Introduction
Squamous cell carcinoma (SCC) is the most common
type of esophageal cancer, followed by adenocarcinoma; these two
histological types account for >90% of primary esophageal cancer
cases (1–5). Adenocarcinoma arises chiefly in Barrett
mucosa and rarely from ectopic gastric mucosa or esophageal glands
(2). However, focal
adenocarcinomatous differentiation occurs in ~20% of cases of
esophageal invasive SCC (1,6). When the adenocarcinomatous features
occupy considerable amounts of the esophageal SCC, tumors are
classified as either adenosquamous carcinoma or mucoepidermoid
carcinoma (1,2,7–11). Adenocarcinomatous differentiation in
SCC should be distinguished from acantholytic SCC (also called
pseudoglandular or adenoid SCC) (12–19). Here
was encountered a unique autopsy case of esophageal adenosquamous
carcinoma mimicking acantholytic SCC, and herein is describe the
clinicopathological findings of this case.
Case report
A 53-year-old Japanese male was hospitalized firstly
to Japan Self-Defense Force Hanshin Hospital (Hyogo, Japan) on
April 1, 2011, and subsequently to Japan Self-Defense Forces
Central Hospital (Tokyo, Japan) on April 14, 2011, for evaluation
of fever and hoarseness. Laboratory examination revealed increased
serum levels of C-reactive protein and carcinoembryonic antigen
(CEA). Endoscopic examination disclosed an ulcerating esophageal
tumor. The biopsy specimen exhibited features of moderate-poorly
differentiated SCC. A computed tomography scan confirmed the
presence of an esophageal tumor and revealed possible metastatic
nodules in the liver and mediastinal and intra-abdominal lymph
nodes (Fig. 1). The fever and
hoarseness were considered to be caused by tumor necrosis and lymph
node metastasis-associated recurrent nerve palsy, respectively. The
patient rapidly deteriorated and succumbed to respiratory
insufficiency one month following first hospitalization.
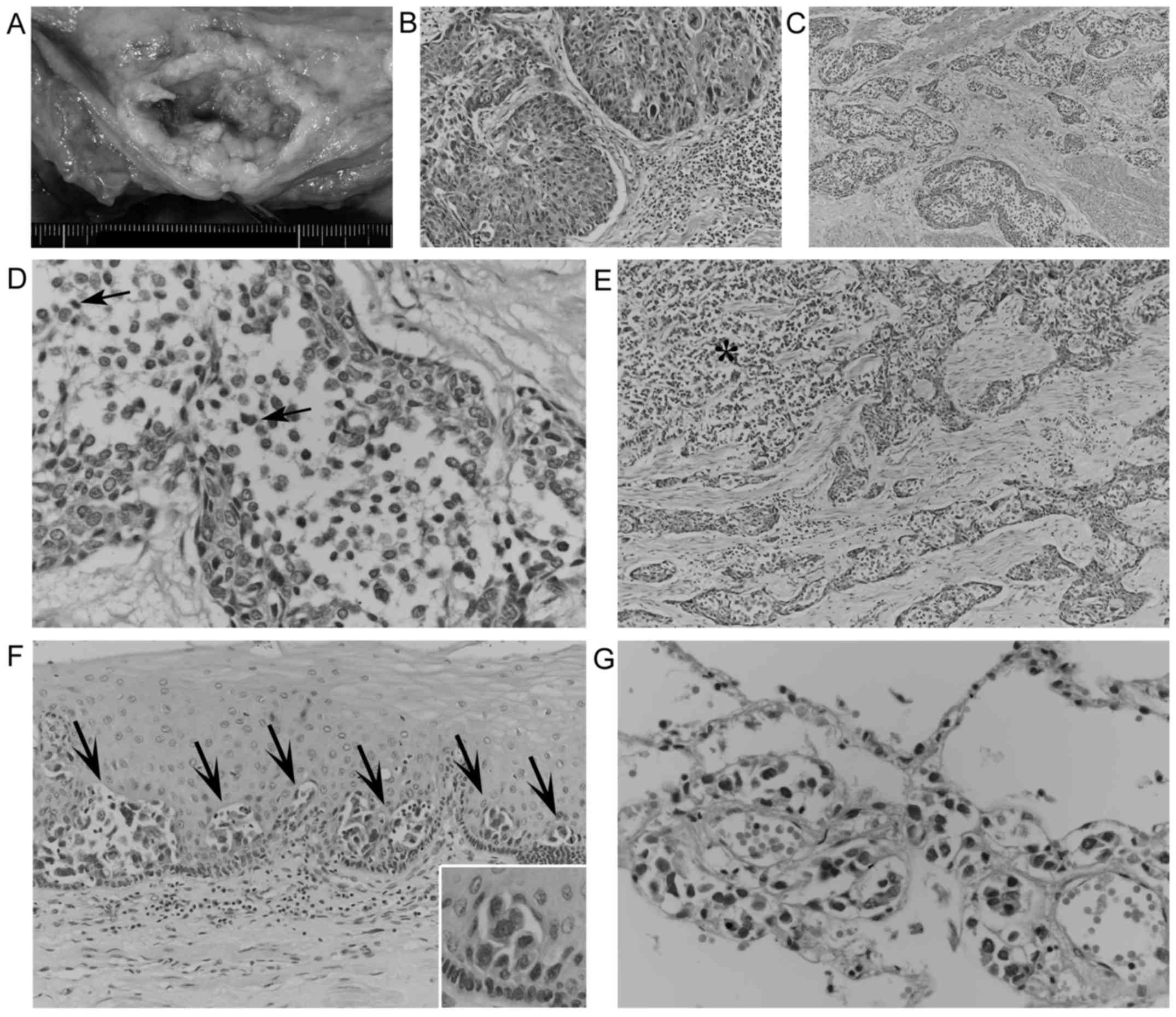
Autopsy revealed a 10-cm ulcerating esophageal tumor
(Fig. 2A) directly invading the
trachea. Microscopically, this tumor partially exhibited nested
proliferation composed of polygonal and/or short spindle cells
containing swollen pleomorphic nuclei without distinct
keratinization (Fig. 2B). These
features indicate a diagnosis of poor-moderately differentiated
SCC. However, invading cancerous nests frequently exhibited
geographic acantholysis-like changes (Fig. 2C), chiefly composed of poorly
cohesive, monomorphic neoplastic cells with relatively scant
cytoplasm. These acantholytic areas were slightly demarcated,
occasionally contained necrotic cells (Fig. 2D), and lacked columnar cells,
intermediate cells and neoplastic cells forming tubules or
intracytoplasmic lumina. These discohesive cancer cells also
invaded in an alveolar fashion (Fig.
2E), reminiscent of micropapillary carcinoma, and focally
involved the adjacent esophageal epithelium in a pagetoid manner
(Fig. 2F). In total, these
discohesive cancer cells comprised ~60% of the tumor volume, and
had widely metastasized to the lungs, chest wall, liver, spleen,
right adrenal gland, vertebral bones and generalized lymph nodes
with multifocal necrosis. Notably, in both lungs, metastatic
discohesive cells exhibited prominent lymphangiosis (Fig. 2G), which would have contributed to the
respiratory failure and causing the patient's mortality. Metastatic
SCC cells were not observed.
In the primary esophageal tumor, SCC cells were
negative for mucin staining and CEA and were weakly positive for
keratin 5/6 (K5/6); scattered positivity for epithelial membrane
antigen (EMA) in SCC cells was also observed. By contrast,
discohesive cancer cells commonly expressed mucin, which was
detected by alcian blue and/or periodic acid-Schiff (PAS) staining
with or without diastase digestion (Fig.
3A and F). In addition, discohesive cancer cells exhibited
strong positivity for EMA (Fig. 3B)
and CEA (Fig. 3C), and negativity for
K5/6 (Fig. 3D). In the primary tumor,
SCC cells exhibited weak cytoplasmic positivity for E-cadherin. By
contrast, discohesive cancer cells exhibited reduced or no
E-cadherin immunostaining as compared with the weak staining of the
surrounding cohesive cancer cells (Fig.
3E). In metastatic lesions, cancer cells exhibited occasional
expression of mucin, and EMA positivity, CEA positivity and K5/6
negativity were observed via immunohistochemistry (Fig. 4). The majority of metastatic cancer
cells exhibited negative or reduced E-cadherin immunostaining
(Fig. 4C), although a minority of
metastatic cancer cells were positive for E-cadherin. No positivity
for p63, α-fetoprotein, placental alkaline phosphatase or vimentin
was observed in any of the primary or metastatic cancerous
lesions.
 | Figure 3.Histochemical and immunohistochemical
findings of the primary esophageal cancer. (A-D) In the
‘pseudo’-acantholytic areas (asterisks) of the primary tumor,
discohesive cancer cells exhibit (A) alcian blue positive mucin,
and (B) strong positivity for EMA (C) and CEA, and no staining for
K5/6. By contrast, the surrounding cohesive squamoid cells exhibit
no staining for CEA (C) and weak positivity for K5/6 (D, arrows;
magnification, ×400). (E) Diminished E-cadherin staining of
discohesive cells (asterisks) in the acantholysis-like area of the
primary tumor, compared with the weak positivity of the surrounding
cohesive squamoid cells (magnification, ×400). (F) Certain cancer
cells (arrowhead) within the ‘pseudo’-acantholytic area (arrows)
have PAS stain-positive mucin (magnification, ×400). CEA,
carcinoembryonic antigen; EMA, epithelial membrane antigen; K5/6,
keratin 5/6; PAS, periodic acid-Schiff. |
Discussion
The present esophageal tumor exhibited not only
nested growth of SCC, but also frequent, loosely arranged
acantholysis-like areas, which occasionally contained necrotic
cells and lacked columnar cells and neoplastic tubules. These
features closely mimicked acantholytic SCC, which is an uncommon
but distinctive variant of SCC with dyskeratosis-associated
acantholytic changes (3,13–18).
However, in the present acantholysis-like areas, the discohesive
cancer cells exhibited alcian blue+ and/or PAS+ mucin, and were
strongly positive for EMA and CEA. No K5/6 positivity was observed
in these discohesive cells; however, K5/6 positivity was scattered
in the SCC components and cohesive cells around the
acantholysis-areas. These findings indicate that these
acantholysis-like areas represent true adenocarcinomatous
differentiation rather than acantholytic changes of SCC. The
esophageal tumor involved both these adenocarcinoma (60%) and SCC
(40%) cells, and lacked intermediate cells suggestive of
mucoepidermoid carcinoma (1,2,7).
Therefore, it was concluded that this tumor was esophageal
adenosquamous carcinoma with ‘pseudo’-acantholytic adenocarcinoma
components.
In the present case, the alveolar growth of the
discohesive adenocarcinoma cells focally resembled those of
micropapillary carcinoma in other sites (20–22).
However, the present alveolar cancer cells formed no distinctive
micropapillary tufts or aggregations with a reversed polarity,
eliminating a possible diagnosis of micropapillary carcinomatous
components. Alveolar or prominently discohesive changes of
adenocarcinoma cells have been rarely described in the lung
(23), but these cases had no SCC
components. Alwaheeb and Chetty (24)
reported a case of acantholytic adenosquamous carcinoma of the
pancreas. However, these acantholytic changes were observed in the
SCC components, and not in the adenocarcinomatous components. Lee
(19) described ‘signet ring cells’
in a case of esophageal acantholytic SCC, but these were negative
for mucin and were considered to have arisen from vacuolar changes.
The present review of the literature failed to reveal any cases of
adenosquamous carcinoma with ‘pseudo’-acantholytic adenocarcinoma
components in the esophagus or other sites.
Acantholytic changes of SCC are known to be
associated with reduced E-cadherin immunoreactivity (14,15). In
the present case, the discohesive adenocarcinoma cells exhibited
diminished or negative staining for E-cadherin despite weak
positivity in SCC cells. Furthermore, the discohesive
adenocarcinoma cells aggressively metastasized to several organs,
directly contributing to the patient's mortality, although SCC
cells were not observed in the metastatic lesions. Furthermore, the
majority of the metastatic cancer cells exhibited little or no
E-cadherin staining. These findings suggest that the loss or
alteration of E-cadherin in adenocarcinoma cells causes
‘pseudo’-acantholysis and also serves a role in aggressive
metastasis (25). Notably, the
adenocarcinoma cells in the present case focally involved the
adjacent esophageal epithelium in a pagetoid manner, which is
exceptionally rare in the esophagus (26,27).
Abraham et al (27) also noted
similar diminished E-cadherin immunostaining in esophageal Paget
cells.
In conclusion, herein is described an autopsy case
of esophageal adenosquamous carcinoma with ‘pseudo’-acantholytic
adenocarcinoma components, possibly associated with the loss of
E-cadherin. Therefore, this tumor should be considered as a rare
but aggressive type of cancer in the differential diagnoses of
esophageal neoplasms.
Glossary
Abbreviations
Abbreviations:
|
CEA
|
carcinoembryonic antigen
|
|
di-PAS
|
periodic acid-Schiff stain after
diastase digestion
|
|
K5/6
|
keratin 5/6
|
|
EMA
|
epithelial membrane antigen
|
|
H&E
|
hematoxylin and eosin
|
|
SCC
|
squamous cell carcinoma
|
References
|
1
|
Lewin KJ and Appelman HD: Tumors of the
esophagus and stomach. In: Atlas of Tumor Pathology3rd. Fascicle
18. Rosai J and Sobin LH: American Registry of Pathology;
Washington, DC: pp. 43–144. 1996
|
|
2
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO Classification of Tumours of the Digestive System.
4th. International Agency for Research on Cancer; Lyon: pp. 18–31.
2010
|
|
3
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown LM, Devesa SS and Chow WH: Incidence
of adenocarcinoma of the esophagus among white Americans by sex,
stage, and age. J Natl Cancer Inst. 100:1184–1187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang CS, Chen X and Tu S: Etiology and
prevention of esophageal cancer. Gastrointest Tumors. 3:3–16. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takubo K, Sasajima K, Yamashita K, Tanaka
Y, Fujita K, Mafune K and Wang QH: Morphological heterogeneity of
esophageal carcinoma. Acta Pathol Jpn. 39:180–189. 1989.PubMed/NCBI
|
|
7
|
Lam KY, Loke SL and Ma LT: Histochemistry
of mucin secreting components in mucoepidermoid and adenosquamous
carcinoma of the oesophagus. J Clin Pathol. 46:1011–1015. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bombí JA, Riverola A, Bordas JM and
Cardesa A: Adenosquamous carcinoma of the esophagus. A case report.
Pathol Res Pract. 187:514–521. 1991.PubMed/NCBI
|
|
9
|
Yachida S, Nakanishi Y, Shimoda T, Nimura
S, Igaki H, Tachimori Y and Kato H: Adenosquamous carcinoma of the
esophagus. Clinicopathologic study of 18 cases. Oncology.
66:218–225. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen SB, Weng HR, Wang G, Yang JS, Yang
WP, Liu DT, Chen YP and Zhang H: Primary adenosquamous carcinoma of
the esophagus. World J Gastroenterol. 19:8382–8390. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ni PZ, Yang YS, Hu WP, Wang WP, Yuan Y and
Chen LQ: Primary adenosquamous carcinoma of the esophagus: An
analysis of 39 cases. J Thorac Dis. 8:2689–2696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cardesa A, Zidar N and Alos L:
Acantholytic squamous cell carcinoma. In: WHO Classification of
TumoursPathology and Genetics of Head and Neck Tumours. Barnes L,
Eveson JW, Reichart P and Sidransky D: IARC press; Lyon: pp.
1292005
|
|
13
|
Elder DE, Elenitsas R, Rosenbach M, Murphy
GF, Rubin AI and Xu X: Lever's Histopathology of the Skin. 11th.
Wolters Kluwer; Philaderphia, PA: pp. 10032015
|
|
14
|
Zidar N, Gale N, Župevc A and Dovšak D:
Pseudovascular adenoid squamous-cell carcinoma of the oral cavity-a
report of two cases. J Clin Pathol. 59:1206–1208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu X, Jiang R and Fowler MR: Acantholytic
squamous cell carcinoma in upper aerodigestive tract:
Histopathology, immunohistochemical profile and epithelial
mesenchymal transition phenotype change. Head Neck Pathol.
6:438–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Terada T: Adenoid squamous cell carcinoma
of the oral cavity. Int J Exp Pathol. 5:442–447. 2012.
|
|
17
|
Cunha IW, Guimaraes GC, Soares F,
Velazquez E, Torres JJ, Chaux A, Ayala G and Cubilla AL:
Pseudoglandular (adenoid, acantholytic) penile squamous cell
carcinoma: A clinicopathologic and outcome study of 7 patients. Am
J Surg Pathol. 33:551–555. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shah AA, Jeffus SK and Stelow EB: Squamous
cell carcinoma variants of the upper aerodigestive tract: A
comprehensive review with a focus on genetic alterations. Arch
Pathol Lab Med. 138:731–744. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee D: Acantholytic squamous cell
carcinoma of the esophagus with prevalent single isolated tumour
cells including signet ring cells and many osteoclast-like giant
cells. Pathology. 48:281–283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luna-Moré S, Gonzalez B, Acedo C, Rodrigo
I and Luna C: Invasive micropapillary carcinoma of the breast. A
new special type of invasive mammary carcinoma. Path Res Pract.
190:668–674. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sánchez-Mora N, Presmanes MC, Monroy V,
Moreno N, Lara-Martínez JM, Aladro MH and Álvarez-Fernández E:
Micropapillary lung adenocarcinoma: A distinctive histologic
subtype with prognostic significance. Case series. Hum Pathol.
39:324–330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reis-Filho JS and Ellis IO: Invasive
micropapillary carcinoma. In: WHO Classification of Tumours of the
Breast4th. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH and van de
Vijver MJ: International Agency for Research on Cancer. Lyon: pp.
65–66. 2012
|
|
23
|
Kozu Y, Isaka M, Ohde Y and Nakajima T:
Aggressive adenocarcinoma of the lung consisting solely of
discohesive cells. J Cardiothoracic Surg. 8:892013. View Article : Google Scholar
|
|
24
|
Alwaheeb S and Chetty R: Adenosquamous
carcinoma of the pancreas with an acantholytic pattern together
with osteoclast-like and pleomorphic giant cells. J Clin Pathol.
58:987–990. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shiozaki H, Tahara H, Oka H, Miyata M,
Kobayashi K, Tamura S, Iihara K, Doki Y, Hirano S, Takeichi M, et
al: Expression of immunoreactive E-cadherin adhesion molecules in
human cancers. Am J Pathol. 139:17–23. 1991.PubMed/NCBI
|
|
26
|
Matsukuma S, Aida S, Shima S and Tamai S:
Paget's disease of the esophagus. A case report with review of the
literature. Am J Surg Pathol. 19:948–955. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abraham SC, Wang H, Wang KK and Wu TT:
Paget cells in the esophagus: Assessment of their histopathologic
features and near-universal association with underlying esophageal
adenocarcinoma. Am J Surg Pathol. 32:1068–1074. 2008. View Article : Google Scholar : PubMed/NCBI
|