Introduction
Renal cell carcinoma (RCC) is one of the most common
types of adult malignancies, accounting for >61,000 new cases
and >14,000 mortalities each year (1). Clear cell renal cell carcinoma (cc-RCC),
which originates from the renal proximal tubule, is the most common
histological subtype of RCC (2).
cc-RCC accounts for 80–90% all RCC cases (2). Extensive efforts have been made to
investigate the pathogenesis of cc-RCC and have led to important
insights into the biology of the disease. However, there is a lack
of effective treatment for patients with advanced cc-RCC. Patients
with cc-RCC may exhibit no clinical symptoms in the early stage,
and 20–30% of cases are initially diagnosed at the advanced stage
(3). Despite the development of
targeted therapies, the mortality rate of patients with advanced
cc-RCC remains high (4). Surgical
resection remains the principal therapeutic method for localized
cc-RCC and early diagnosis of localized cc-RCC is associated with a
favorable outcome (5).
Engrailed-2 (EN2) is one member of the homeobox
(HOX) gene family and has an important function in the development
of the nervous system at the embryonic stage (6). Previous studies have demonstrated that
the EN2 gene is highly expressed in a number of types of malignant
tumor, including prostate (7), breast
(8) and bladder (9) cancer. However, a previous study by the
present authors revealed that the expression of EN2 gene was
relatively decreased in cc-RCC tissues compared with normal kidney
tissue (10). A previous study
demonstrated that the level of EN2 mRNA in cc-RCC was negatively
associated with tumor differentiation (10). Therefore, one of the main aims of the
present study was to investigate the reasons underlying why the EN2
gene is downregulated in c-RCC.
Numerous studies (11–14) have
demonstrated that aberrant DNA methylation, a typical epigenetic
modification, serves an important function in the tumor suppressor
genes inactivated. The present study hypothesized that promoter DNA
methylation may be the mechanism that leads to the downregulation
of the EN2 gene in cc-RCC. Therefore, the present study analyzed
the promoter methylation status of the EN2 gene using the
methylation-specific polymerase chain reaction (MSP). Following
demethylation, performed using the DNA methyltransferase inhibitor
5-Aza-dc, alterations in the status of promoter methylation and the
level of EN2 expression were analyzed. Furthermore, the effects of
the re-activation of EN2 on cell proliferation, apoptosis and
invasion were analyzed.
Materials and methods
Cell lines and cell culture
A total of three human cc-RCC cell lines (786-O,
ACHN and A498) and one normal human proximal tubule epithelial cell
line (HK-2) were obtained from the Cell Bank of Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China). HK-2
cells were cultured in Dulbecco's modified Eagle's medium/F12
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The three tumor cell lines were cultured in RPMI 1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc.). All medium was
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin solution (Gibco;
Thermo Fisher Scientific, Inc.). All cell lines were incubated in a
humidified atmosphere containing 5% CO2 at 37°C.
Tissue samples
A total of 40 pairs of tumor tissues and matched
adjacent non-malignant tissues were obtained from patients with
cc-RCC, who underwent radical or partial nephrectomy at the First
Affiliated Hospital of Jinan University (Guangzhou, China) between
January 2007 and August 2015. The average age of the patients was
54.5 years. All patients with cc-RCC were diagnosed by pathological
examination. None of the patients had undergone chemotherapy or
radiotherapy prior to surgery. The clinicopathological features of
these enrolled patients was classified according to the 1997 TNM
system (15) and the General Rules
for Clinical and Pathological Studies on Renal Cell Carcinoma of
the Japanese Urological Association, 3rd edition, 1999 (16). The present study was approved by the
Institutional Review Board of Jinan University, and each patient
signed informed consent.
DNA methyltransferase inhibitor
(5-aza-2-deoxycytidine/5-Aza-dc) treatment
The cc-RCC cell line 786-O was incubated at 37°C
with various concentrations of 5-Aza-dc (0, 2.5, 5, 10 and 20
µmol/l; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 5 days.
Following incubation, changes in EN2 gene promoter methylation and
the restoration of EN2 gene expression were analyzed.
DNA extraction and
methylation-specific PCR (MSP)
Genomic DNA from cell lines and tissue specimens was
extracted using a standard treatment of digestion with
SDS/proteinase K solution, followed by phenol-chloroform extraction
and ethanol precipitation. Subsequently, bisulfite modification of
genomic DNA was performed using the EZ DNA Methylation-Gold kit
(Zymo Research Corp., Irvine, CA, USA) according to the
manufacturer's protocol. The total PCR reaction (25 µl) consisted
of: 0.5 mM primers, 2.5 mM dNTPs, 1X buffer, 2 µl bisulfite-treated
DNA and 0.25 µl (5 U/µl) TaqTM HS (Takara Biotechnology Co., Ltd.,
Dalian, China). Methylation-specific PCR amplification was
performed for 5 min initial denaturation at 95°C, followed by 35
cycles of 94°C for 30 sec, 60°C for 40 sec, 72°C for 30 sec and
72°C extension for 10 min. MSP analysis was performed for EN2 using
primers specific for methylated and unmethylated DNA products. The
primer sequences are as follows: Methylated DNA product: Forward,
5′-AACGCGTTGTTTGTATGC-3′; and reverse, 5′-AAACAATCGCAATAATCCG-3′
(product size, 138 bp) and unmethylated DNA product forward,
5′-GGGAATGTGTTGTTTGTATGT-3′ and reverse
5′-AAACAATCACAATAATCCAACA-3′ (product size, 138 bp). For each set
of DNA modification and PCR, normal human lymphocyte DNA was used
as an unmethylated positive control. The lymphocyte DNA that had
been methylated by SssI methylase served as a methylated positive
control. Distilled water without DNA served as a control for
contamination. PCR products were separated and analyzed using a 3%
agarose gel with appropriate size markers.
RNA extraction and reverse
transcription-quantitative (RT-qPCR)
The 786-O cells were harvested using centrifugation
(300 × g) at 37°C for 5 min, following 5-Aza-dc treatment. The
total RNA was extracted and reverse transcribed into cDNA as
described previously (10). qPCR was
performed using the Applied Biosystems 7500 Fast Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
reaction mixture contained 5 µl cDNA, 0.5 mM primer, 10 µl 2X SYBR
Green PCR SuperMix (Invitrogen; Thermo Fisher Scientific, Inc.) and
4 µl dH2O. The mRNA expression of EN2 gene was
determined using qPCR using the following protocol: 50°C for 2 min,
95°C for 2 min, followed by 40 cycles of 95°C for 15 sec and 60°C
for 32 sec. 18S rRNA was selected as a reference and internal
control. The sequences of the primers are as follows: EN2 forward,
5′-AGAAGAACCCGAACAAAGAG-3′; reverse, 5′-GTACCTGTTGGTCTGGAACTC-3′;
18S ribosomal (r)RNA forward, 5′-CCTGGATACCGCAGCTAGGA-3′ and
reverse, 5′-GCGGCGCAATACGAATGCCCC-3′. The primers were synthesized
by Sangon Biotech Co., Ltd. (Shanghai, China). The relative mRNA
expression of EN2 gene was calculated using the 2−ΔΔCq
method (17).
Protein isolation and western
blotting
Following 5-Aza-dc treatment, the 786-O cells were
submitted to western blot assay as described previously (10). Briefly, total protein was extracted
from cells using radioimmunoprecipitation assay buffer (BestBio,
Shanghai, China). The protein content was quantified using the BCA
Protein Assay kit (BestBio) according to the manufacturer's
protocol. Western blot analysis was performed using 60 µg of
protein lysate isolated from the cells. Subsequently, proteins were
separated using 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (Merck KGaA). The membranes were blocked using
5% bovine serum albumin (Beijing Dingguo Changsheng Biotechnology
Co., Ltd., Beijing, China) in Tris-Buffered Saline with Tween-20
and incubated with a rabbit polyclonal anti-EN2 antibody at a
dilution of 1:1,000 (Abcam, Cambridge, UK; cat. no., ab28731)
overnight at 4°C, or a rabbit monoclonal anti-GAPDH antibody (cat.
no., BM3875; Wuhan Boster Biological Technology, Wuhan, China) at a
dilution of 1:3,000 overnight at 4°C. Subsequently, the membranes
were incubated with horseradish peroxidase-conjugated secondary
antibodies (dilution, 1:2,000; Wuhan Boster Biological Technology;
cat. no., BA1054) at room temperature for 1 h 30 min. Finally, the
membranes were visualized using an enhanced chemiluminescence
detection kit (BestBio).
Cell proliferation assay and apoptosis
assay
Cell proliferation was determined using MTT
colorimetric assay. The 786-O cells were seeded in 96-well plates
(5×103 cells/well) and incubated in RPMI 1640 medium
containing various concentrations of 5-Aza-dc (0, 2.5, 5, 10 and 20
µmol/l) at 37°C for 1, 2, 3 and 5 days. Subsequently, 20 µl MTT (5
mg/ml; Sigma-Aldrich; Merck KGaA) was added to each well, and the
cells were incubated at 37°C for 4 h. Subsequently, the
MTT-containing medium was removed and replaced with 100 µl dimethyl
sulfoxide to dissolve the formazan crystals. Absorbance was
measured at 570 nm using a microplate reader. Following the
deduction of the blank cell absorbance, the cell proliferation rate
(%) was calculated using the following formula: Cell proliferation
rate (%) = [optical density (OD) value of treatment / OD value of
control] × 100.
Following treatment with 5-Aza-dc for 5 days,
apoptotic cells were quantified using the Annexin V-FITC Apoptosis
Detection kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China)
according to the manufacturer's protocol. The cells were analyzed
using a FACS Calibur flow cytometer (BD, Biosciences, Franklin
Lakes, NJ, USA) and apoptosis profiles were calculated using
CellQuest software (V1.0.2; BD, Biosciences).
Cell invasion assay
Cell invasion assays were performed using Transwell
inserts. The 786-O cells were treated with 5-Aza-dc as
aforementioned. To prepare the Transwell inserts (Corning, Inc.,
Corning, NY, USA), the Matrigel (BD Biosciences) was dissolved at
4°C overnight, then diluted in RPMI-1640 medium without FBS.
Subsequently, the Matrigel was added to pre-cooled Transwell
inserts and incubated at 37°C for 2 h to solidify the Matrigel.
Following this, the cells for each group were suspended in
RPMI-1640 medium without serum. Subsequently, 1×104
cells were seeded in the upper Transwell chamber and incubated with
RPMI-1640 medium without FBS for 24 h. The cells on the inner
surface were fixed in methanol and stained with 0.1% crystal violet
at 37°C for 10 min. Invasive cells were observed in six randomly
selected fields, and the number of stained cells was counted at
×200 magnification using an inverted microscope (Olympus
Corporation, Tokyo, Japan).
Statistical analysis
Each experiment was repeated three times. All data
were expressed as the mean ± standard deviation of three
independent experiments. All statistical calculations were
performed using SPSS statistical software (version 20.0; IBM Corp.,
Armonk, NY, USA). The χ2 test or Fisher's exact test
were used to analyze categorical variables, and the one-way
analysis of variance or Wilcoxon rank sum test were used for
analysis of continuous variables. P<0.05 was considered to
indicate a statistically significant difference.
Results
Aberrant methylation status of EN2
gene in cc-RCC samples and cell lines
To investigate whether the promoter of the EN2 gene
in cc-RCC was hyper-methylated, MSP was performed in 40 pairs of
cc-RCC tissues and matched adjacent non-malignant tissues. Aberrant
methylation of EN2 gene was observed in 12/40 cc-RCC samples, but
no methylation of the EN2 gene was observed in adjacent
non-malignant tissues (Fig. 1A). This
difference was considered to be significant (P<0.01).
Subsequently, the methylation status of EN2 gene was evaluated in
cc-RCC cell lines (786-O, ACHN and A498) and a normal human
proximal tubule epithelial cell line (HK-2). Hyper-methylation of
the EN2 gene was identified in all cc-RCC cell lines compared with
HK-2. The results of the present study revealed complete EN2
promoter methylation in 786-O cells, partial methylation in ACHN
and A498, and in HK-2 cells the EN2 promoter was unmethylated
(Fig. 1B).
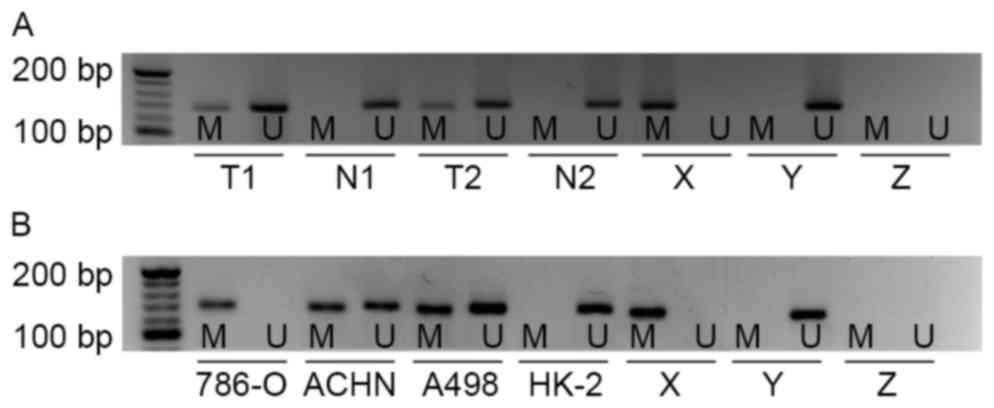 | Figure 1.EN2 gene promoter methylation status
determined in primary kidney tissues and cell lines. (A) EN2 gene
promoter methylation in primary cc-RCC tissues and corresponding
non-malignant kidney tissues as indicated by MSP analysis. (B) EN2
gene promoter methylation in cc-RCC cell lines: 786-O, ACHN, A498
and normal human proximal tubule epithelial cell line HK-2 as
indicated by MSP analysis. cc-RCC, clear cell renal cell carcinoma;
EN2, engrailed-2; MSP, methylation-specific polymerase chain
reaction; M, presence of methylated DNA; U, presence of
unmethylated DNA; T, primary cc-RCC tissue, T1, tissue sample 1,
T2, tissue sample 2; N, non-malignant kidney tissue; X, methylated
positive control; Y, unmethylated positive control; Z, negative
control. |
The association between the frequency of EN2
promoter methylation and the clinical pathological parameters of
cc-RCC was analyzed. The frequency of EN2 promoter methylation was
identified to be positively associated with histological grade
(P=0.043) and tumor size (P=0.001) of cc-RCC (Table I). However, there was no statistical
association identified between the frequency of EN2 promoter
methylation and sex, age, tumor stage or lymph node metastasis
(Table I).
 | Table I.Association between methylation
status of EN2 with clinicopathological variables in patients with
cc-RCC. |
Table I.
Association between methylation
status of EN2 with clinicopathological variables in patients with
cc-RCC.
|
|
| No. of patients
with EN2 DNA methylation |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | Patients
(n=40) | M | U | χ2 |
P-valuea |
|---|
| Tumor tissues | 40 | 12 | 28 | 14.118 |
<0.0001a |
| Normal tissues | 40 | 0 | 40 |
|
|
| Age, years |
|
|
|
|
|
|
≤60 | 25 | 10 | 15 |
2.032 | 0.154 |
|
>60 | 15 | 2 | 13 |
|
|
| Sex |
|
|
|
|
|
|
Male | 26 | 5 | 21 |
2.768 | 0.096 |
|
Female | 14 | 7 | 7 |
|
|
| Size of tumor,
cm |
|
|
|
|
|
| ≤4 | 13 | 2 | 11 |
13.808 | 0.001a |
|
4–7 | 19 | 3 | 16 |
|
|
|
>7 | 8 | 7 | 1 |
|
|
| TNM stage |
|
|
|
|
|
|
I/II | 34 | 9 | 25 |
0.458 | 0.499 |
|
III/IV | 6 | 3 | 3 |
|
|
| Grade |
|
|
|
|
|
| G1 | 6 | 1 | 5 |
5.926 | 0.043a |
| G2 | 22 | 4 | 18 |
|
|
| G3 | 12 | 7 | 5 |
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
Yes | 5 | 3 | 2 |
0.798 | 0.372 |
| No | 35 | 10 | 25 |
|
|
Demethylation and re-expression of EN2
gene is induced following 5-Aza-dc treatment
Based on the MSP results of cc-RCC cell lines, the
786-O cell line was selected for further study. To validate whether
downregulation of EN2 gene was associated with aberrant promoter
methylation, the expression of EN2 gene was analyzed using RT-qPCR
and western blot following treatment with different concentrations
of 5-Aza-dc (0, 2.5, 5, 10 and 20 µM) for 5 days. As determined
using RT-qPCR, the mRNA expression of EN2 was increased in a
concentration-dependent manner following treatment with 5-Aza-dc
(Fig. 2A). The expression of EN2 in
the 20 µM group was 5.04-fold higher compared with the 0 µM group
(2−∆∆Cq, 5.15±0.25 vs. 1.02±0.21; P<0.01). Consistent
with the results of RT-qPCR, an increase in the levels of EN2 was
observed following treatment with 5-Aza-dc (Fig. 2B). Furthermore, the methylation status
of EN2 gene was decreased following treatment with 5-Aza-dc, in a
dose-dependent manner (Fig. 2C). The
results of the present study indicated that downregulation of the
EN2 gene may be regulated by hyper-methylation of the EN2
promoter.
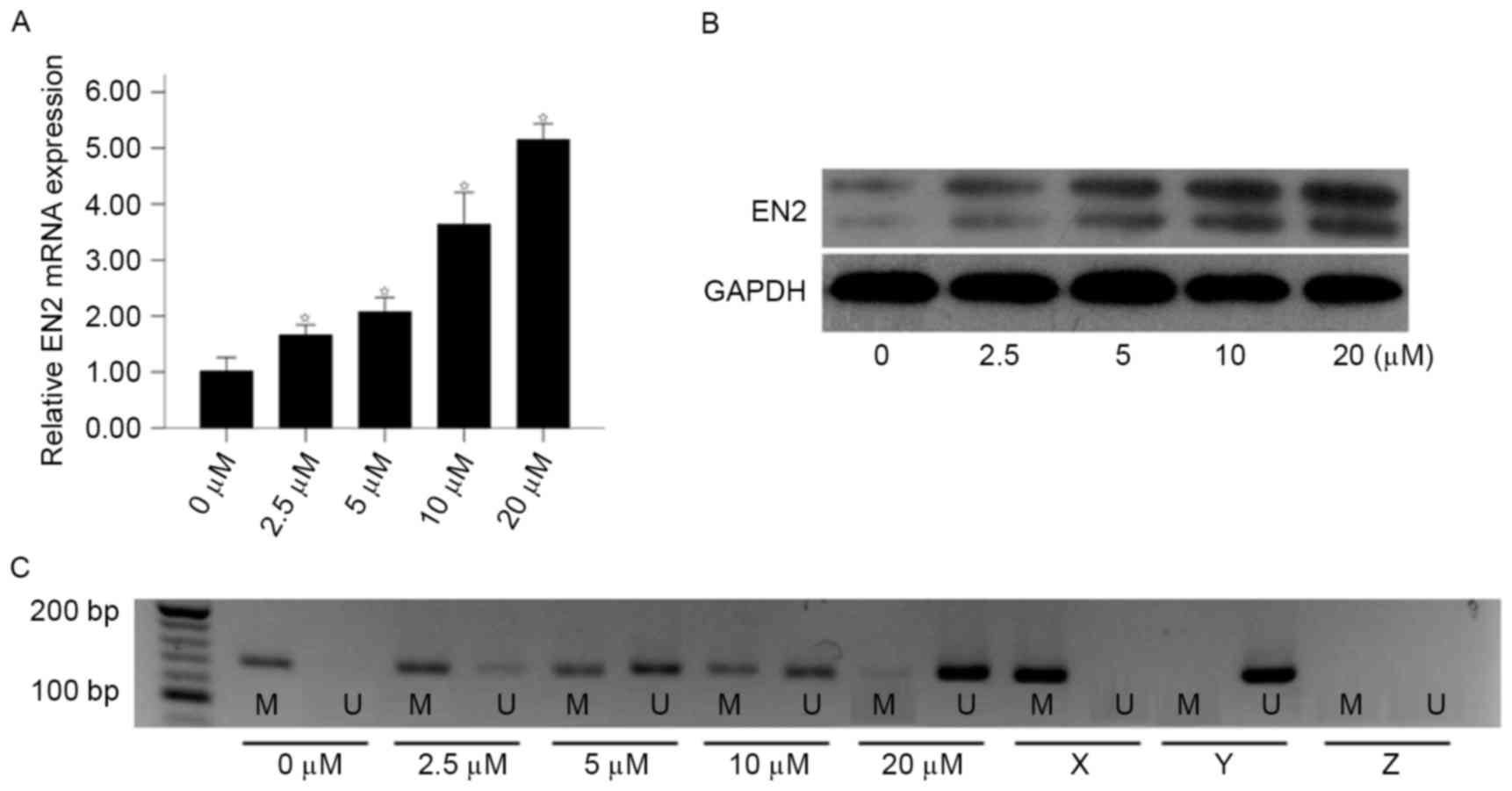 | Figure 2.Effect of 5-Aza-dc treatment on the
expression of EN2 and promoter methylation status in 786-O cells.
786-O cells were treated with various concentrations of 5-Aza-dc
(0, 2.5, 5, 10 or 20 µM) for 5 days. (A) The relative EN2 mRNA
expression increased following treatment with 5-Aza-dc, analyzed
using reverse transcription-quantitative polymerase chain reaction.
(B) Effect of 5-Aza-dc treatment on EN2 protein expression as
analyzed by western blotting. (C) Treatment with 5-Aza-dc induced
EN2 gene promoter demethylation as analyzed using MSP. *P<0.05
vs. control (0 µM group). 5-Aza-dc, 5-aza-2-deoxycytidine; EN2,
engrailed-2; MSP, methylation-specific polymerase chain reaction;
M, presence of methylated DNA; U, presence of unmethylated DNA; X,
methylated positive control; Y, unmethylated positive control; Z,
negative control. |
Expression of EN2 induced by 5-Aza-d
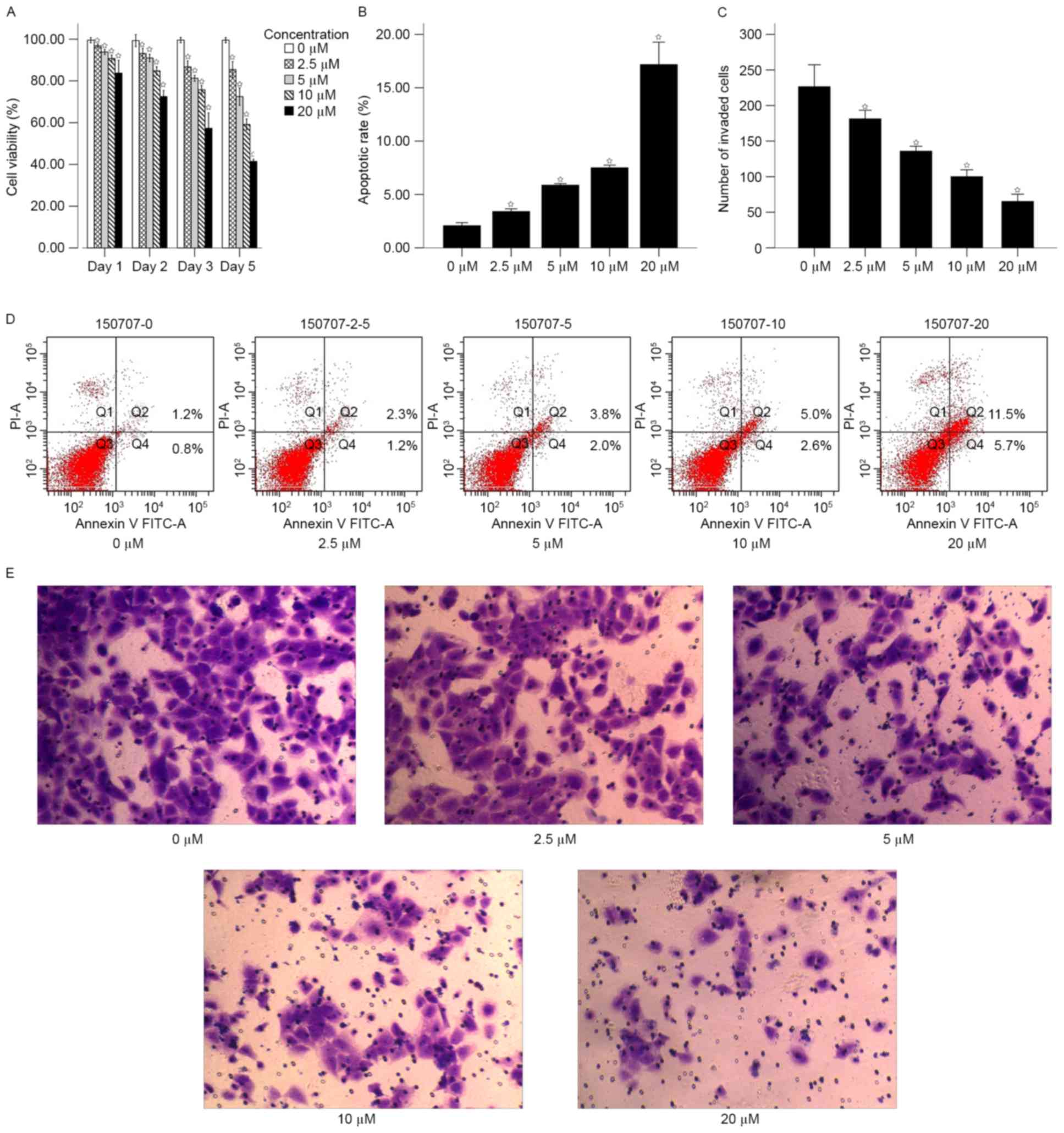
treatment inhibits cell proliferation and invasion
The proliferation of 786-O cells was analyzed
following treatment with 5-Aza-dc (Fig.
3). The results of the MTT assay demonstrated that the
proliferation rate of the cells significantly decreased with the
increase in concentration of 5-Aza-dc compared with the untreated
control after 1, 2, 3 and 5 days (P<0.05; Fig. 3A). The proliferation rate of untreated
control cells were on average 1.19, 1.37, 1.74 and 2.4-fold
increased, compared with the 20 µM groups at 1, 2, 3 and 5 days,
respectively.
Cell apoptosis was subsequently analyzed using flow
cytometry following treatment with 5-Aza-dc for 5 days. The results
suggested that the apoptotic rate increased in a dose-dependent
manner (Fig. 3B and D). The mean
apoptotic rates of for different concentrations of 5-Aza-dc were as
follows: 0 µM, 2.07±0.13; 2.5 µM, 3.40±0.10; 5 µM, 5.87±0.06; 10
µM, 7.50±0.10; and 20 µM, 17.17±0.85. The apoptotic rates of cells
treated with 2.5, 5, 10 and 20 µM were significantly increased
compared with the untreated control (P<0.05; Fig. 3B).
Transwell assay was performed to analyze the effect
of 5-Aza-dc treatment on cell invasion. Following treatment with
5-Aza-dc for 5 days, the mean number of cells passing through the
membrane for different concentrations were as follows: 0 µM,
226.50±29.34; 2.5 µM, 181.33±11.48; 5 µM, 135.83±6.56; 10 µM,
100.00±9.17; and 20 µM, 65.17±9.66. The number of cells invaded in
the 2.5, 5, 10 and 20 µM 5-Aza-dc treatment groups were
significantly increased compared with the untreated control
(P<0.05; Fig. 3C and E).
Discussion
HOX genes act as transcription factors (18,19). HOX
genes can bind to specific DNA regions to activate or repress the
expression of targeted genes, which are involved in a number of
biological processes including cell apoptosis, motility and
differentiation, angiogenesis and maintenance of organ function
(20,21). The EN2 gene belongs to the HOX family
and serve a crucial function in the development of the nervous
system at the human embryonic stage (22). In adults, aberrant expression of EN2
gene may be associated with tumorigenesis of a number of types of
solid tumor (8,9,23–25). Morgan et al (25) observed EN2 was expressed in, and
secreted by prostate cancer (PCa) cell lines and tissues, but not
by normal prostate tissue. In addition, it was reported that
urinary EN2 may be a potential diagnostic biomarker for PCa, with a
sensitivity of 66% and a specificity of 88.2% (25). These observations were consistent with
the results of Marszałł et al (23). Furthermore, a previous study
identified a statistical significant association between
pre-surgical urinary EN2 levels and cancer volume in radical
prostatectomy specimens (24).
Additionally, the upregulation of EN2 gene was associated with
progression in breast (8) and bladder
(9) cancer.
A preliminary study by the present authors revealed
that EN2 expression was downregulated in cc-RCC primary tissues and
cell lines, whereas it was relatively highly expressed in normal
proximal convoluted renal tubules and distal convoluted renal
tubule epithelial cells (10). In
another study by the present authors, EN2 expression was inhibited
in HK-2 cells using lentivirus-mediated transfection, and
overexpression of EN2 in 786-O cells was induced via plasmid
transfection (26). Following the
inhibition of the EN2 gene, proliferation and invasion of HK-2
cells were promoted. However the inhibition of the EN2 gene also
resulted in a shortened cell cycle and inhibition of apoptosis. By
contrast, overexpression of EN2 gene was able to promote apoptosis
and reduce invasive ability and suppress proliferative ability of
786-O cells (26). These findings
indicated that EN2 may have an anti-oncogenic function in cc-RCC.
However, the mechanism leading to the downregulation of the EN2
gene in cc-RCC remains unknown.
There are several molecules that serve as two-way
regulatory factors by either activating or repressing oncogenesis
in different types of cancer (27–30). Most
members of the HOX gene family are regarded as bidirectional
regulation factors due to their tissue-specific expression and
regulation (31). For example, HOXB13
was observed to be overexpressed in ovarian (32) and breast (33) cancer. Hwever, Jung et al
(34) also reported that HOXB13 was
downregulated in prostate cancer. Abate-Shen (35) proposed three mechanisms that drive the
deregulation of HOX genes. One of these mechanisms was an
epigenetic modification that leads to downregulation or silencing
of HOX genes (35).
Hyper-methylation of EN2 has been identified in
other diseases (36,37). Abollo-Jiménez et al (36) identified that the EN2 gene promoter
was methylated during the progression of chronic myelogenous
leukemia to T-cell lineage-specific blast crisis. In addition,
Lambert et al (37) reported
that the EN2 gene was hyper-methylated in pilocytic astrocytoma
(PA). The methylation status of the EN2 gene was associated with
the location of PA (infratentorial vs. supratentorial) (37). Therefore, in the present study, it was
hypothesized that aberrant methylation of the promoter may be the
mechanism leading to the downregulation of the EN2 gene in
cc-RCC.
To validate the aforementioned hypothesis, the
methylation status of EN2 was analyzed in cc-RCC tissue specimens
and cell lines, using MSP analysis in the present study. The
results of the present study demonstrated that EN2 gene promoter
was hyper-methylated in 12/40 primary cc-RCC tissues and all tested
cc-RCC cell lines, whereas none of the 40 adjacent non-malignant
tissues revealed hyper-methylation. The results of the present
study were similar to the findings reported by Slater et al
(38). Slater et al observed
that EN2 was hyper-methylated in chromophobe RCC (6/20) but not
renal oncocytoma (0/21). However, they did not investigate the
function of EN2 methylation in chromophobe RCC (38). In the present study, the analysis of
association between EN2 methylation and clinicopathological
parameters indicated that EN2 methylation was significantly
associated with increased malignant behavior in cc-RCC.
Subsequently, 786-O cells, which revealed complete methylation,
were selected to asses whether promoter methylation underlie the
silencing of the EN2 gene in cc-RCC. Compared with untreated
methylated 786-O cells, treatment of the cells with 5-Aza-dc
increased the expression of EN2 mRNA and decreased the methylation
of the EN2 promoter. These results suggested that promoter
methylation was involved in the silencing of EN2 in cc-RCC.
Following 5-Aza-dc treatment, EN2 was re-expressed and methylation
status of the EN2 promoter was decreased. These results suggested
that promoter methylation was involved in the silencing of EN2 gene
in cc-RCC.
Following EN2 gene re-activation, proliferation and
invasion of 786-O cells were markedly inhibited, whereas the
apoptosis of 786-O cell was increased. It indicated that silencing
of EN2 gene may promote the progression of cc-RCC.
However, there are several limitations of the
present study that should be clarified. One of the limitations is
the small sample size used. Therefore, additional studies with
larger numbers of patients would be useful to demonstrate the use
of EN2 as a biomarker. In addition, the present study was not able
to determine the exact sites of CpG methylation of the EN2 gene
promoter. In conclusion, the present study used tissue samples and
cell lines to confirm that the EN2 gene promoter was
hyper-methylated in cc-RCC, which may underlie the silencing of the
EN2 gene.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81460386), the
Natural Science Foundation of JiangXi Province (grant no.
20151BAB205014) and the Major Research Fund for the People's
Livelihood of Guangzhou Science and Technology Plan (grant no.
2011Y-00003).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vincenzi B, Santini D and Tonini G:
Renal-cell carcinoma. N Engl J Med. 354:1095–1096. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lam JS, Leppert JT, Belldegrun AS and
Figlin RA: Novel approaches in the therapy of metastatic renal cell
carcinoma. World J Urol. 23:202–212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Volpe A and Patard JJ: Prognostic factors
in renal cell carcinoma. World J Urol. 28:319–327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simon HH, Thuret S and Alberi L: Midbrain
dopaminergic neurons: Control of their cell fate by the engrailed
transcription factors. Cell Tissue Res. 318:53–61. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morgan R, Boxall A, Bhatt A, Bailey M,
Hindley R, Langley S, Whitaker HC, Neal DE, Ismail M, Whitaker H,
et al: Engrailed-2 (En2): A highly specific urinary biomarker for
the early diagnosis of prostate cancer. Clin Cancer Res.
17:1090–1098. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin NL, Saba-El-Leil MK, Sadekova S,
Meloche S and Sauvageau G: EN2 is a candidate oncogene in human
breast cancer. Oncogene. 24:6890–6901. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morgan R, Bryan RT, Javed S, Launchbury F,
Zeegers MP, Cheng KK, James ND, Wallace DM, Hurst CD, Ward DG, et
al: Expression of Engrailed-2 (EN2) protein in bladder cancer and
its potential utility as a urinary diagnostic biomarker. Eur J
Cancer. 49:2214–2222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai CY, Pan B, Luo Y, Liang WB, Chen J, Ye
DM, Guo JN, Li L and Su ZX: Engrailed-2 is down-regulated but also
ectopically expressed in clear cell renal cell carcinoma. Mol Biol
Rep. 41:3651–3657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang JC, Zhang H and Jin SF: Epigenetics
and cervical cancer: From pathogenesis to therapy. Tumor Biol.
35:5083–5093. 2014. View Article : Google Scholar
|
|
12
|
Hauser S, Zahalka T, Fechner G, Muller SC
and Ellinger J: Serum DNA hypermethylation in patients with kidney
cancer: Results of a prospective study. Anticancer Res.
33:4651–4656. 2013.PubMed/NCBI
|
|
13
|
Chmelarova M, Krepinska E, Spacek J, Laco
J, Beranek M and Palicka V: Methylation in the p53 promoter in
epithelial ovarian cancer. Clin Transl Oncol. 15:160–163. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cancer Genome Atlas Research Network:
Comprehensive molecular characterization of clear cell renal cell
carcinoma. Nature. 499:43–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guinan P, Sobin LH, Algaba F, Badellino F,
Kameyama S, MacLennan G and Novick A: TNM staging of renal cell
carcinoma: Workgroup No. 3. Union international contre le cancer
(UICC) and the American joint committee on cancer (AJCC). Cancer.
80:992–993. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Japanease Urological Association, .
General Rule for Clinical and Pathological Studies on Renal Cell
Carcinoma. 3rd. Kanehara Company; Tokyo: 1999
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grier DG, Thompson A, Kwasniewska A,
McGonigle GJ, Halliday HL and Lappin TR: The pathophysiology of HOX
genes and their role in cancer. J Pathol. 205:154–171. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lewis MT: Homeobox genes in mammary gland
development and neoplasia. Breast Cancer Res. 2:158–169. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kelly ZL, Michael A, Butler-Manuel S,
Pandha HS and Morgan RG: HOX genes in ovarian cancer. J Ovarian
Res. 4:162011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Veraksa A, Del Campo M and McGinnis W:
Developmental patterning genes and their conserved functions: From
model organisms to humans. Mol Genet Metab. 69:85–100. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanks M, Wurst W, Anson-Cartwright L,
Auerbach AB and Joyner AL: Rescue of the En-1 mutant phenotype by
replacement of En-1 with En-2. Science. 269:679–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marszałł MP, Sroka W, Adamowski M, Słupski
P, Jarzemski P, Siódmiak J and Odrowąż-Sypniewska G: Engrailed-2
protein as a potential urinary prostate cancer biomarker: A
comparison study before and after digital rectal examination. Eur J
Cancer Prev. 24:51–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pandha H, Sorensen KD, Orntoft TF, Langley
S, Hoyer S, Borre M and Morgan R: Urinary engrailed-2 (EN2) levels
predict tumour volume in men undergoing radical prostatectomy for
prostate cancer. BJU Int. 110:E287–E292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morgan R, Boxall A, Bhatt A, Bailey M,
Hindley R, Langley S, Whitaker HC, Neal DE, Ismail M, Whitaker H,
et al: Engrailed-2 (EN2): A tumor specific urinary biomarker for
the early diagnosis of prostate cancer. Clin Cancer Res.
17:1090–1098. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lai CY, Xu Y, Yu GS, Wu X, Li YF, Pan B,
Heng BL, Xue YJ and Su ZX: Engrailed-2 might play an anti-oncogenic
role in clear-cell renal cell carcinoma. J Mol Histol. 47:229–237.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Farago M, Dominguez I, Landesman-Bollag E,
Xu X, Rosner A, Cardiff RD and Seldin DC: Kinase-inactive glycogen
synthase kinase 3beta promotes Wnt signaling and mammary
tumorigenesis. Cancer Res. 65:5792–5801. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao Q, Lu X and Feng YJ: Glycogen synthase
kinase-3beta positively regulates the proliferation of human
ovarian cancer cells. Cell Res. 16:671–677. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang T, Wei Y, Honaker Y, Yamaguchi H,
Appella E, Hung MC and Piwnica-Worms H: GSK-3 beta targets Cdc25A
for ubiquitin-mediated proteolysis and GSK-3 beta inactivation
correlates with Cdc25A overproduction in human cancers. Cancer
Cell. 13:36–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mai W, Kawakami K, Shakoori A, Kyo S,
Miyashita K, Yokoi K, Jin M, Shimasaki T, Motoo Y and Minamoto T:
Deregulated GSK3{beta} sustains gastrointestinal cancer cells
survival by modulating human telomerase reverse transcriptase and
telomerase. Clin Cancer Res. 15:6810–6819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shah N and Sukumar S: The Hox genes and
their roles in oncogenesis. Nat Rev Cancer. 10:361–371. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miao J, Wang Z, Provencher H, Muir B,
Dahiya S, Carney E, Leong CO, Sgroi DC and Orsulic S: HOXB13
promotes ovarian cancer progression. Proc Natl Acad Sci USA.
104:17093–17098. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Z, Dahiya S, Provencher H, Muir B,
Carney E, Coser K, Shioda T, Ma XJ and Sgroi DC: The prognostic
biomarkers HOXB13, IL17BR, and CHDH are regulated by estrogen in
breast cancer. Clin Cancer Res. 13:6327–6334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jung C, Kim RS, Zhang HJ, Lee SJ and Jeng
MH: HOXB13 induces growth suppression of prostate cancer cells as a
repressor of hormone-activated androgen receptor signaling. Cancer
Res. 64:9185–9192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abate-Shen C: Deregulated homeobox gene
expression in cancer: Cause or consequence? Nat Rev Cancer.
2:777–785. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abollo-Jiménez F, Campos-Sánchez E,
Toboso-Navasa A, Vicente-Dueñas C, González-Herrero I,
Alonso-Escudero E, González M, Segura V, Blanco O, Martínez-Climent
JA, et al: Lineage-specific function of Engrailed-2 in the
progression of chronic myelogenous leukemia to T-cell blast crisis.
Cell Cycle. 13:1717–1726. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lambert SR, Witt H, Hovestadt V, Zucknick
M, Kool M, Pearson DM, Korshunov A, Ryzhova M, Ichimura K, Jabado
N, et al: Differential expression and methylation of brain
developmental genes define location-specific subsets of pilocytic
astrocytoma. Acta Neuropathol. 126:291–301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Slater AA, Alokail M, Gentle D, Yao M,
Kovacs G, Maher ER and Latif F: DNA methylation profiling
distinguishes histological subtypes of renal cell carcinoma.
Epigenetics. 8:252–267. 2013. View Article : Google Scholar : PubMed/NCBI
|