Introduction
The mortality rate from colorectal cancer is the
third highest in men (being behind that of lung and prostate
cancer) and second in women (being behind breast cancer) in the
United States (1). Even when patients
undergo curative surgery for advanced cancer, recurrence can still
occur. Markers that relate closely to cancer progression and
metastasis would enable early diagnosis and intervention. Thus, the
identification of novel markers that predict cancer progression is
important for planning clinical strategies. In addition, the
identification of such markers could lead to the development of
novel therapeutic agents. In colorectal cancer, various
molecular-targeted drugs have been developed and clinically applied
in previous years (2–8). In addition, the assessment of specific
genes including cancer progression gene sets via development of
chip technology has led to tailor-made therapy.
The present study focused on the Golgi-associated
PDZ- and coiled-coil motif-containing (GOPC) since it has been
reported that the knockdown of GOPC in cells increases activation
of the mitogen-activated protein kinase (MAPK)-extracellular
signal-regulated kinase (Erk) 1/2 pathway. The MAPK-Erk1/2 pathway
is a chief cellular signal transduction pathway that regulates cell
differentiation, proliferation, survival and migration in
colorectal cancer (9–13). The present study aimed to elucidate
the correlation between GOPC expression and clinicopathological
factors and prognosis in colorectal cancer.
Materials and methods
Patients and samples
GOPC expression was assessed for each of nine
clinical samples of colorectal cancer and normal mucosa using
reverse transcription-quantitative PCR (RT-qPCR). An additional 153
clinical colorectal cancer samples were used to assess the
correlation of GOPC expression and clinicopathological factors or
prognosis. For immunohistochemical analysis, 10 normal colorectal
mucosa and 10 colorectal cancer tissue specimens were used. All
samples were obtained by surgery between March 2003 and June 2006
at Osaka University, Minoh City Hospital, Kansai Rosai Hospital,
Kinki Central Hospital of the Mutual Aid Association of Public
School Teachers, National Hospital Organization Osaka National
Hospital, NTT (Nippon Telegraph And Telephone) West Osaka Hospital,
Osaka Medical Center for Cancer and Cardiovascular Diseases,
Saiseikai Suita Hospital, Sakai City Medical Center and Toyonaka
Municipal Hospital (all in Osaka, Japan). Every patient provided
informed consent and the present study was approved by the Research
Ethics Board of each institution.
Assessment of tumor stage
Tumor stages were defined according to the tumor
node metastasis (TNM) staging system (14).
Assessment of clinicopathological and
prognostic factors
The present study assessed the correlation between
GOPC expression and clinical characteristics, venous invasion,
lymph invasion, tumor invasion, lymph node metastasis, TNM stage,
overall survival (OS) and recurrence-free survival (RFS). The 153
colorectal cancer samples included 32 TNM stage 0/I cases, 45 stage
II cases, 58 stage III cases and 18 stage IV cases according to the
UICC classification for colorectal cancer.
Processing mRNA and RT-qPCR
Total RNA was extracted from frozen tumor tissue
using miRNeasy Mini kit (Qiagen AB, Sollentuna, Sweden). No DNase
treatment was performed. Total RNA was then reverse transcribed to
cDNA using the High Capacity RNA-to-cDNA™ kit (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. cDNA was the amplified by RT-qPCR using
the Light Cycler® 2.0 DX400 (Roche Diagnostics, Basel,
Switzerland). The PCR reaction mixture consisted of 0.5 µl of cDNA,
5.0 µl of THUNDERBIRD™ SYBR® qPCR Mix (Toyobo Co., Ltd.,
Osaka, Japan), 4.0 µl of water and 0.5 µl of each primer. The GOPC
primers were: Forward, 5′-GTGGATGTGGATCTGCTCCT-3′ and reverse,
5′-CCTCCAGCTTGTGGTTGATT-3′. Primers for GAPDH, the internal
control, were: Forward, 5′-CAACTACATGGTTTACATGTTC-3′ and reverse,
5′-GCCAGTGGACTCCACGAC-3′. The normalization was performed by
standard curve method (15). The
amplification protocol consisted of 55 cycles of: Denaturation at
95°C for 5 sec, annealing at 60°C for 5 sec and extension at 72°C
for 30 sec. The RT-qPCR experiment was performed 7 times.
Immunohistochemical staining
The expression of the GOPC protein was assessed by
immunohistochemical staining of formalin-fixed and
paraffin-embedded normal colorectal mucosa and colorectal cancer
tissue sections. The surgical tissue samples were placed overnight
at room temperature in 10% formalin before paraffin embedding.
Briefly, 3.5 µm thick sections were incubated overnight at 4°C
using the rabbit polyclonal anti-GOPC antibody (dilution, 1:1,000;
#ab37036; Abcam, Cambridge, UK) subsequent to immersion and
blockade of endogenous peroxidase activity. The blocking was for 20
min at room temperature using VECTASTAIN Elite ABC horseradish
peroxidase kit (Rabbit IgG; #PK-6101; Vector Laboratories,
Burlingame, CA, USA). Hematoxylin was used for nuclear staining for
1 min. Dehydration was performed using 60, 70, 80, 90 and 95%
ethanol for 1 min each, 100% ethanol for 2 min twice and xylene for
5 min, 3 times. The specimens were visualized on the light field
using a confocal microscope BZ-X710 (Keyence Corporation, Osaka,
Japan) and BZ-X analyzer (v. 1.3.0.3; Keyence Corporation).
Statistical analyses
Statistical analyses were performed using Fisher's
exact tests to compare the differences between the two groups. The
cumulative probabilities of OS or RFS were compared between these
two groups by the Kaplan-Meier method with the log-rank test to
calculate significant differences. Cases of non-curative resection
were excluded from the RFS analyses. Univariate and multivariate
analyses for OS and RFS were performed to evaluate independent
prognostic factors using a Cox proportional hazards model. All
statistical analyses were performed with JMP Pro software (version
11; JMP, Buckinghamshire, UK). P<0.05 was considered to indicate
a statistically significant difference.
Results
Correlation between GOPC mRNA
expression and clinicopathological factors
Firstly, RT-qPCR was used to assess the expression
of GOPC in normal colorectal mucosa and colorectal cancer tissue in
nine clinical samples. The Wilcoxon rank-sum test was used to
assess the statistical significance. GOPC expression in normal
colorectal mucosa specimens was significantly increased compared
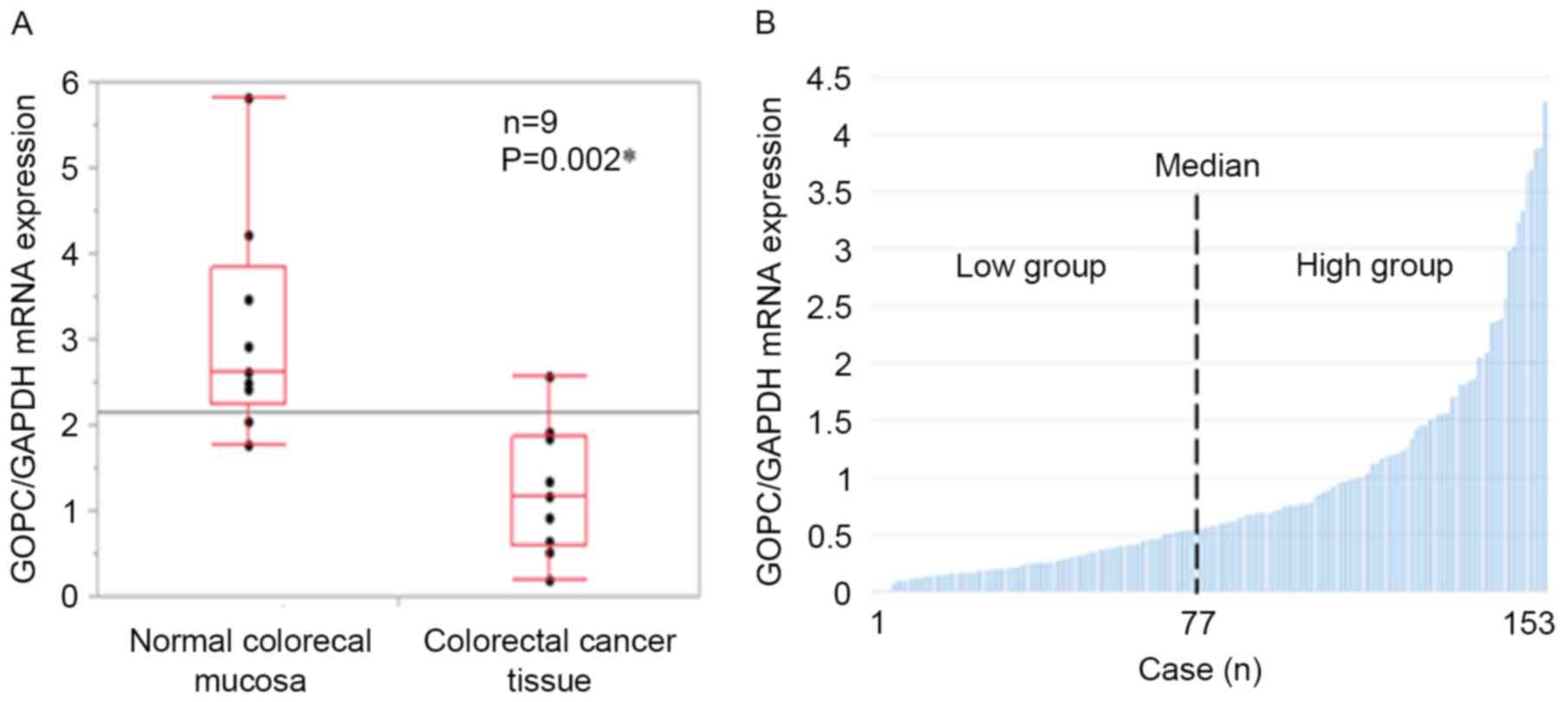
with colorectal cancer specimens (P=0.002; Fig. 1A). The GOPC expression in the
additional 153 colorectal cancer specimens was then assessed. Data
obtained from RT-qPCR was investigated to see if it fit Gaussian
distribution with the Shapiro-Wilk test, and it did not. Thus, the
median value was used to classify the higher (GOPC high) and lower
(GOPC low) expression groups and clinicopathological
characteristics were assessed based on the level of GOPC
expression. Based on the median score to separate the GOPC high and
low groups (Fig. 1B), there were 77
GOPC high cases, and 76 GOPC low cases. The baseline
characteristics are presented in Table
I. The GOPC high group included 48 men and 29 women whereas the
GOPC low group included 47 men and 29 women. The groups did not
differ in the site of primary disease or size of the primary tumor,
and CEA and CA19-9 levels also did not differ. In the analysis of
clinicopathological factors, the proportion of positive venous
invasion was significantly increased in the GOPC low group compared
with the GOPC high group (P=0.001). Histological type, lymphatic
invasion, depth of tumor invasion, lymphatic nodule metastasis, and
TNM stages were not observed to differ between the two groups
(Table II).
 | Table I.Baseline characteristics of the GOPC
high and low groups. |
Table I.
Baseline characteristics of the GOPC
high and low groups.
|
| GOPC expression |
|
|---|
|
|
|
|
|---|
| Clinical
characteristics | High group
(n=77) | Low group (n=76) | P-value |
|---|
| Gender |
|
|
|
| Male | 48 | 47 | NS |
|
Female | 29 | 29 |
| Primary site |
|
|
|
|
Colon | 37 | 48 | 0.191 |
|
Rectum | 40 | 28 |
| Tumor size, cm |
|
|
|
| Median
(range) | 6 (2–9.5) | 4.7 (1.3–15.5) | 0.552 |
| CEA, ng/ml |
|
|
|
| Median
(range) | 4 (1–204)
0.432 | 4.8
(0.9–7,636) |
|
| CA19-9, U/ml |
|
|
|
| Median
(range) | 13 (2–10,740) | 15 (0–186,061) | 0.140 |
 | Table II.Correlation between GOPC expression
and pathological characteristics. |
Table II.
Correlation between GOPC expression
and pathological characteristics.
|
| GOPC
expression |
|
|---|
|
|
|
|
|---|
| Pathological
characteristics | High group
(n=77) | Low group
(n=76) | P-value |
|---|
| Histological
type |
|
|
|
| tub1,
tub2 | 75 | 69 | 0.097 |
| por,
sig | 2 | 7 |
|
| Lymph invasion |
|
|
|
|
Negative | 32 | 25 | 0.316 |
|
Positive | 45 | 51 |
|
| Venous
invasion |
|
|
|
|
Negative | 50 | 29 | 0.001a |
|
Positive | 27 | 47 |
|
| Tumor invasion |
|
|
|
|
T0-2 | 18 | 18 | NS |
|
T3-4 | 59 | 58 |
| Lymph node
metastasis |
|
|
|
|
Negative | 41 | 39 | 0.871 |
|
Positive | 36 | 37 |
|
| TNM stage |
|
|
|
|
0-II | 40 | 37 | 0.747 |
|
III–IV | 37 | 39 |
|
| Metastasis
site |
|
|
|
|
Liver | 5 | 9 |
|
|
Pleura | 1 | 3 |
|
|
Other | 1 | 1 |
|
| Curability |
|
|
|
|
Curative | 67 | 64 | 0.651 |
|
Non-curative | 10 | 12 |
|
Correlation between GOPC mRNA
expression and clinical outcome
The correlation between GOPC expression and clinical
outcome was assessed by comparison of the GOPC high and low groups.
OS and RFS were assessed by the Kaplan-Meier method using the
log-rank test. The Kaplan-Meier curves demonstrated that there was
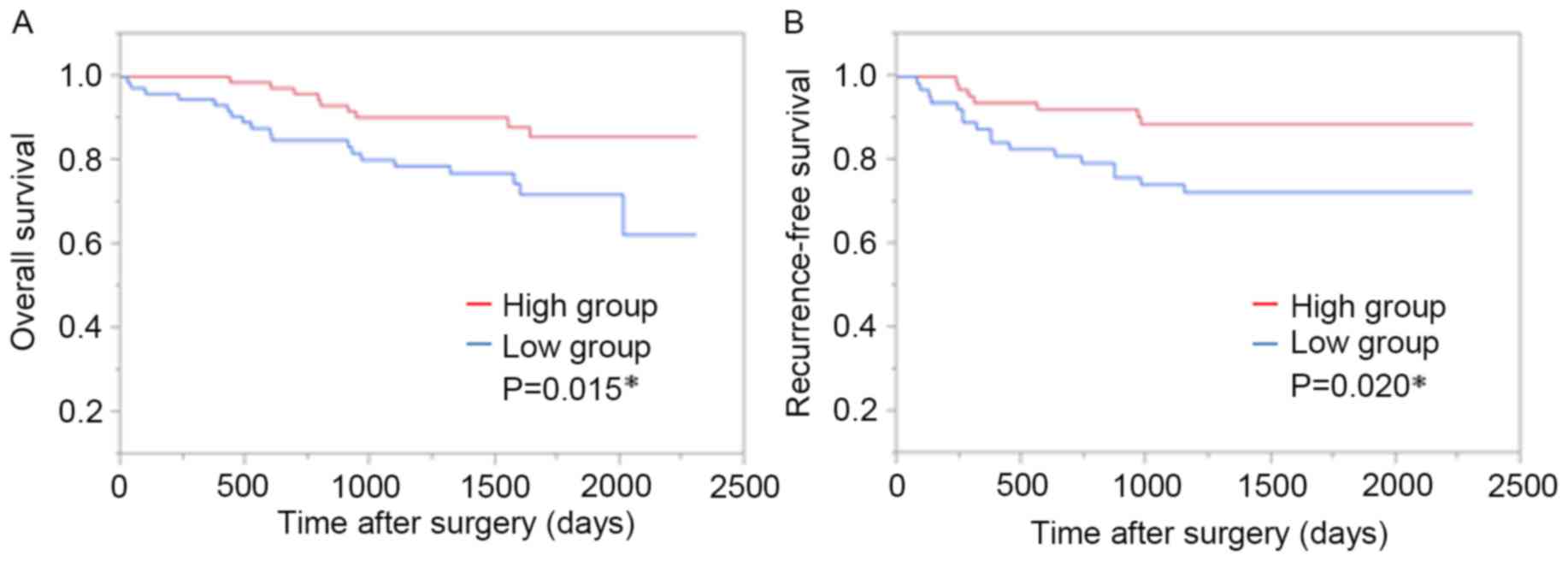
a significantly poorer OS in the GOPC low group compared with the
GOPC high group (P=0.015; Fig. 2A).
Univariate and multivariate analyses identified lymphatic invasion
to be an independent prognostic factor for OS [hazard ratio
(HR)=7.628; 95% confidence interval (CI), 1.441–141.2; P=0.012;
Table III].
 | Table IIIResults of univariate and
multivariate analyses for overall survival in a Cox proportional
hazards model. |
Table III
Results of univariate and
multivariate analyses for overall survival in a Cox proportional
hazards model.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
|
Characteristics | n | HR | 95% CI | P value | HR | 95% CI | P-value |
|---|
| Gender |
|
|
|
|
|
|
|
|
Male/Female | 95/58 | 0.821 | 0.398–1.779 | 0.621 |
|
|
|
| Pathological
type |
|
|
|
|
|
|
|
| por,
sig/tub1, tub2 | 9/144 | 6.240 | 1.793–16.84 | 0.006a | 2.099 | 0.585–5.991 | 0.230 |
| Lymph invasion |
|
|
|
|
|
|
|
|
Positive/Negative | 96/57 | 19.18 | 4.095–342.0 |
<0.001a | 7.628 | 1.441–141.2 | 0.012a |
| Venous
invasion |
|
|
|
|
|
|
|
|
Positive/Negative | 74/49 | 6.571 | 2.713–19.55 |
<0.001a | 2.345 | 0.896–7.406 | 0.084 |
| Tumor invasion |
|
|
|
|
|
|
|
|
T3-4/T0-2 | 117/36 | 4.820 | 1.443–29.89 | 0.007a | 2.000 | 0.555–12.87 | 0.322 |
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
Positive/Negative | 73/80 | 5.236 | 2.264–14.20 |
<0.001a | 2.465 | 0.998–7.059 | 0.050 |
| GOPC
expression |
|
|
|
|
|
|
|
|
Low/High | 76/77 | 2.558 | 1.198–5.912 | 0.014a | 1.902 | 0.853–4.552 | 0.117 |
The correlation of GOPC expression and RFS was
assessed in 131 patients (22 of the 153 patients had undergone
non-curative surgery and were excluded from RFS analysis). Adjuvant
chemotherapy was used in 27 GOPC high and 24 GOPC low cases. The 4
regimens of adjuvant chemotherapy were: Uracil-tegafur with
leucovorin; Uracil-tegafur with doxifluridine; Uracil-tegafur with
irinotecan; 5-fluorouracil with l-leucovorin. Relapse was observed
in 25 patients: 8 in the GOPC high group and 17 in the GOPC low
group. The proportion of recurrence was significantly higher in the
GOPC low group (P=0.049; Table IV).
The Kaplan-Meier curves indicated that RFS was significantly
reduced in the GOPC low group compared with the GOPC high group
(P=0.020; Fig. 2B). Univariate and
multivariate analyses identified lymph node metastasis (HR=2.861;
95% CI, 1.138–7.880; P=0.024) and lower GOPC expression (HR=2.800;
95% CI, 1.121–7.648; P=0.027) to be independent prognostic factors
for RFS (Table V).
 | Table IV.Correlation between GOPC expression
and adjuvant chemotherapy or recurrence. |
Table IV.
Correlation between GOPC expression
and adjuvant chemotherapy or recurrence.
|
| GOPC
expression |
|
|---|
|
|
|
|
|---|
| Variables | High group
(n=67) | Low group
(n=64) | P-value |
|---|
| Adjuvant
chemotherapy |
|
|
|
|
Yes | 27 | 24 | 0.857 |
| No | 40 | 40 |
|
| Recurrence |
|
|
|
|
Yes | 8 | 17 | 0.049a |
| No | 59 | 47 |
|
| Site |
|
|
|
|
Local | 2 | 3 |
|
| Lymph
node | 1 | 4 |
|
|
Liver | 3 | 3 |
|
|
Lung | 1 | 5 |
|
|
Pleura | 1 | 2 |
|
|
Other | 0 | 0 |
|
 | Table V.Results of univariate and
multivariate analyses for recurrence-free survival in a Cox
proportional hazards model. |
Table V.
Results of univariate and
multivariate analyses for recurrence-free survival in a Cox
proportional hazards model.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
|
Characteristics | n | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender |
|
|
|
|
|
|
|
|
Male/Female | 85/46 | 1.072 | 0.471–2.646 | 0.870 |
|
|
|
| Pathological
type |
|
|
|
|
|
|
|
| Por,
sig/tub1, tub2 | 5/126 | 5.208 | 1.228–15.15 | 0.028a | 2.618 | 0.578–8.711 | 0.187 |
| Lymph invasion |
|
|
|
|
|
|
|
|
Positive/Negative | 77/54 | 3.841 | 1.453–13.20 | 0.005a | 2.966 | 0.9903–10.96 | 0.052 |
| Venous
invasion |
|
|
|
|
|
|
|
|
Positive/Negative | 57/74 | 1.654 | 0.739–3.767 | 0.218 | 1.672 | 0.639–4.365 | 0.290 |
| Tumor invasion |
|
|
|
|
|
|
|
|
T3-4/T0-2 | 95/36 | 2.837 | 0.978–12.04 | 0.055 |
|
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
Positive/Negative | 55/76 | 3.555 | 1.533–9.195 | 0.002a | 2.861 | 1.138–7.880 | 0.024a |
| GOPC
expression |
|
|
|
|
|
|
|
|
Low/High | 64/67 | 2.706 | 1.167–7.000 | 0.019a | 2.800 | 1.121–7.648 | 0.027a |
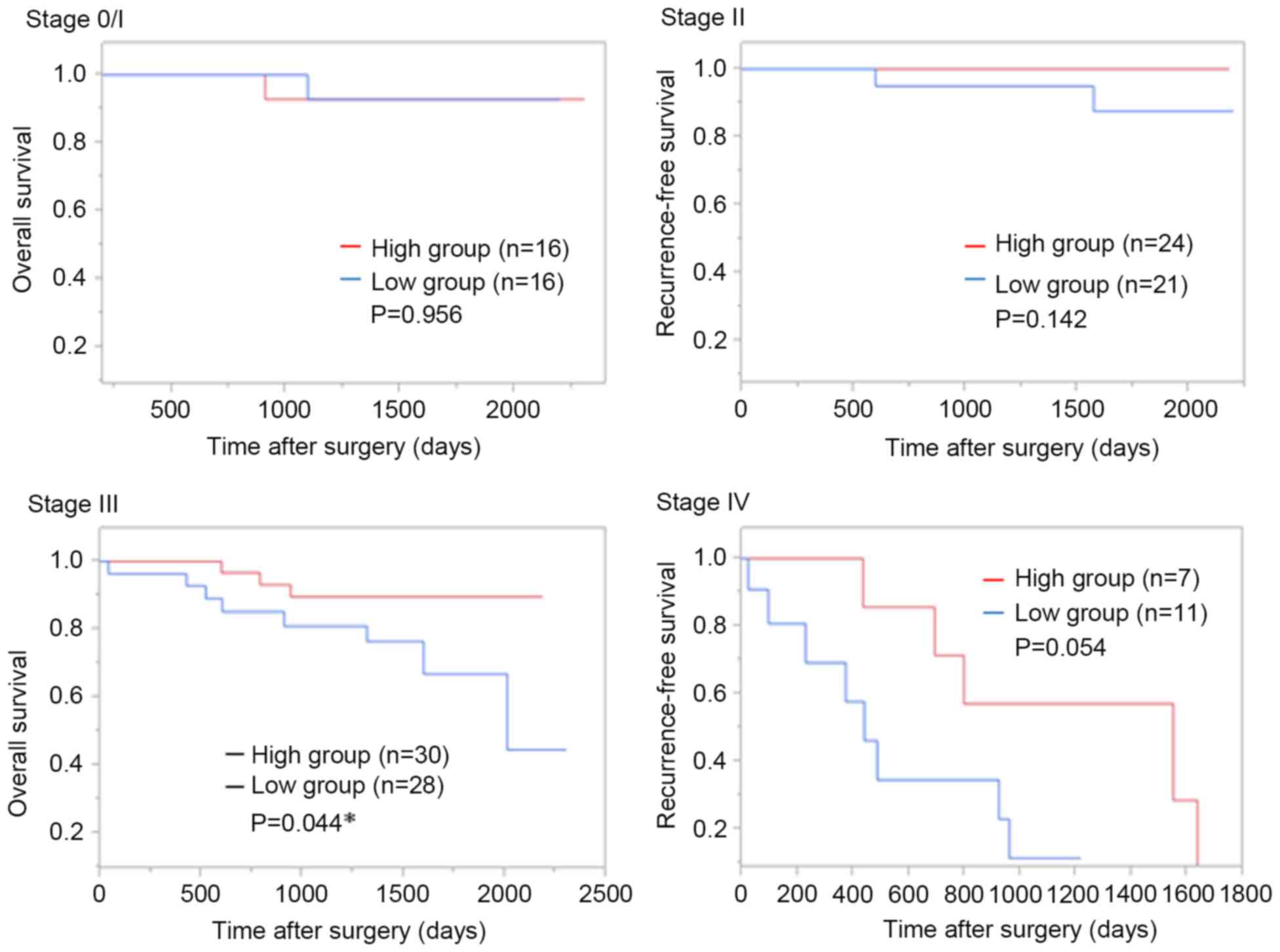
In analyses according to each stage, OS in the GOPC
low group was poorer compared with in the GOPC high group at stage
III (P=0.044) and stage IV (P=0.054; Fig.
3).
Expression of GOPC protein in normal
colorectal mucosa and colorectal cancer tissue
Immunohistochemical analysis was performed to assess
the protein expression of GOPC in 10 sections each of normal
colorectal mucosa and colorectal cancer tissue. Representative
staining of GOPC in the normal colorectal mucosa and colorectal
cancer tissue was observed (Fig. 4).
GOPC protein expression in normal mucosa was increased compared
with in cancer tissue and expression localized in the cytoplasm or
cell surface membrane (Fig. 4A and
B). As for the GOPC expression in cancer tissue, a high
expression was observed at the surface of cancerous tissue, whilst
low expression was observed at the invasive front (Fig. 4C and D).
Discussion
GOPC, also known as PDZ domain protein interacting
specifically with TC10 or Fused in Glioblastoma (FIG), and cystic
fibrosis transmembrane conductance regulator-associated ligand,
controls the trafficking of numerous integral membrane proteins
from the trans-Golgi network to the cell surface (16–19). Its
domain structure consists of an N-terminal region with two
coiled-coil domains and a C-terminal PDZ domain (16). The PDZ domain mediates interactions
with frizzled, a Wnt receptor (16),
and TC10, a member of the Rho-family GTPases (17). In addition, GOPC regulates various
proteins including the soluble N-ethylmaleimide sensitive fusion
protein attachment protein receptor (Q-SNARE) protein syntaxin-6
involved in endocytosis (19),
cluster of differentiation-46 in autophagy (20) and claudin-1 and claudin-2 in tight
junctions (21). In glioblastoma,
GOPC (or FIG) is reported to fuse with the c-ros-oncogene 1 (ROS),
a type of receptor tyrosine kinase, yielding the so-called FIG-ROS
(22). Certain studies have indicated
that FIG-ROS performs oncogenic roles in several processes,
including cellular proliferation, colony formation, cell cycle
progression, migration and invasion in intrahepatic
cholangiocarcinoma (23,24). To the best of our knowledge, no study
has previously been published regarding GOPC and FIG-ROS in
colorectal cancer.
GOPC mRNA expression was evaluated in 153 colorectal
cancer specimens by RT-qPCR and the correlation between GOPC
expression and prognosis was analyzed. In the analyses of the
clinicopathological factors, the proportion of venous invasion was
significantly increased in the GOPC low group compared with in the
GOPC high group, as was the proportion of cancer recurrence. The
number of stage IV cases (11 in GOPC low, 7 in GOPC high) and the
number of hematogenous metastasis cases (8 in GOPC low, 4 in GOPC
high) were greater in the GOPC low group. Multivariate analysis for
RFS identified lower expression of GOPC to be an independent
prognostic factor.
To compare the expression of GOPC mRNA and protein
between normal colorectal mucosa and cancerous tissue, RT-qPCR and
immunohistochemical analysis were performed. The expression of GOPC
mRNA and protein in the normal colorectal mucosa was increased
compared with cancer tissue, suggesting that the colorectal mucosa
loses GOPC expression during carcinogenesis events.
Immunohistochemical analysis demonstrated that the expression of
GOPC protein in cancer tissue, particularly in front invasion of
cancer, was lower compared with normal mucosa. Combined with the
RT-qPCR and immunohistochemical findings of GOPC expression, this
result suggests that loss of GOPC performs an important role in
cancer malignancy.
GOPC also controls postendocytic sorting of several
receptors toward lysosomal degradation (25–28) and
reduces the amount of cell surface receptors (29,30). GOPC
binds to G protein-coupled receptors with a PDZ ligand motif,
including metabotropic glutamate receptors (31,32), the
somatostatin receptor subtype 5 (30,33). It
was recently reported that GOPC knockdown in the HEK293 cell line
reduces internalized β1-AR and increases cell surface β1-AR
(34). Thus, activation of the
MAPK-Erk1/2 pathway was induced increasingly by β1-AR agonists. The
MAPK-Erk1/2 pathway is a cellular signal transduction pathway that
can regulate cell differentiation, proliferation and cell cycle
progression and is a major pathway inducing the progression of
colorectal cancer (9–13). The present study revealed that lower
expression of GOPC increases the risk of recurrence, metastasis,
and a poor prognosis in colorectal cancer. In colorectal cancer,
whether GOPC expression increases activation of MAPK-Erk1/2 via the
β1-AR cascade remains unknown. In addition, although the present
study suggested that the lower expression of GOPC increases the
proportion of recurrence subsequent to chemotherapy, there is no
report that has clarified the correlation of GOPC expression and
chemoresistance. The authors are now preparing in vitro and
in vivo assays focusing on the GOPC-β1-AR-MAPK-Erk1/2
pathway in colorectal cancer.
The present study demonstrated that lower GOPC
expression significantly correlates with poorer OS and RFS. To the
best of our knowledge, the present study is the first to clarify
the correlation between GOPC expression and prognosis in colorectal
cancer and demonstrates that GOPC is a possible marker for poor
prognosis in this disease.
Acknowledgements
The authors would like to express special thanks to
all surgeons working at Minoh City Hospital, Kansai Rosai Hospital,
Kinki Central Hospital of the Mutual Aid Association of Public
School Teachers, National Hospital Organization Osaka National
Hospital, NTT (Nippon Telegraph And Telephone) West Osaka Hospital,
Osaka Medical Center for Cancer and Cardiovascular Diseases,
Saiseikai Suita Hospital, Sakai City Medical Center, Toyonaka
Municipal Hospital (all in Osaka, Japan). The authors would also
like to thank Ms. Yurika Nakamura for technical assistance.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jonker DJ, O'Callaghan CJ, Karapetis CS,
Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, et
al: Cetuximab for the treatment of colorectal cancer. N Engl J Med.
357:2040–2048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karapetis CS, Khambata-Ford S, Jonker DJ,
O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD,
Robitaille S, et al: K-ras mutations and benefit from cetuximab in
advanced colorectal cancer. N Engl J Med. 359:1757–1765. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E, Peeters M, Siena S, Humblet
Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J,
Richardson G, et al: Open-label phase III trial of panitumumab plus
best supportive care compared with best supportive care alone in
patients with chemotherapy-refractory metastatic colorectal cancer.
J Clin Oncol. 25:1658–1664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adachi T, Hinoi T, Egi H, Shimomura M and
Ohdan H: Oxaliplatin and molecular-targeted drug therapies improved
the overall survival in colorectal cancer patients with synchronous
peritoneal carcinomatosis undergoing incomplete cytoreductive
surgery. Surg Today. 45:986–992. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beppu N, Yoshie H, Kimura F, Aihara T, Doi
H, Kamikonya N, Matsubara N, Tomita N, Yanagi H and Yamanaka N: The
short-term outcomes of induction SOX (S-1 + oxaliplatin) +/−
cetuximab chemotherapy followed by short-course chemoradiotherapy
in patients with poor-risk locally advanced rectal cancer. Surg
Today. 46:1123–1131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Keyse SM: Dual-specificity MAP kinase
phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 27:253–261.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dunn KL, Espino PS, Drobic B, He S and
Davie JR: The Ras-MAPK signal transduction pathway, cancer and
chromatin remodeling. Biochem Cell Biol. 83:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoon S and Seger R: The extracellular
signal-regulated kinase: Multiple substrates regulate diverse
cellular functions. Growth Factors. 24:21–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leslie H, Sobin MKG and Christian
Wittekind: TNM classification of malignant tumors. 7th.
Wiley-Blackwell; 2011
|
|
15
|
Larionov A, Krause A and Miller W: A
standard curve based method for relative real time PCR data
processing. BMC Bioinformatics. 6:622005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yao R, Maeda T, Takada S and Noda T:
Identification of a PDZ domain containing Golgi protein, GOPC, as
an interaction partner of frizzled. Biochem Biophys Res Commun.
286:771–778. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Neudauer CL, Joberty G and Macara IG:
PIST: A novel PDZ/coiled-coil domain binding partner for the
rho-family GTPase TC10. Biochem Biophys Res Commun. 280:541–547.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pelaseyed T and Hansson GC: CFTR anion
channel modulates expression of human transmembrane mucin MUC3
through the PDZ protein GOPC. J Cell Sci. 124:3074–3083. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Charest A, Lane K, McMahon K and Housman
DE: Association of a novel PDZ domain-containing peripheral Golgi
protein with the Q-SNARE (Q-soluble N-ethylmaleimide-sensitive
fusion protein (NSF) attachment protein receptor) protein syntaxin
6. J Biol Chem. 276:29456–29465. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meiffren G, Joubert PE, Gregoire IP,
Codogno P, Rabourdin-Combe C and Faure M: Pathogen recognition by
the cell surface receptor CD46 induces autophagy. Autophagy.
6:299–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu R, Stewart L and Wilson JM: Scaffolding
protein GOPC regulates tight junction structure. Cell Tissue Res.
360:321–332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Charest A, Lane K, McMahon K, Park J,
Preisinger E, Conroy H and Housman D: Fusion of FIG to the receptor
tyrosine kinase ROS in a glioblastoma with an interstitial
del(6)(q21q21). Genes Chromosomes Cancer. 37:58–71. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saborowski A, Saborowski M, Davare MA,
Druker BJ, Klimstra DS and Lowe SW: Mouse model of intrahepatic
cholangiocarcinoma validates FIG-ROS as a potent fusion oncogene
and therapeutic target. Proc Natl Acad Sci USA. 110:19513–19518.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng G, Hu C, Zhu L, Huang F, Huang W, Xu
H and Nie W: Downregulation of ROS-FIG inhibits cell proliferation,
colony formation, cell cycle progression, migration and invasion,
while inducing apoptosis in intrahepatic cholangiocarcinoma cells.
Int J Mol Med. 34:661–668. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng J, Cebotaru V, Cebotaru L and
Guggino WB: Syntaxin 6 and CAL mediate the degradation of the
cystic fibrosis transmembrane conductance regulator. Mol Biol Cell.
21:1178–1187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng J, Moyer BD, Milewski M, Loffing J,
Ikeda M, Mickle JE, Cutting GR, Li M, Stanton BA and Guggino WB: A
Golgi-associated PDZ domain protein modulates cystic fibrosis
transmembrane regulator plasma membrane expression. J Biol Chem.
277:3520–3529. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng J, Wang H and Guggino WB: Regulation
of cystic fibrosis transmembrane regulator trafficking and protein
expression by a Rho family small GTPase TC10. J Biol Chem.
280:3731–3739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao TT, Deacon HW, Reczek D, Bretscher A
and von Zastrow M: A kinase-regulated PDZ-domain interaction
controls endocytic sorting of the beta2-adrenergic receptor.
Nature. 401:286–290. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
He J, Bellini M, Xu J, Castleberry AM and
Hall RA: Interaction with cystic fibrosis transmembrane conductance
regulator-associated ligand (CAL) inhibits beta1-adrenergic
receptor surface expression. J Biol Chem. 279:50190–50196. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bauch C, Koliwer J, Buck F, Hönck HH and
Kreienkamp HJ: Subcellular sorting of the G-protein coupled mouse
somatostatin receptor 5 by a network of PDZ-domain containing
proteins. PLoS One. 9:e885292014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng S, Zhang J, Zhu P, Ma Y, Xiong Y,
Sun L, Xu J, Zhang H and He J: The PDZ domain protein CAL interacts
with mGluR5a and modulates receptor expression. J Neurochem.
112:588–598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Cheng S, Xiong Y, Ma Y, Luo D,
Jeromin A, Zhang H and He J: A novel association of mGluR1a with
the PDZ scaffold protein CAL modulates receptor activity. FEBS
Lett. 582:4117–4124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wente W, Stroh T, Beaudet A, Richter D and
Kreienkamp HJ: Interactions with PDZ domain proteins PIST/GOPC and
PDZK1 regulate intracellular sorting of the somatostatin receptor
subtype 5. J Biol Chem. 280:32419–32425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koliwer J, Park M, Bauch C, von Zastrow M
and Kreienkamp HJ: The golgi-associated PDZ domain protein
PIST/GOPC stabilizes the β1-adrenergic receptor in intracellular
compartments after internalization. J Biol Chem. 290:6120–6129.
2015. View Article : Google Scholar : PubMed/NCBI
|