Introduction
Bladder cancer (BC) is the fifth most common cancer
and the second most diagnosed genitourinary cancer in Western
countries (1). In China, BC is the
leading cause of mortality of all urinary malignancies (2,3). BC may be
non-muscle invasive or muscle invasive. Patients with non-muscle
invasive BC have a high risk of experiencing tumor recurrence and
progression into muscle invasive BC and the prognosis of patients
with muscle invasive BC is poor due to its high rate of metastasis
(4). It has previously been
demonstrated that the epithelial-mesenchymal transition (EMT) is
important in BC (5). However, the
precise mechanism of how the EMT regulates BC invasion remains
unclear.
Zinc finger and BTB domain containing 7A (ZBTB7) is
a ZBTB protein family member that critically influences cellular
differentiation in a pleiotropic manner (6). ZBTB7 consists of four COOH-terminal
krüppel-type zinc fingers and an NH2-terminal POZ/BTB domain
(6). The POZ/BTB domain is involved
in homodimerization and heterodimerization and recruits
corepressors including B-cell lymphoma 6 protein (BCL-6) and
nuclear receptor corepressors 1 and 2. The krüppel-type zinc finger
domain mediates specific DNA recognition and binding (7). ZBTB7 is overexpressed in different types
of human cancer, including non-small cell lung, prostate, ovarian
and breast carcinoma, as well as glioma, T-cell and B-cell lymphoma
(8–13). A previous study by the current authors
suggested that the expression of E-cadherin in bladder cancer cells
may be inhibited by ZBTB7 and that low E-cadherin expression may
alter the phenotype and apical-base polarity of epithelial cells in
the T24 cell line (4). Therefore,
ZBTB7 may regulate the EMT in bladder cancer cells. However, the
levels of ZBTB7 expression in bladder cancer tissues and how ZBTB7
regulates EMT remain unknown.
The present study investigated the expression of
ZBTB7 in transitional cell carcinoma of the bladder (TCC). The
expression of ZBTB7 in TCC and normal human bladder tissues was
evaluated using immunohistochemistry, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting to investigate whether ZBTB7 expression was
associated with the clinicopathological characteristics and
prognosis of patients with TCC.
Patients and methods
Patients
Between September 2008 and June 2014, 100 patients
(72 males and 28 females; mean age 66.3) pathologically diagnosed
with TCC were enrolled in the current study at the Department of
Shanghai Tenth People's Hospital, Tongji University (Shanghai,
China). Pre-operative clinical data for each patient, including
complete blood count, liver biochemistry, renal function, and tumor
size were assembled in a computerized database. Two different types
of tissues from each patient with TCC, including tissue taken from
the TCC tumor itself and tumor-free tissue taken >5 cm from the
tumor edge, were collected immediately following surgical
resection. All tissue samples included the mucous and muscular
layers. Areas of hemorrhage and tissue necrosis were excluded.
Specimens from each patient were divided into two sections. One
section was snap-frozen immediately following resection and stored
in liquid nitrogen until required. The other section was preserved
in 10% formaldehyde solution (at 62°C for 60 min) and
paraffin-embedded. The current study was performed following a
protocol approved by the Ethics Committee of Shanghai Tenth
People's Hospital, Tongji University School of Medicine (Shanghai,
China). Written informed consent for participation was obtained
from each patient.
RT-qPCR
ZBTB7 primers were designed using Primer 3
(http://bioinfo.ut.ee/primer3-0.4.0/primer3/) according
to the sequence of ZBTB7 taken from Genbank (https://www.ncbi.nlm.nih.gov/nuccore). β-actin acted
as an internal control and its primers were synthesized by Shanghai
Sangon Biological Engineering Technology & Services Co., Ltd.
(Shanghai, China). The primer sequences were as follows: β-actin
forward, 5′-TGAAGGTGACAGCAGTCGGTT-3′ and reverse,
5′-AGAAGTGGGGTGGCTTTTAGGA-3′; ZBTB7 forward,
5′-TTCACCAGGCAGGACAAG−3′ and reverse,
5′-GGTTCTTCAGGTCGTAGTTG-3′.
Total RNA was isolated from the tissues using a
Tiangen RNA isolation kit (Tiangen, Biotech, Co., Ltd., Beijing,
China), following the manufacturer's protocol. The density of the
bands was measured using a UV spectrophotometer. Total RNA was
reverse-transcribed into cDNA using Takara PrimeScript™ RT reagent
kit (Takara Bio, Inc., Otsu, Japan) following the manufacturer's
protocol. qPCR was subsequently performed in triplicate for each
sample using a SYBR® ExScript Real-time PCR kit (Takara
Biotechnology Co., Ltd., Dalian, China). A 20 µl reaction mixture
was used, containing 2 µl template DNA, 1 µl primers, 10 µl SYBR
premix and 7 µl ddH2O. PCR was performed using a 7900HT
Fast Real-Time PCR machine (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) under the following conditions:
95°C for 30 sec, 40 cycles of 95°C for 5 sec and 60°C for 30 sec.
PCR results were quantified using the −2ΔΔCq method
(14).
Western blotting
Total protein was extracted using
radioimmunoprecipitation assay lysis buffer (Sangon Biotech Co.,
Ltd., Shanghai, China). Protein concentration was determined using
the bicinchoninic acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Equal quantities of protein (30 µg) were
separated by 8% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis with Tris-glycine running buffer and were
subsequently transferred to nitrocellulose membranes. Membranes
were blocked with 0.1% Tween in Tris-buffered saline for 60 min at
room temperature and incubated with monoclonal antibodies against
ZBTB7 (cat. no. ab70208) (1:200 dilution) and glyceraldehyde
3-phosphate dehydrogenase [GAPDH; (cat. no. ab8245); 1:500
dilution, both from Abcam, Cambridge, UK] at 4°C overnight. GAPDH
acted as a control. The membranes were washed with PBS-0.05%
Tween-20 and then incubated with appropriate goat-anti-rabbit
peroxidase-conjugated secondary antibody (cat. no. A0545; 1:1,000)
or goat-anti-mouse peroxidase-conjugated secondary antibody (cat.
no. A9044; 1:5,000) (both from Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 1 h at 37°C, washed again and developed
using an enhanced chemiluminescence (ECL) western blotting system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunohistochemical staining
Sections (5 µm) were stained using the Sp method
(15) using the Vectorstain Elite ABC
kit (Vector Laboratories, Inc., Burlingame, CA, USA) following the
manufacturer's protocol, and underwent high temperature and
pressure antigen retrieval (the sections were immersed in 0.01 M
citric acid salt retrieval solution, pH 6.0 for 10 min) and were
blocked with 2% goat serum (Gibco; Thermo Fisher Scientific, Inc.)
for 20 min at room temperature. Following the addition of 50 µl
primary antibody against ZBTB7 (cat. no. ab70208; dilution, 1:100;
Abcam), the sections were incubated at 4°C overnight. Negative
controls were established using phosphate buffered saline instead
of primary antibody. Subsequently, 50 µl secondary antibody
(Vectorstain Elite ABC kit; dilution, 1:100; Vector Laboratories,
Inc., Burlingame, CA, USA) was added to the sections, which were
incubated for 30 min at room temperature. Staining was performed
using diaminobenzidine for 5 min at room temperature followed by
counterstaining with hematoxylin for 1 min at room temperature.
Following dehydration, sections were fixed using a graded ethanol
series (70% ethanol 5 min, 80% ethanol 5 min, 90% ethanol 5 min,
100% ethanol 5 min) then treat with xylene for 10 min at room
temperature and mounted with Permount™ mounting medium (Thermo
Fisher Scientific, Inc.).
The slides were observed using a light microscope
under five random high-power fields and 100 cells were counted in
each field. The number of ZBTB7-positive cells with brownish
granular staining in the nucleus and cytoplasm were estimated. The
observer was blinded to the study and analysis was based upon the
percentage of positively stained cells and the degree of staining.
Positively stained cells were scored as follows: <5% staining,
0; 6–25, 1; 26–50, 2; 51–75, 3 and >75%, 4. Cells were also
given a score according to the degree of the staining exhibited:
Light yellow staining, 1; yellow, 2 and brown, 3. A positive
reaction was determined if the product of the two scores was >1
(16).
The cancer genome atlas (TCGA)
database analysis
The TCGA database (http://cancergenome.nih.gov) was analyzed using the
UCSC Cancer Genome Brower (https://genome-cancer.soe.ucsc.edu/). These data
included gene expression data from 436 patients with bladder
cancer, which facilitated the further investigation of the overall
and recurrence free survival in groups with low and high levels of
ZBTB7 expression.
Statistical analysis
Statistical analysis was performed using the IBM
SPSS software v20.0 (IBM Corp., Armonk, NY, USA). Student's t-test
was used as statistical methods to compare the difference in ZBTB7
mRNA expression between normal bladder tissues and TCC tissues of
different grades and stages, and between primary and recurrent
cases. To compare the level of ZBTB7 protein expression (two group:
Positive and negative group) between TCC tissues of different
grades and stages, and between primary and recurrent cases, the χ2
test was used. The log-rank test was used to analyze the
significant of low expression and high expression of ZBTB7.
P<0.05 was considered to indicate a statistically significant
difference. Comparison of survival curves was conducted using the
log-rank test.
Results
Clinicopathological characteristics of
selected patients
A total of 100 patients with TCC, recruited from the
Department of Urology, Shanghai Tenth People's Hospital between
September 2010 and June 2014, were included in the present study.
The clinicopathological characteristics of these patients are
summarized in Table I.
 | Table I.Clinicopathological characteristics of
patients with TCC. |
Table I.
Clinicopathological characteristics of
patients with TCC.
|
|
| Grade | TNM stage |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Variables | Patients | Potential | Low grade | High grade | T1 | T2 | T3 | Primary
carcinoma | Recurrent |
|---|
| Total | 100 | 24 | 36 | 40 | 40 | 48 | 12 | 81 | 19 |
| Sex |
|
|
|
|
|
|
|
|
|
| Male | 72 | 19 | 25 | 28 | 29 | 34 | 9 | 63 | 9 |
|
Female | 28 | 5 | 11 | 12 | 11 | 14 | 3 | 18 | 10 |
| Age |
|
|
|
|
|
|
|
|
|
|
<45 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
|
45–65 | 65 | 9 | 27 | 29 | 25 | 29 | 11 | 57 | 8 |
|
>65 | 34 | 15 | 9 | 10 | 15 | 19 | 0 | 23 | 11 |
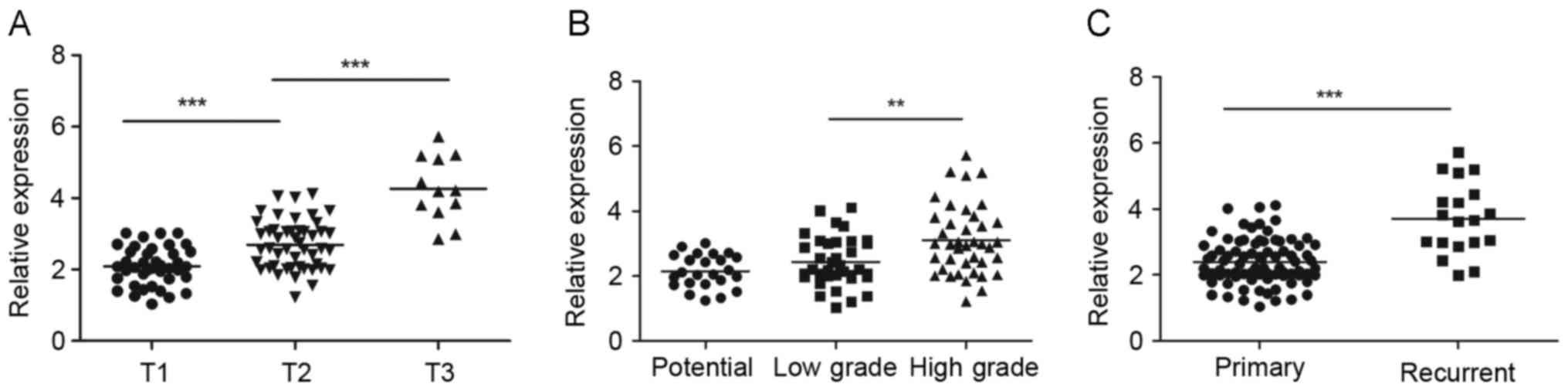
The expression of ZBTB7 mRNA in
patients with TCC
The association between ZBTB7 mRNA expression in TCC
samples and patient clinicopathological characteristics was
analyzed (Fig. 1). Firstly, the
association between tumor size, as determined by the
tumor-node-metastasis (TNM) classification system was analyzed
(17). The expression of ZBTB7 mRNA
was significantly higher in larger than smaller tumors. The
relative expression of ZBTB7 in T1 stage tumors was 2.08±0.53, in
T2 it 2.69±0.68, significantly higher than in T1 stage tumors
(P<0.01) and in T3 it was 4.26±0.90, significantly higher than
in T2 stage tumors (P<0.01; Fig.
1A). ZBTB7 expression was significantly higher in high grade
tumors compared with low-grade tumors (P<0.05; Fig. 1B). However, there is no significant
difference between the low and potential grade groups. In terms of
grade, as determined by the WHO 1973 grading system and the new WHO
classification of malignant tumors of the urinary tract 2004
(18), the relative expression of
ZBTB7 was 2.15±0.51 in potential grade tumors, 2.44±0.76 in low
grade tumors and 3.10±1.06 in high grade tumors. Additionally,
ZBTB7 expression was significantly higher in recurrent tumors
(3.70±1.09) compared with primary tumors (2.39±0.69; P<0.01;
Fig. 1C).
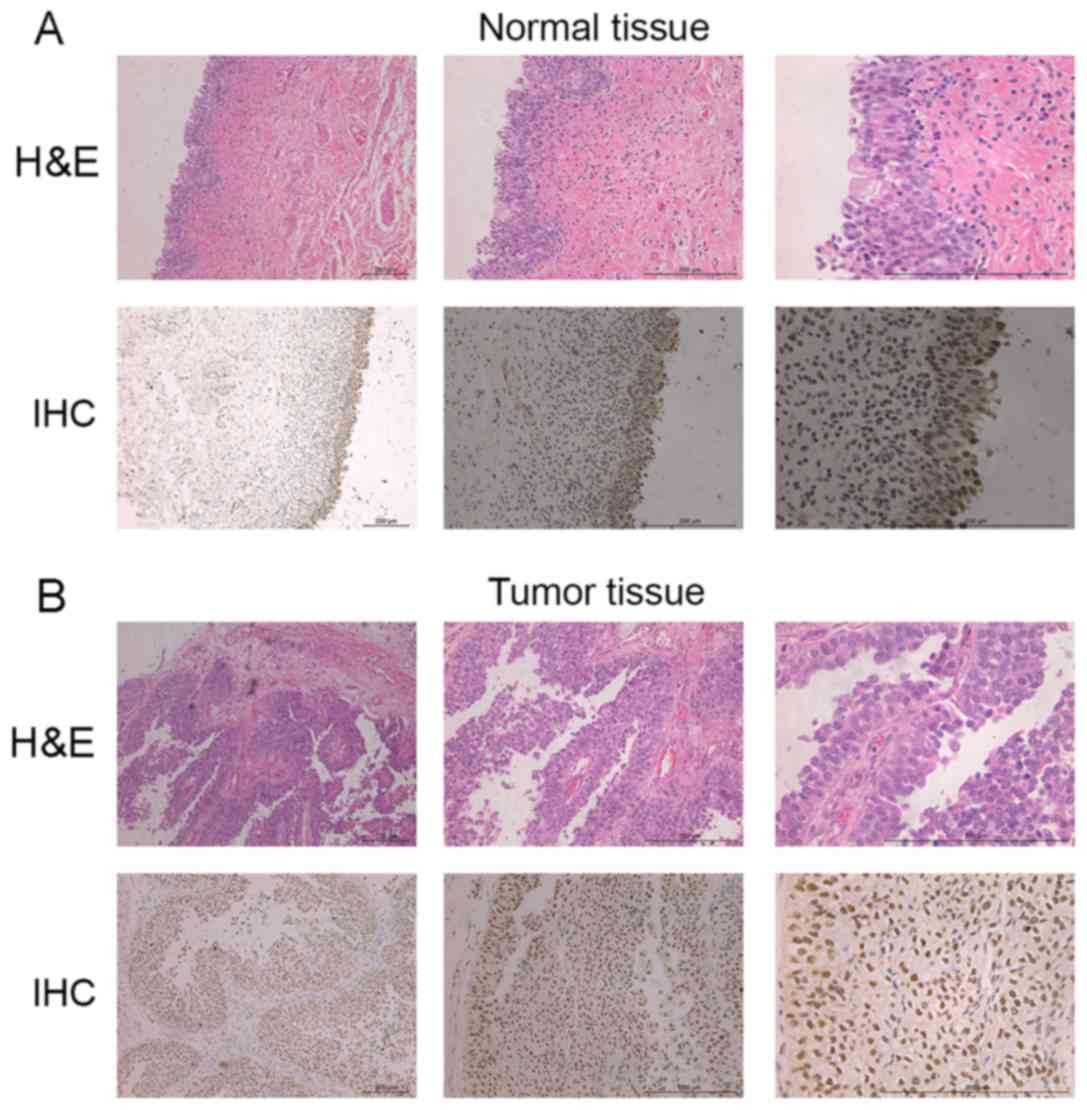
The expression of ZBTB7 protein in
normal human bladder and TCC tissues
ZBTB7 protein was measured in the nucleus and
cytoplasm. Following immunohistochemical staining, cells with high
ZBTB7 expression were stained a brownish yellow granular color
(Fig. 2). The expression of ZBTB7
protein in normal bladder tissue was positive only in the mucous
layer. However, in TCC tumor tissue, the mucous and muscular layer
exhibited positive ZBTB7 expression. The positive rate of ZBTB7
protein expression in TCC tumors of different stages and grades
were as follows (Table II): T1, 70%;
T2, 87.5%; T3, 83.3%; potential grade, 16.6%; low grade, 83.33%;
high grade, 80%; recurrent tumor, 100% and primary tumor, 75%. The
expression of ZBTB7 protein differed significantly between TCC and
normal bladder tissues (P<0.05) (Fig.
2) and between tumors of different TCC grades (potential grade
vs. low grade, P<0.05) (Table
II). The associations between the expression of ZBTB7 protein
in TCC tissues and patient clinicopathological indicators are
presented in Table II. No
significant differences were found between tumors in different TNM
stages (P>0.05), however ZBTB7 protein expression differed
significantly among tumors with different histological grades
(potential grade vs. low grade, P<0.05).
 | Table II.The associations between ZBTB7 IHC
and clinicopathological indicators. |
Table II.
The associations between ZBTB7 IHC
and clinicopathological indicators.
| Pathological
feature | Number | Positive | Negative | Rate of expression,
% |
P-valuesa |
|---|
| TNM stage |
|
|
|
|
|
| T1 | 40 | 28 | 12 | 70 |
|
| T2 | 32 | 28 | 4 | 87.50 | 0.424 |
| T3 | 28 | 20 | 8 | 71.43 |
|
| Grade |
|
|
|
|
|
|
Potential grade | 12 | 2 | 10 | 16.60 |
|
| Low
grade | 48 | 40 | 8 | 83.33 | 0.003 |
| High
grade | 40 | 32 | 8 | 80 |
|
| Primary or
recurrent |
|
|
|
|
|
|
Primary | 19 | 19 | 0 | 100 |
|
|
Recurrent | 81 | 60 | 19 | 75 | 0.185 |
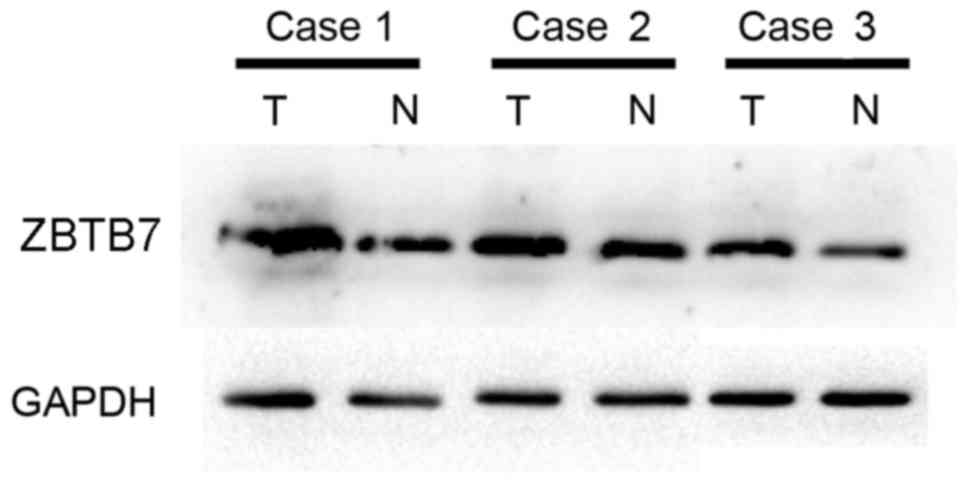
Levels of ZBTB7 protein in TCC bladder and normal
tissue were detected by western blot analysis and it was observed
that the expression of ZBTB7 protein was markedly higher in tumor
tissue than normal tissue (Fig.
3).
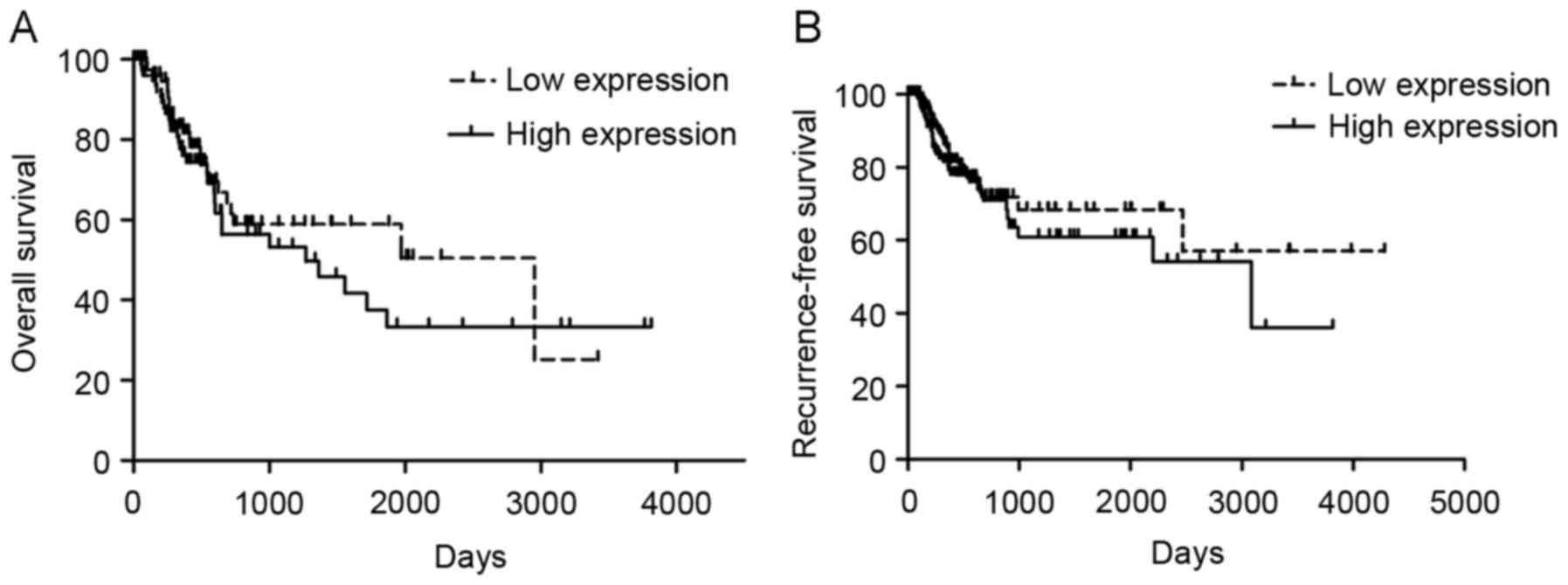
ZBTB7 gene expression is not
associated with the clinical prognosis of TCC
Information from 436 patients with BC patients from
the TCGA database were analyzed and patients with higher ZBTB7
expression exhibited a lower overall survival rates compared with
patients with low ZBTB7 expression (Fig.
4A). However, this difference was not statistically significant
(P=0.75). Recurrence free survival in patients with low expression
of ZBTB7 compared with patients with high expression of ZBTB7 was
also analyzed. Recurrence-free survival was generally lower in
patients with high ZBTB7 expression. However, this difference was
not significant (P=0.43).
Discussion
BC is responsible for 150,000 cases of
cancer-associated mortality annually and is the fourth most common
malignant neoplasm diagnosed in males worldwide (19). Approximately 70–80% cases of BCs are
non-muscle invasive and the remaining 20–30% are muscle invasive at
initial diagnosis. Between 30 and 50% of patients with non-muscle
invasive BC experience recurrence following transurethral resection
of the primary tumor and 10–20% progress to muscle invasive BC
(4). Therefore, patients with
non-muscle invasive BC have a high risk of experiencing recurrence
and progression to muscle invasive BC. Despite therapeutic
advancements in BC treatment, including surgery and neoadjuvant
chemotherapy, there were 16,000 cases of BC-associated mortality in
the USA in 2015 alone (20).
A number of potential markers for BC have been
identified, including zinc finger protein 671 (21) and C-X-C motif chemokine ligand 1
(22). However, there are still no
characterized, non-invasive molecular markers able to detect BC
tumors and predict patient outcomes. Therefore, improved
understanding of the molecular carcinogenesis of BC tumor
progression may aid the development of novel, less invasive
diagnostic markers. In the current study, high expression of ZBTB7
in BC tumor tissue was detected and it was determined that ZBTB7
expression may be associated with tumor size, grade and stage.
ZBTB7, a member of the ZBTB family, was identified
by Maeda et al (6) in 2005.
ZBTB7 is located at 19p13.3 and possesses two exons and two
introns. It was initially considered to bind to HIV-1 promoter
elements and was subsequently identified as a protein that
interacts with BCL-6 and leads to the inactivation of mouse ZBTB7,
inhibition of multicellular differentiation and embryonic lethality
(23,24). ZBTB7 is regarded as a master regulator
as it binds specifically to p19 ARF, which is an important
anti-oncogene. Chen et al (25) demonstrated that ZBTB7 is able to
combine directly with the p19 ARF promoter and inactivate it in
vivo. In addition, ZBTB7 is able to act upstream of many types
of proto- and anti-oncogenes, which serve an important role in
tumorigenesis and tumor biological behavior, whereas non-specific
proto-oncogenes simply promote tumor cell growth (9).
Previous studies have indicated that ZBTB7 is highly
expressed in different types of cancer, including lung and colon
cancer (6,8,11,26–28). In
these neoplasms, it may promote tumorigenesis, acting as a
proto-oncogene by repressing or enhancing the expression of genes
involved in apoptosis, cell proliferation, differentiation and
invasion. The present study demonstrated that ZBTB7 expression in
TCC tissue was positive, indicating that ZBTB7 may promote the
initiation and progression of TCC. By assessing the association
between ZBTB7 gene expression in TCC and the clinicopathological
features of patients, it was determined that the higher expression
of ZBTB7 was associated with a more severe tumor TNM stage, tumor
recurrence and a higher histological grade. These results are in
concordance with previous studies that investigated the prognostic
impact of ZBTB7 in TCC (4,29). Therefore, high expression of ZBTB7 in
TCC may be important in tumor initiation, progression and
invasion.
The present study used the TCGA database to analyze
the association between ZBTB7 expression and patient prognosis.
Patients with high expression of ZBTB7 generally exhibited lower
overall survival rates compared to those with low ZBTB7 expression.
However, this difference was not statistically significant
(P>0.05). This may be explained by two reasons. Firstly, no
follow-up information existed on >100 patients out of the 436
patients included in the database. This loss of patients to
follow-up may have skewed the end result. Secondly, the number of
patients included in the current study was relatively small.
Therefore, further studies involving larger cohorts are required to
confirm the prognostic impact of ZBTB7 in TCC.
In conclusion, positive immunoreactivity of ZBTB7 is
frequently observed in TCC and there was a significant association
between high ZBTB7 expression and a higher tumor histological stage
and grade. ZBTB7 expression may serve an important role in the
initiation and progression of TCC and routine assessment of ZBTB7
expression may improve the identification of patients with
high-risk TCC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 31100702/C100307) and
Specialized Research Fund for the Doctoral Program of Higher
Education in China (grant no. 20110072120054).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo C, Zhu K, Sun W, Yang B, Gu W, Luo J,
Peng B and Zheng J: The effect of ZBTB7 on bladder cancer
epithelial-mesenchymal transition. Biochem Biophys Res Commun.
443:1226–1231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Geng J, Fan J, Ouyang Q, Zhang X, Zhang X,
Yu J, Xu Z, Li Q, Yao X, Liu X and Zheng J: Loss of PPM1A
expression enhances invasion and the epithelial-to-mesenchymal
transition in bladder cancer by activating the TGF-β/Smad signaling
pathway. Oncotarget. 5:5700–5711. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maeda T, Hobbs RM, Merghoub T, Guernah I,
Zelent A, Cordon-Cardo C, Teruya-Feldstein J and Pandolfi PP: Role
of the proto-oncogene Pokemon in cellular transformation and ARF
repression. Nature. 433:278–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang QL, Tian DA and Xu XJ: Depletion of
Pokemon gene inhibits hepatocellular carcinoma cell growth through
inhibition of H-ras. Onkologie. 34:526–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Apostolopoulou K, Pateras IS, Evangelou K,
Tsantoulis PK, Liontos M, Kittas C, Tiniakos DG, Kotsinas A,
Cordon-Cardo C and Gorgoulis VG: Gene amplification is a relatively
frequent event leading to ZBTB7A (Pokemon) overexpression in
non-small cell lung cancer. J Pathol. 213:294–302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aggarwal A, Hunter WJ III, Aggarwal H,
Silva ED, Davey MS, Murphy RF and Agrawal DK: Expression of
leukemia/lymphoma-related factor (LRF/ZBTB7) in human breast
carcinoma and other cancers. Exp Mol Pathol. 89:140–148. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rovin RA and Winn R: Pokemon expression in
malignant glioma: An application of bioinformatics methods.
Neurosurg Focus. 19:E82005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu H, Qu D, Chen F, Zhang Z, Liu B and Liu
H: ZBTB7 overexpression contributes to malignancy in breast cancer.
Cancer Invest. 28:672–678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maeda T, Merghoub T, Hobbs RM, Dong L,
Maeda M, Zakrzewski J, van den Brink MR, Zelent A, Shigematsu H,
Akashi K, et al: Regulation of B versus T lymphoid lineage fate
decision by the proto-oncogene LRF. Science. 316:860–866. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang L, Siu MK, Wong OG, Tam KF, Lam EW,
Ngan HY, Le XF, Wong ES, Chan HY and Cheung AN: Overexpression of
proto-oncogene FBI-1 activates membrane type 1-matrix
metalloproteinase in association with adverse outcome in ovarian
cancers. Mol Cancer. 9:3182010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi SR, Key ME and Kalra KL: Antigen
retrieval in formalin-fixed, paraffin-embedded tissues: An
enhancement method for immunohistochemical staining based on
microwave oven heating of tissue sections. J Histochem Cytochem.
39:741–748. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao ZH, Wang SF, Yu L, Wang J, Chang H,
Yan WL, Fu K and Zhang J: Expression of transcription factor
Pokemon in non-small cell lung cancer and its clinical
significance. Chin Med J (Engl). 121:445–449. 2008.PubMed/NCBI
|
|
17
|
Sobin LH and Fleming ID: TNM
classification of malignant tumors, fifth edition (1997). Union
Internationale Contre le Cancer and the American Joint Committee on
Cancer. Cancer. 80:1803–1804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
World Health Organization Classification
of Tumours: Pathology and Genetics of Tumours of the Urinary
Systemand Male Genital Organs. Eble JN, Sauter G, Epstein JI and
Sesterhenn IA: IARC Press; Lyon: 2004
|
|
19
|
Chavan S, Bray F, Lortet-Tieulent J,
Goodman M and Jemal A: International variations in bladder cancer
incidence and mortality. Eur Urol. 66:59–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scarpato KR, Morgans AK and Moses KA:
Optimal management of muscle-invasive bladder cancer-a review. Res
Rep Urol. 7:143–151. 2015.PubMed/NCBI
|
|
21
|
Yeh CM, Chen PC, Hsieh HY, Jou YC, Lin CT,
Tsai MH, Huang WY, Wang YT, Lin RI, Chen SS, et al: Methylomics
analysis identifies ZNF671 as an epigenetically repressed novel
tumor suppressor and a potential non-invasive biomarker for the
detection of urothelial carcinoma. Oncotarget. 6:29555–29572. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakashima M, Matsui Y, Kobayashi T, Saito
R, Hatahira S, Kawakami K, Nakamura E, Nishiyama H and Ogawa O:
Urine CXCL1 as a biomarker for tumor detection and outcome
prediction in bladder cancer. Cancer Biomark. 15:357–364. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kelly KF and Daniel JM: POZ for
effect-POZ-ZF transcription factors in cancer and development.
Trends Cell Biol. 16:578–587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stogios PJ, Chen L and Privé GG: Crystal
structure of the BTB domain from the LRF/ZBTB7 transcriptional
regulator. Protein Sci. 16:336–342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen WY, Zeng X, Carter MG, Morrell CN,
Yen Chiu RW, Esteller M, Watkins DN, Herman JG, Mankowski JL and
Baylin SB: Heterozygous disruption of Hic1 predisposes mice to a
gender-dependent spectrum of malignant tumors. Nat Genet.
33:197–202. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao ZH, Wang SF, Yu L, Wang J, Chang H,
Yan WL, Zhang J and Fu K: Overexpression of Pokemon in non-small
cell lung cancer and foreshowing tumor biological behavior as well
as clinical results. Lung Cancer. 62:113–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiao W, Liu F, Tang FZ, Lan J, Xiao RP,
Chen XZ, Ye HL and Cai YL: Expression of the Pokemon proto-oncogene
in nasopharyngeal carcinoma cell lines and tissues. Asian Pac J
Cancer Prev. 14:6315–6319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao GT, Yang LJ, Li XX, Cui HL and Guo R:
Expression of the proto-oncogene Pokemon in colorectal
cancer-inhibitory effects of an siRNA. Asian Pac J Cancer Prev.
14:4999–5005. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li W, Kidiyoor A, Hu Y, Guo C, Liu M, Yao
X, Zhang Y, Peng B and Zheng J: Evaluation of transforming growth
factor-β1 suppress Pokemon/epithelial-mesenchymal transition
expression in human bladder cancer cells. Tumour Biol.
36:1155–1162. 2015. View Article : Google Scholar : PubMed/NCBI
|