Introduction
Cancer is the second leading cause of death
worldwide, accounting for 24% of total mortalities (1). Approximately 90% of cancer-associated
mortalities are caused by local invasion and distant metastasis of
tumor cells; the prognosis of patients with advanced cancer is
associated with the degree of aggressive metastasis (2–4). However,
the mechanism underlying metastasis of cancer remained unclear,
until certain genes associated with metastasis were identified in a
previous study (5). A previous study
suggested that the epithelial-mesenchymal transition (EMT), an
important morphological event in which polarized epithelial cells
convert to contractile and motile mesenchymal cells, is recognized
as an important process during metastasis (6). EMT induced the generation of cancer
cells with stem cell-like characteristics, including increasing
their self-renewal, tumor-initiating capabilities and resistance to
apoptosis and chemotherapy, which promoted tumor cell invasion and
metastasis (7). During the EMT of
cancer cells in situ, epithelial cell layers lose their
polarity and cell-cell contacts, undergoing a remodeling of the
cytoskeleton (8). The expression
levels of proteins, including E-cadherins, that promote cell-cell
contact may be lost, enhancing their capacity for cell migration
and invasion, which are pivotal events in the initial stage of
metastasis (9,10). Therefore, previous studies have
investigated the inhibition of EMT of cancer cells as a novel
therapeutic target for cancer metastasis (11,12).
Hispolon
[6-(3,4-dihydroxyphenyl)-4-hydroxyhexa-3,5-dien-2-one] (HPL), one
of the bioactive components isolated from Phellinus linteus,
has been reported to possess antioxidant, anti-inflammatory and
anti-proliferative properties and to exert protective effects
against acute liver damage (13–15). In
addition, HPL has demonstrated antitumor effects in various types
of cancer cells, including melanoma, leukemia, hepatocarcinoma,
bladder cancer and gastric cancer cells (16–20).
The present study hypothesized that HPL is an
effective inhibitor of EMT during cancer progression, and may
therefore be used as an agent for epithelial tumors. It was
revealed in the present study that HPL significantly inhibited the
invasion and migration of MCF-7 and A549 human epithelial cancer
cells during TGF-β-induced EMT, by co-regulating the
TGF-β-Snail/Twist signaling axis. Therefore, it was suggested that
HPL may be an effective candidate agent for use against tumors due
to its inhibition of metastasis.
Materials and methods
Cell culture and reagents
MCF-7 and A549 cells (American Type Culture
Collection, Manassas, VA, USA) were maintained in Dulbecco's
modified Eagle's medium (DMEM, HyClone, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS, HyClone) and 1%
penicillin/streptomycin antibiotics. HPL was purchased from Santa
Cruz Biotechnology (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). The antibody for β-actin was supplied by Santa Cruz
Biotechnology, Inc., Snail antibody was purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA) and Twist antibody
was obtained from Abcam (Cambridge, MA, USA).
Cell proliferation assay
All proliferation assays were based on the MTT
method. Cells were seeded in a 96-well plate (1×104
cells/well). Following overnight culture, HPL was added to the
cells and further cultured for 24 h at 37°C. Cells cultured without
HPL were used as a control. The media was removed and dimethyl
sulfoxide was added to the MTT (Sigma-Aldrich; Merck KGaG,
Darmstadt, Germany) solubilization solution. Absorbance was
measured at 550 nm. For the colony formation assay, single-cell
suspensions of 5×103 cells were seeded onto a 6-well
plate and allowed to attach for 24 h at 37°C in culture medium.
Cells were then treated with 20 or 200 µM HPL at 37°C. Medium
containing HPL was refreshed every two days. Following 10 days,
colonies were fixed with 100% methanol for 10 min and stained with
0.1% crystal violet at room temperature. Plates were washed with
PBS and imaged.
Cell migration assay
Migration was assessed by a wound-healing assay.
MCF-7 and A549 cells were seeded at 2×104 cells/well and
were cultured for 24 h. Following scraping the cell monolayer with
a sterile micropipette tip, the wells were washed with PBS, and
treated with TGF-β (10 ng/ml, R&D Systems, Inc., Minneapolis,
MN, USA) or co-treated with TGF-β (10 ng/ml) and HPL (20 µM) for 24
h at 37°C. The first image of each scratch was acquired at time
zero. At 24 h, each scratch was examined and captured at the same
location and the healed area was determined. All captured images
were obtained by using a light microscope (Eclipse, Ti-S, Nikon
Instruments Inc., NY, USA).
Transwell invasion assay
The invasion of tumor cells was assessed in
Transwell chambers (Corning Incorporated, Corning, NY, USA) with 8
µm pore size, 6.5 mm diameter polyvinylpyrrolidone-free
polycarbonated membranes that were coated with 1 mg/ml fibronectin
(R&D Systems, Inc.). The cells were seeded onto the upper wells
at a concentration 1×105 MCF-7 and A549 cells/well and
were cultured for 24 h at 37°C following treatment with TGF-β (10
ng/ml) or co-treatment with TGF-β (10 ng/ml) and HPL (20 µM). The
bottom chambers of the Transwell were filled with conditioned
medium, DMEM. Following incubation for 24 h, cells were fixed with
100% methanol for 10 min, stained with 0.1% crystal violet for 5
min at room temperature and counted under a light microscope.
Western blotting
MCF-7 and A549 cells were treated with TGF-β (10
ng/ml) or co-treated with TGF-β (10 ng/ml) and HPL (20 µM) for 24 h
at 37°C. Following lysis in radioimmunoprecipitation assay buffer
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), proteins
quantified with BCA protein assay kit (Thermo Fisher Scientific,
Inc.) were resolved by 10% SDS-PAGE gel and immunoblotted with
Immobilon-P transfer membrane (EMD Millipore, Billerica, MA, USA)
using primary antibodies including anti-E-cadherin (1:1,000;
catalog no. ab184633; Abcam, Cambridge, UK), anti-Snail (1:1,000;
Cell Signaling Technology, Inc., catalog no. 3895), anti-Twist
(1:1,000; catalog no. ab175430; Abcam) and anti-β-actin (1:1,000;
catalog no. sc47778; Santa Cruz Biotechnology, Inc.) for 2 h at
room temperature. Following treatment with secondary antibodies,
goat anti-mouse IgG (1:2,000; catalog no. sc2005; Santa Cruz
Biotechnology, Inc.) for 2 h at room temperature, the
immunoreactive bands were visualized using the standard enhanced
chemiluminescence method (SuperSignal Est Pico; Thermo Scientific,
Inc.).
Immunofluorescence staining
MCF-7 and A549 cells were grown in 4-chamber slides
in serum-free media, and were treated with TGF-β (10 ng/ml) or
co-treated with TGF-β (10 ng/ml) and HPL (20 µM) at 37°C. Following
24 h, the cells were fixed with 4% paraformaldehyde for 15 min at
4°C. Cells were washed with PBS containing 0.1% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) and incubated with
anti-E-cadherin antibody (1:100; catalog no. ab184633; Santa Cruz
Biotechnology, Inc.) for 1 h followed by 1 h incubation with
FITC-tagged goat anti-mouse IgG (1:200; catalog no. sc2010; Santa
Cruz Biotechnology, Inc.), then counter-stained with DAPI for 5
min. All staining were procedures performed at room temperature.
Cell images were captured at ×400 magnification on a Leica
fluorescence microscope.
Statistical analysis
The results are presented as the mean ± standard
error, and statistical comparisons between groups were performed
using the Student's t-test using SigmaPlot (version 10.0; Systat
Software, Inc., San Jose, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of HPL on the growth of human
cancer cells in vitro
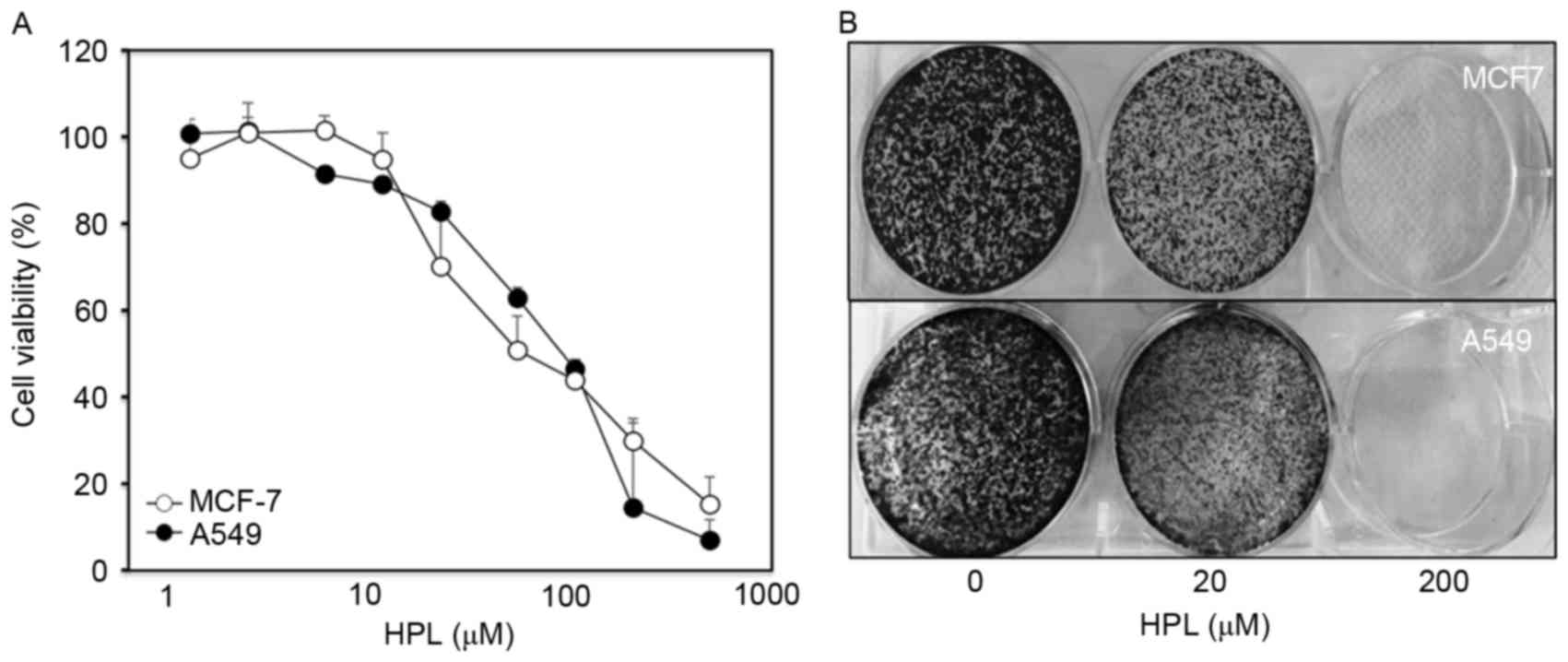
The present study initially examined the effect of
HPL on the proliferation of the MCF-7 and A549 human cancer cell
lines. To determine the drug concentration that induced 50% growth
inhibition (IC50), cells were treated with various
concentrations of HPL (1, 2, 5, 10, 20, 50, 100, 200 and 500 mM)
for 24 h and cell viability was evaluated by MTT assay. As
presented in Fig. 1A, IC50
values for both cell types were similar (~65 µM). The long-term
effects of HPL were determined by culturing MCF-7 and A549 cells
with or without HPL for 10 days and then performing colony
formation assays. At a concentration of 20 µM, HPL demonstrated a
slight inhibitory effect, whereas 200 µM HPL almost completely
inhibited colony formation (Fig. 1B).
Therefore, 20 µM HPL was considered to be a suitable dose for
subsequent experiments.
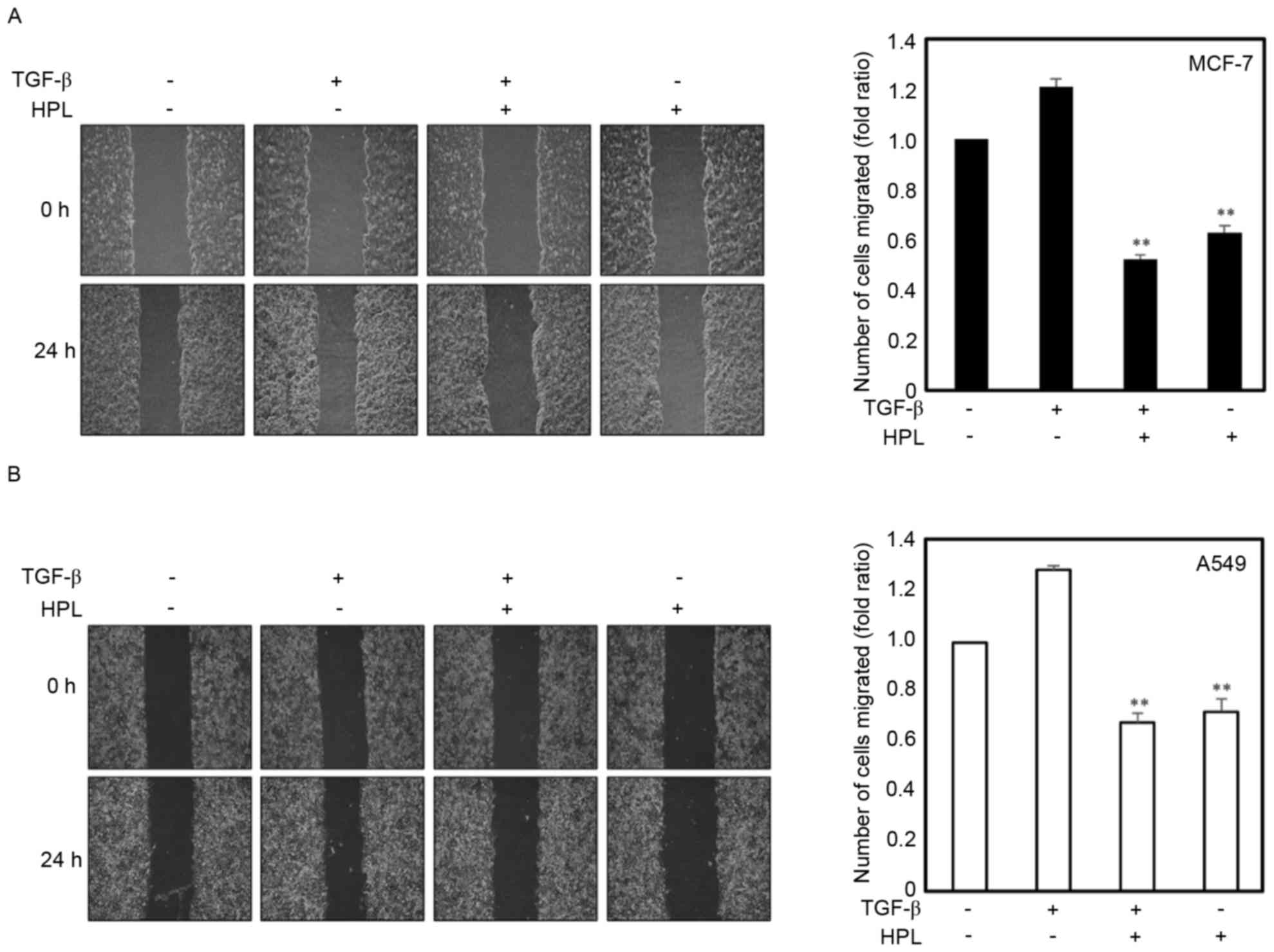
Effect of HPL on TGF-β-induced
migration of human cancer cells
TGF-β (10 ng/ml) may function as a pro-oncogenic
factor through the induction of the EMT process, as previously
reported (21). The present study
investigated the effects of HPL on cell migration to demonstrate
that HPL inhibited TGF-β-induced EMT as EMT is associated with
enhanced tumor progression. Cancer cell lines were treated with
TGF-β alone, TGF-β plus HPL (20 µM) or HPL alone (20 µM), and
wound-healing assays were performed. The TGF-β-treated cancer cells
exhibited a ≥1.2-fold increase in migration, whereas treatment with
20 µM HPL inhibited this TGF-β-induced migration by 45% for MCF-7
and 50% for A549 cells (Fig. 2A and
B). The inhibition of migration was also observed in the HPL
alone treatment group, HPL decreased the migration by 60% for MCF-7
and 65% for A549 cells compared with the untreated control group.
These results revealed that HPL inhibited the migration of cancer
cells during EMT induced by TGF-β.
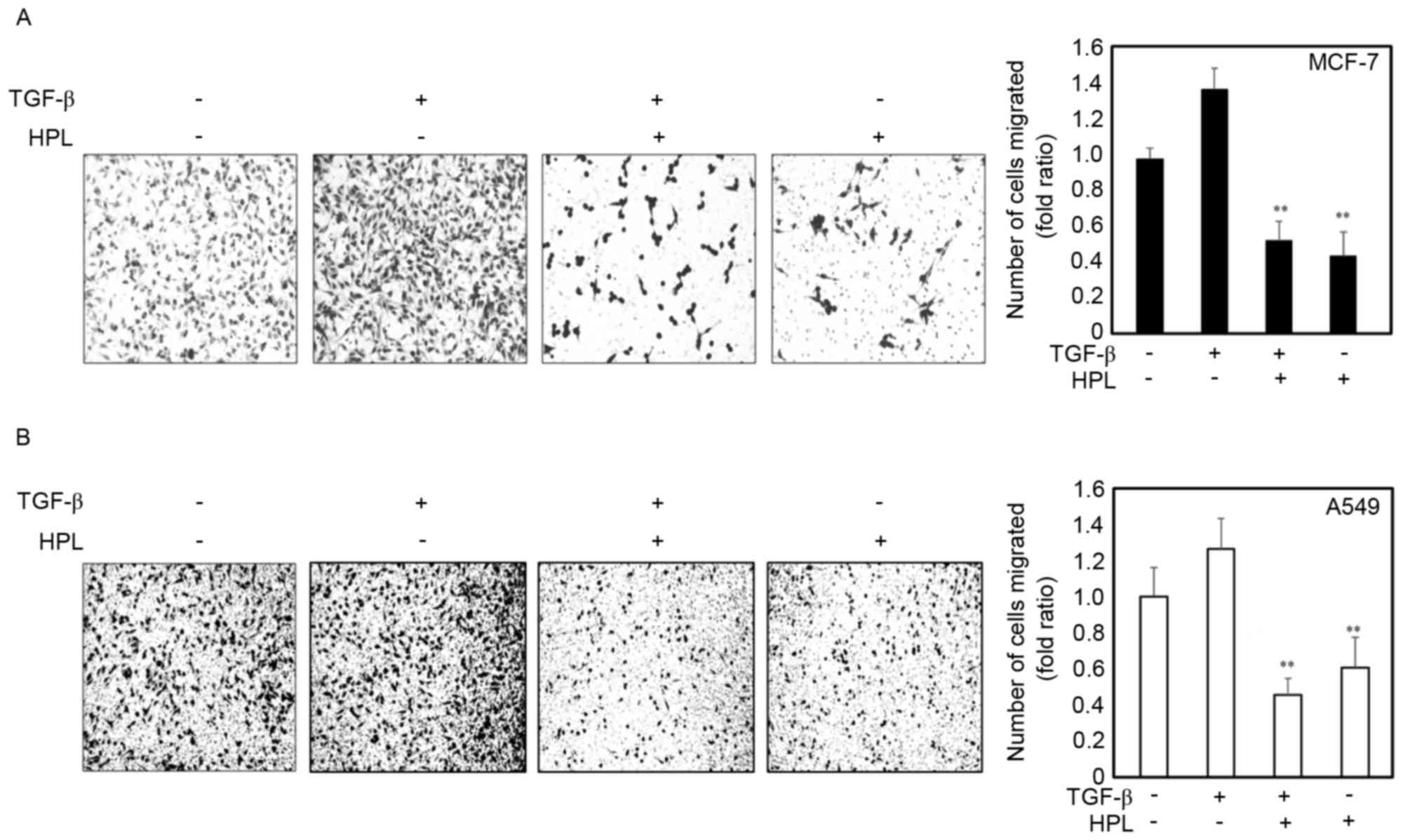
Effect of HPL on the TGF-β-induced
invasion of human cancer cells
The present study next investigated whether HPL
inhibited the TGF-β-induced invasiveness of cancer cells. Following
treatment with TGF-β alone, the number of invasive cells
significantly increased compared with the untreated cells. However,
the number of invasive cells was significantly reduced in the cells
treated with the combination of TGF-β plus HPL (Fig. 3). The quantitative analysis is
presented in Fig. 3. HPL
significantly inhibited TGF-β-induced invasion of cancer cells by
50% for MCF-7 and 40% for A549 cells, compared with the untreated
control group. These results suggested that HPL inhibits the effect
of TGF-β, increasing the invasiveness of human cancer cells, as
occurs during the EMT.
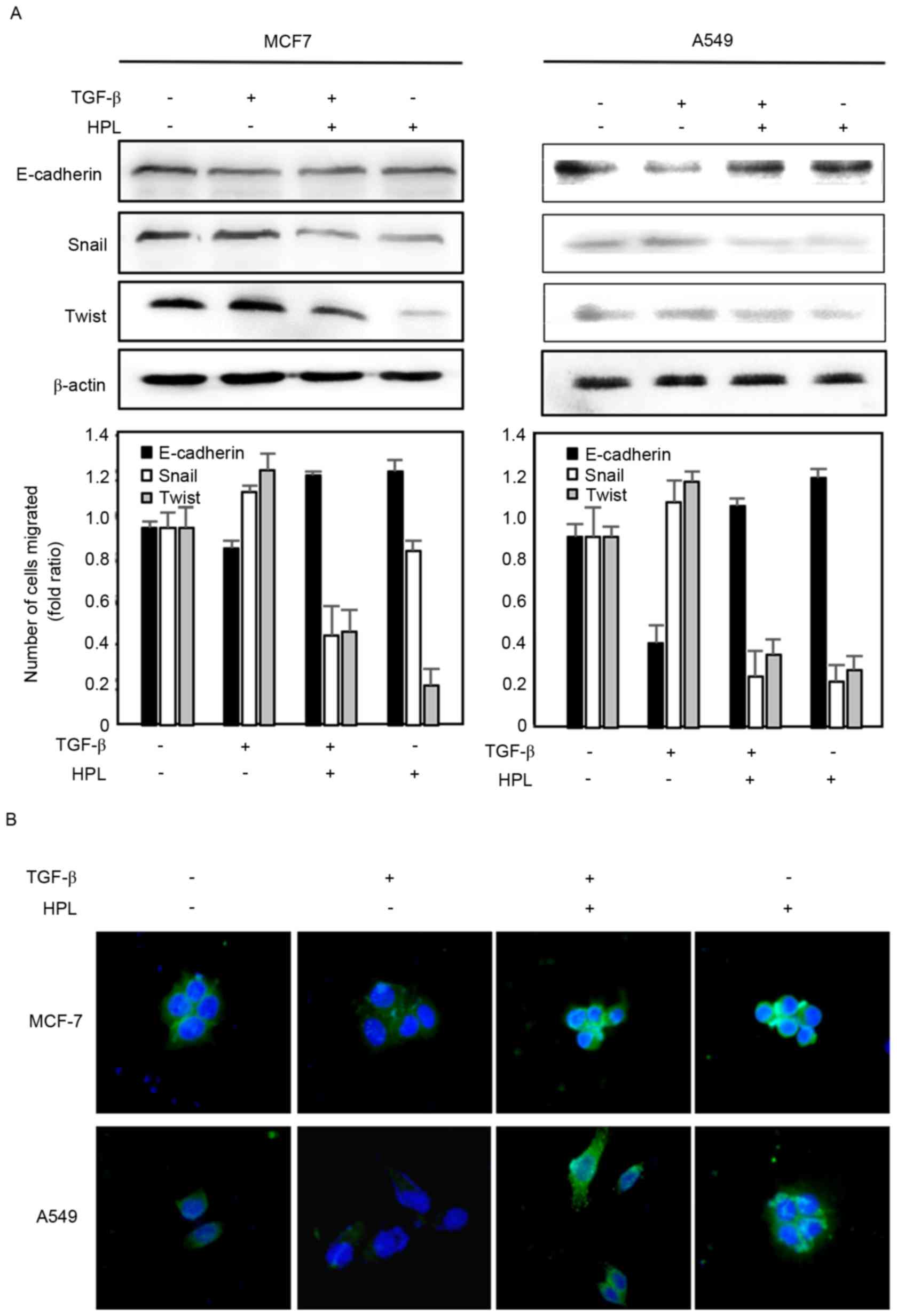
Effects of HPL on the expression level
of E-cadherin
To further investigate the effect of HPL on
TGF-β-induced EMT, the present study evaluated the expression
levels of the EMT-associated protein, E-cadherin, by western
blotting (Fig. 4A). The expression of
E-cadherin was downregulated in the TGF-β-treated group compared
with the controls. However, HPL reversed the TGF-β-induced EMT by
reducing E-cadherin expression levels. The present study also
determined the E-cadherin expression level in cancer cells by
immunofluorescence (Fig. 4B).
Consistent with the western blotting results, in the two cell
types, E-cadherin was seldom expressed following TGF-β treatment,
but was significantly recovered by co-treatment with HPL. Taken
together, the western blotting and fluorescence imaging results
suggested that HPL has an inhibitory effect on EMT.
TGF-β-Snail/Twist signaling axis for
reversal of TGF-β-induced EMT
Numerous previous studies have reported that drugs
may inhibit the invasion and migration of cancer cells by
suppressing TGF-β activation and Snail/Twist induction, which
results in recovering E-cadherin expression (22,23). It
was suggested that the TGF-β signaling pathway is critically
involved in the acquisition of EMT by its downstream target, the
transcription factor Snail and Twist (24). The present study investigated the
expression levels of the Snail and Twist proteins by western
blotting to determine whether the effect of HPL described above is
associated with the inhibition of the TGF-β-Snail/Twist axis. As
presented in Fig. 4A, TGF-β
significantly upregulated the expression levels of Snail and Twist
proteins, which reduced the expression level of E-cadherin. These
effects were reduced by HPL, suggesting that HPL suppressed
TGF-β-induced EMT by co-regulating Snail and Twist.
Discussion
The EMT is the most established example of the
changes that occur in the patterns and functions of cancer cells
(25). During the EMT, epithelial
cells acquire mesenchymal features including increased motility,
invasiveness and a heightened resistance to apoptosis, instead of
losing their differentiated characteristics including cell-cell
adhesion and apical-basal polarity (26). These alterations, particularly the
reduction in intercellular adhesion and increase in motility,
result in metastasis, enabling these cells to break through the
basal membrane and migrate over long distances (27). In addition, EMT is considered to be an
important process in the invasive cascade, facilitating the
migration of tumor cells from their site of origin and their
dissemination to distant tissues (28). As EMT serves a role in enhancing the
invasive and metastatic behavior of cancer cells, inhibition of EMT
is a suitable strategy for cancer chemotherapy, particularly
metastasis.
As previously reported, TGF-β induces EMT in various
types of cancer cells, increasing their invasion and migration and
resulting in enhanced metastasis (22,23,29). The
present study demonstrated that MCF-7 and A549 human cancer cells
may be induced by TGF-β to undergo a stimulated EMT, reducing
E-cadherin expression level in cancer cells and increasing their
invasiveness and migration. HPL inhibited the action of TGF-β in
inducing the EMT, reversing the altered expression level of
proteins associated with cell invasion and migration. The present
study also revealed that Snail/Twist signaling may be required for
TGF-β-induced EMT in cancer cells, which further elucidates the
mechanism underlying HPL inhibition of cancer cell metastasis.
HPL, derived from Phellinus linteus, is known
for its anticancer properties, particularly with breast cancer
modulating estrogen receptor α, as previously reported (30). However, HPL has not been associated
with cancer metastasis via the EMT, although its strong antitumor
effects have been reported (31). To
the best of our knowledge, this is the first study to demonstrate
that the anti-metastatic effects of HPL are associated with the EMT
in cultured human cancer cells. Therefore, the results of the
present study suggested a novel anticancer activity for HPL in
inhibiting the progression of cancer metastasis.
The present study demonstrated that HPL inhibited
the TGF-β-induced EMT, and thus cell migration and invasion, which
result from the dysregulation of cell-cell adhesion proteins and
the expression levels of E-cadherin, an EMT-associated protein.
E-cadherin is expressed by the majority of epithelial tissues,
facilitates tight cell-cell adhesion and suppresses the
dissociation of epithelial cells from their locations. The loss of
E-cadherin expression correlates with the invasiveness and
undifferentiated phenotype of numerous epithelium-derived cancer
cells (32). Therefore, the loss of
E-cadherin expression in cancer cells has functional significance
in cancer progression and metastasis.
The results of the present study also revealed that
the mechanism underlying HPL may involve suppression of
TGF-β-Snail/Twist signaling axis. The changes in gene expression
that contributed to the repression of the epithelial phenotype and
activation of the mesenchymal phenotype involves the regulators
Snail and Twist (33). Induction of
Snail expression has been noted in all EMT processes that have been
previously studied, and increased Snail levels have been correlated
with more invasive tumor types (34–36). Snail
regulated the expression level of epithelial or mesenchymal genes
and it regulated the expression level of E-cadherin, which is
downregulated during EMT (37).
Twist, a helix-loop-helix protein, is a major regulator of mesoderm
formation in Drosophila and neural tube closure in mice,
suggesting its involvement in developmental EMT (38). Twist decreases E-cadherin expression
levels and enhances cell migration and invasion (39,40). The
results of the present study support these previous findings and
provided a mechanistic basis for the inhibition of tumor
progression by HPL.
In conclusion, the present study demonstrated that
HPL inhibition of tumor invasion and migration is associated with
the EMT process during tumor progression, and is possibly mediated
by suppression of the TGF-β-Snail/Twist signaling axis and
regulating the expression level of E-cadherin, an important
downstream EMT marker. Although further in vivo studies are
required to establish the potential of HPL as a therapeutic agent,
the present study suggested that HPL is an effective anticancer
agent with inhibition of metastatic activity against epithelial
tumors.
Acknowledgements
The present study was supported by the Basic Science
Research Program of the National Research Foundation of Korea (NRF)
funded by the Ministry of Education (grant no. 2014R1A1A2057861)
and the Ministry of Science, ICT & Future Planning (grant no.
NRF-2013R1A1A1062292).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sleeman JP, Nazarenko I and Thiele W: Do
all roads lead to Rome? Routes to metastasis development. Int J
Cancer. 128:2511–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y and Zhou BP: Epithelial-mesenchymal
transition-A hallmark of breast cancer metastasis. Cancer Hallm.
1:38–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bogenrieder T and Herlyn M: Axis of evil:
Molecular mechanisms of cancer metastasis. Oncogene. 22:6524–6536.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kong D, Li Y, Wang Z and Sarkar FH: Cancer
stem cells and epithelial-to-mesenchymal transition
(EMT)-phenotypic cells: Are they cousins or twins? Cancers (Basel).
3:716–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Davis FM, Stewart TA, Thompson EW and
Monteith GR: Targeting EMT in cancer: Opportunities for
pharmacological intervention. Trends Pharmacol Sci. 35:479–488.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ginnebaugh KR, Ahmad A and Sarkar FH: The
therapeutic potential of targeting the epithelial-mesenchymal
transition in cancer. Expert Opin Ther Targets. 18:731–745. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ali NA, Lüdtke J, Pilgrim H and Lindequist
U: Inhibition of chemiluminescence response of human mononuclear
cells and suppression of mitogen-induced proliferation of spleen
lymphocytes of mice by hispolon and hispidin. Pharmazie.
51:667–670. 1996.PubMed/NCBI
|
|
14
|
Huang GJ, Deng JS, Chiu CS, Liao JC, Hsieh
WT, Sheu MJ and Wu CH: Hispolon protects against acute liver damage
in the rat by inhibiting lipid peroxidation, proinflammatory
cytokine and oxidative stress and downregulating the expressions of
iNOS, COX-2 and MMP-9. Evid Based Complement Alternat Med.
2012:4807142012.PubMed/NCBI
|
|
15
|
Yang LY, Shen SC, Cheng KT, Subbaraju GV,
Chien CC and Chen YC: Hispolon inhibition of inflammatory apoptosis
through reduction of iNOS/NO production via HO-1 induction in
macrophages. J Ethnopharmacol. 156:61–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen W, Zhao Z, Li L, Wu B, Chen SF, Zhou
H, Wang Y and Li YQ: Hispolon induces apoptosis in human gastric
cancer cells through a ROS-mediated mitochondrial pathway. Free
Radic Biol Med. 45:60–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YC, Chang HY, Deng JS, Chen JJ, Huang
SS, Lin IH, Kuo WL, Chao W and Huang GJ: Hispolon from Phellinus
linteus induces G0/G1 cell cycle arrest and apoptosis in NB4
human leukaemia cells. Am J Chin Med. 41:1439–1457. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen YS, Lee SM, Lin CC and Liu CY:
Hispolon decreases melanin production and induces apoptosis in
melanoma cells through the downregulation of tyrosinase and
microphthalmia-associated transcription factor (MITF) expressions
and the activation of caspase-3, −8 and −9. Int J Mol Sci.
15:1201–1215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang GJ, Deng JS, Huang SS and Hu ML:
Hispolon induces apoptosis and cell cycle arrest of human
hepatocellular carcinoma Hep3B cells by modulating ERK
phosphorylation. J Agric Food Chem. 59:7104–7113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu TL, Huang GJ, Lu TJ, Wu JB, Wu CH, Yang
TC, Iizuka A and Chen YF: Hispolon from Phellinus linteus
has antiproliferative effects via MDM2-recruited ERK1/2 activity in
breast and bladder cancer cells. Food Chem Toxicol. 47:2013–2021.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Derynck R, Muthusamy BP and Saeteurn KY:
Signaling pathway cooperation in TGF-β-induced
epithelial-mesenchymal transition. Curr Opin Cell Biol. 31:56–66.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Drabsch Y and ten Dijke P: TGF-β
signalling and its role in cancer progression and metastasis.
Cancer Metastasis Rev. 31:553–568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fabregat I, Fernando J, Mainez J and
Sancho P: TGF-beta signaling in cancer treatment. Curr Pharm Des.
20:2934–2947. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zavadil J and Böttinger EP: TGF-beta and
epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mulholland DJ, Kobayashi N, Ruscetti M,
Zhi A, Tran LM, Huang J, Gleave M and Wu H: Pten loss and RAS/MAPK
activation cooperate to promote EMT and metastasis initiated from
prostate cancer stem/progenitor cells. Cancer research.
72:1878–1889. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Principe DR, Doll JA, Bauer J, Jung B,
Munshi HG, Bartholin L, Pasche B, Lee C and Grippo PJ: TGF-β:
Duality of function between tumor prevention and carcinogenesis. J
Natl Cancer Inst. 106:djt3692014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jang EH, Jang SY, Cho IH, Hong D, Jung B,
Park MJ and Kim JH: Hispolon inhibits the growth of estrogen
receptor positive human breast cancer cells through modulation of
estrogen receptor alpha. Biochem Biophys Res Commun. 463:917–922.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim JH, Kim YC and Park B: Hispolon from
Phellinus linteus induces apoptosis and sensitizes human
cancer cells to the tumor necrosis factor-related
apoptosis-inducing ligand through upregulation of death receptors.
Oncol Rep. 35:1020–1026. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Araki K, Shimura T, Suzuki H, Tsutsumi S,
Wada W, Yajima T, Kobayahi T, Kubo N and Kuwano H: E/N-cadherin
switch mediates cancer progression via TGF-β-induced
epithelial-to-mesenchymal transition in extrahepatic
cholangiocarcinoma. Br J Cancer. 105:1885–1893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Z, Zhang L, Ma Z, Yang M, Tang J, Fu Y,
Mao Y, Hong X and Zhang Y: ETV1 induces epithelial to mesenchymal
transition in human gastric cancer cells through the upregulation
of Snail expression. Oncol Rep. 30:2859–2863. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Masui T, Ota I, Yook JI, Mikami S, Yane K,
Yamanaka T and Hosoi H: Snail-induced epithelial-mesenchymal
transition promotes cancer stem cell-like phenotype in head and
neck cancer cells. Int J Oncol. 44:693–699. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and De Herreros García A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang
SY, Liu CJ, Teng SC and Wu KJ: Direct regulation of TWIST by
HIF-1alpha promotes metastasis. Nat Cell Biol. 10:295–305. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen ZF and Behringer RR: Twist is
required in head mesenchyme for cranial neural tube morphogenesis.
Genes Dev. 9:686–699. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|