in future following
repeated performance and increased experience of the technique.
In the present study, the tumor was located in the
posterior wall of the upper gastric body, which is more challenging
to approach than a tumor located in the anterior wall of the upper
gastric body. The present study applied a hanging suture to
facilitate approaching the posterior wall. Using a grasper, the
hanging suture is held to permit easier access to the tumor,
although the traction and movement generated during the procedure
may result in an increase in the pinhole size. The pinhole of the
hanging suture was detected by inflating the stomach with air using
endoscopy and pooling normal saline on the gastric serosa to
generate an air bubble in the laparoscopic view. In the present
study, an additional suture was applied to repair the pinhole, and
an air leakage test was subsequently performed. The suture position
was maintained and the results of the air leakage test were
observed.
To conclude, the present study described, to the
best of our knowledge, the first published cases of minimally
invasive NEWS for treating gastric GISTs in Thailand. Further
studies are required to confirm that NEWS can be recommended as a
standard treatment for patients with small gastric GISTs.
Acknowledgements
The authors would like to thank Professor Yuko
Kitagawa (Chairman), Dr Hiroya Takeuchi and other members of the
Department of Surgery (School of Medicine, Keio University, Tokyo,
Japan), Professor Naohisa Yahagi, Dr Osamu Goto and other staff of
the Division of Research and Development for Minimally Invasive
Treatment (Cancer Center, Keio University, Tokyo, Japan) for their
technical assistance with the NEWS procedure, and Ryan St. Clair
for assistance in editing the English version of the original
manuscript.
References
|
1
|
Rubin BP, Henrick MC and Corless CL:
Gastrointestinal stromal tumour. Lancet. 369:1731–1741. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Søreide K, Sanvika OA, Søreide JA,
Giljacac V, Jureckovad A and Bulusue VR: Global epidemiology of
gastrointestinal stromal tumours (GIST): A systematic review of
population-based cohort studies. Cancer Epidemiol. 40:39–46. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishida T, Blay JY, Hirota S, Kitagawa Y
and Kang YK: The standard diagnosis, treatment, and follow-up of
gastrointestinal stromal tumors based on guidelines. Gastric
Cancer. 19:3–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Demetri GD, von Mehren M, Antonescu CR,
DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF,
Schuetze S, et al: NCCN task force report: Update on the management
of patients with gastrointestinal stromal tumors. J Natl Compr Canc
Netw. 8 Suppl 2:S1–S44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maehata T, Goto O, Takeuchi H, Kitagawa Y
and Yahagi N: Cutting edge of endoscopic full-thickness resection
for gastric tumor. World J Gastrointest Endosc. 7:1208–1215.
2015.PubMed/NCBI
|
|
6
|
Goto O, Mitsui T, Fujishiro M, Wada I,
Shimizu N, Seto Y and Koike K: New method of endoscopic
full-thickness resection: A pilot study of non-exposed endoscopic
wall-inversion surgery in an ex vivo porcine model. Gastric Cancer.
14:183–187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitsui T, Nimi K, Yamashita H, Goto O,
Aikou S, Hatao F, Wada I, Shimizu N, Fujishiro M, Koike K and Seto
Y: Non-exposed endoscopic wall-inversion surgery as a novel partial
gastrectomy technique. Gastric Cancer. 17:594–599. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goto O, Takeuchi H, Kawakubo H, Matsuda S,
Kato F, Sasaki M, Fujimoto A, Ochiai Y, Horii J, Uraoka T, et al:
Feasibility of non-exposed endoscopic wall-inversion surgery with
sentinel node basin dissection as a new surgical method for early
gastric cancer: A porcine survival study. Gastric Cancer.
18:440–445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goto O, Takeuchi H, Kawakubo H, Sasaki M,
Matsuda T, Matsuda S, Kigasawa Y, Kadota Y, Fujimoto A, Ochiai Y,
et al: First case of non-exposed endoscopic wall-inversion surgery
with sentinel node basin dissection for early gastric cancer.
Gastric Cancer. 18:434–439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO Classification of Tumours of the Digestive System.
4th. IARC Press; Lyon, France: pp. 4172010
|
|
11
|
Miettinen M, Fletcher CDM, Kindblom LG and
Tsui WMS: Mesenchymal tumours of the stomachWHO Classification of
Tumours of the Digestive System. Bosman FT, Carneiro F, Hruban RH
and Theise ND: 4th. IARC Press; Lyon, France: pp. 74–75. 2010
|
|
12
|
Kim DW, Kim JS, Kim BW, Jung JY, Kim GJ
and Kim JJ: Non-exposed endoscopic wall-inversion surgery for
gastrointestinal stromal tumor of the stomach: First case report in
Korea. Clin Endosc. 49:475–478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goto O, Takeuchi H, Sasaki M, Kawakubo H,
Akimoto T, Fujimoto A, Ochiai Y, Maehata T, Nishizawa T, Kitagawa Y
and Yahagi N: Laparoscopy-assisted endoscopic full-thickness
resection of gastric subepithelial tumors using a nonexposure
technique. Endoscopy. 48:1010–1015. 2016. View Article : Google Scholar : PubMed/NCBI
|