Introduction
Malignant mixed mullerian tumors (MMMTs) are
uncommon and lethal neoplasms. They occur in postmenopausal females
and comprise only 1–2% of uterine cancer and 3–5% of all uterine
malignancies (1). Clinical
presentation usually consists of abdominal pain, distension and
atypical bleeding. Though common in the uterus, these tumors may
also arise epithelial (carcinoma) and mesenchymal (sarcoma)
elements (1).
Two of the organs most frequently affected by cancer
metastasis are the lungs, and at some stage, ~30% of all cancer
patients will develop lung metastases (2). This is mainly as the lung is the first
filter in the general circulation (3,4). Secondly,
the low pressure system and slow blood flow velocities of the
pulmonary circulation are apt to allow tumor cells to stagnate
(3,4).
Metastatic neoplasm often present as multiple lung nodules and
masses when incidental can be detected by a diagnostic chest
radiography of computed tomography (CT) scan (5). Metastatic lung cancer frequently has
non-specific symptoms and CT scans reveal single or multiple
spherical nodules with a smooth periphery (5). However, metastatic lung cancer
presenting with sustained hemoptysis as the initial symptom, with
multiple nodular opacities surrounded by a ground-glass attenuation
halo (halo-sign), which are commonly observed in pulmonary
aspergillosis (6), have not been
reported before.
The present study reports a case of MMMTs with lung
metastasis in a patient who intially presented with multiple
pulmonary nodular opacities surrounded by a ground-glass
attenuation halo, mimicking invasive aspergillosis.
Case report
A 58-year-old woman was referred to the Department
of Respiratory and Critical Care Medicine (Union Hospital, Tongji
Medical College, Huazhong University of Science and Technology,
Wuhan, Hubei, China) with hemoptysis and post-menopausal vaginal
bleeding on January 10th, 2015. At 4 months prior to this
admission, the patient presented with hemoptysis, or bloody sputum,
after a rough cough. The patient reported no fever, night sweats or
chest pain. At 1 month prior to the present admission, the patient
presented with vaginal bleeding, ~5 ml every day, without cervical
pain. The patient was initially treated with empiric antibiotics
for presumptive pneumonia in local hospital. Short-term follow-up
revealed that the hemoptysis and vaginal bleeding of the patient
had progressively worsened over time, thus the patient was admitted
to our hospital for further evaluation.
A chest examination was normal. No detectable
peripheral lymphadenopathy was found. Laboratory results included
the following: normal creatinine, blood urea nitrogen and serum
electrolyte levels; aminotransferase, 8 U/l (5–35 U/l); aspartate
aminotransferase, 12 U/l (8–40 U/l); albumin, 37.2 g/l (35–55 g/l);
total protein, 61.0 g/l (64–83 g/l); leukocyte count,
4.87×109/l (3.5–5.5×109/l); red blood cell
count, 3.27×1012/(3.8–5.1×1012/l);
hemoglobin, 79 g/l (110–150 g/l); and platelet count,
281×109/l (125–350×109/l). Serum test results
were negative for carcinoembryonic antigen (CEA), carbohydrate
antigen (CA125), hepatitis B virus, human immunodeficiency virus,
hepatitis C virus, 1–3-β glucan antigen-D, galactomannan antigen,
tuberculosis antibody, immunoglobulin E and procalcitonin.
Additionally, a serum test showed an erythrocyte sedimentation rate
of 36 mm/h (<20 mm/h) and a C-reactive protein level of 3.48
mg/l (0–8 mg/l). Sputum smear and cultures were negative for
bacteria, fungus and Mycobacterium tuberculosis. Other
results were as follows: Urinary occult blood test, 3+; and urinary
red blood cell quantification, 194.0/µl (<25/µl).
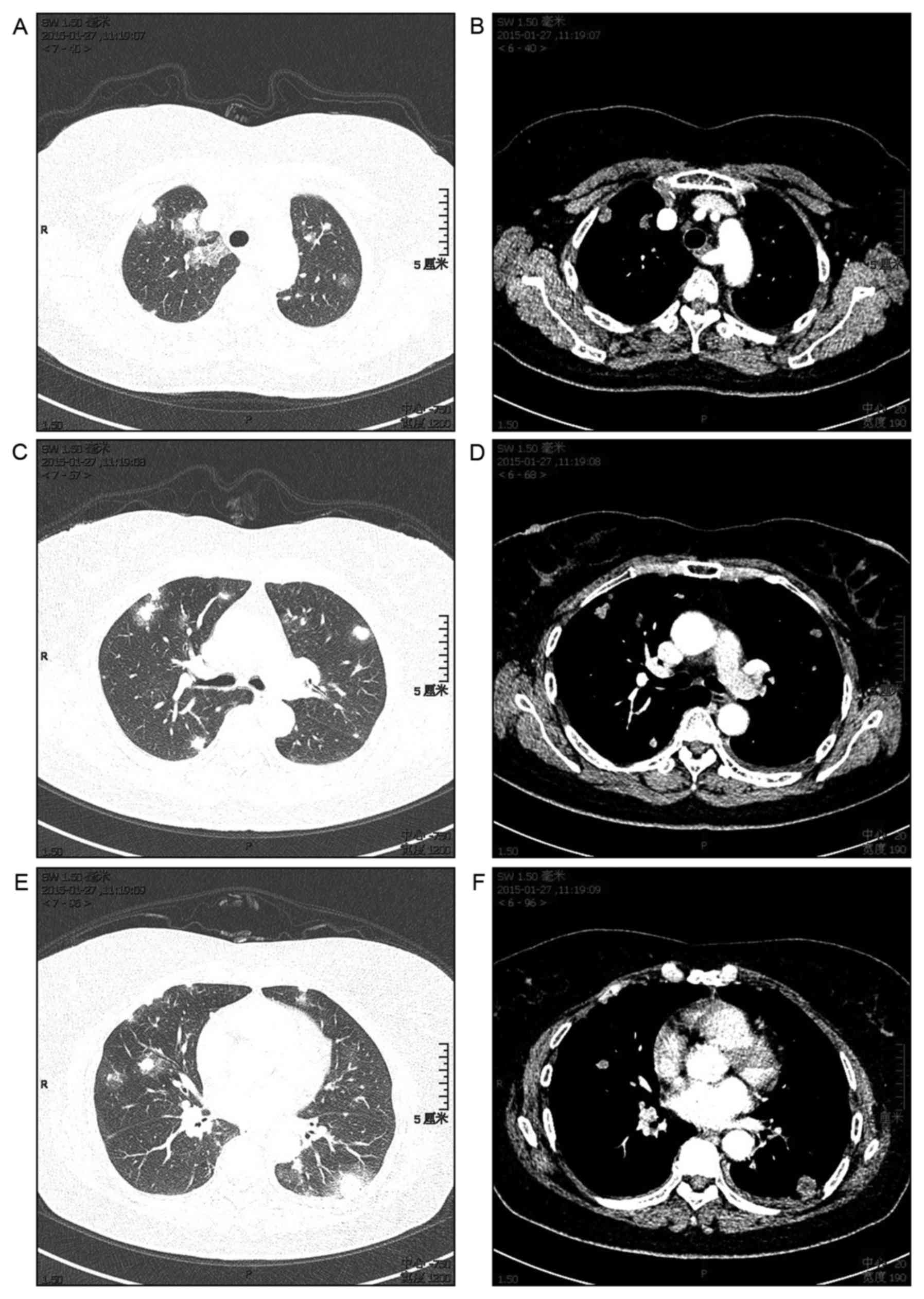
Chest CT revealed multiple nodular opacities
surrounded by a ground-glass attenuation halo (halo-sign) in the
peripheral regions of the bilateral lung field. The majority of the
lesions reached underneath the pleura, interstitial shadows such as
ground-glass-like shadows and thickening of the interlobular walls
were observed, and a small amount of bilateral pleural effusion
existed (Fig. 1).
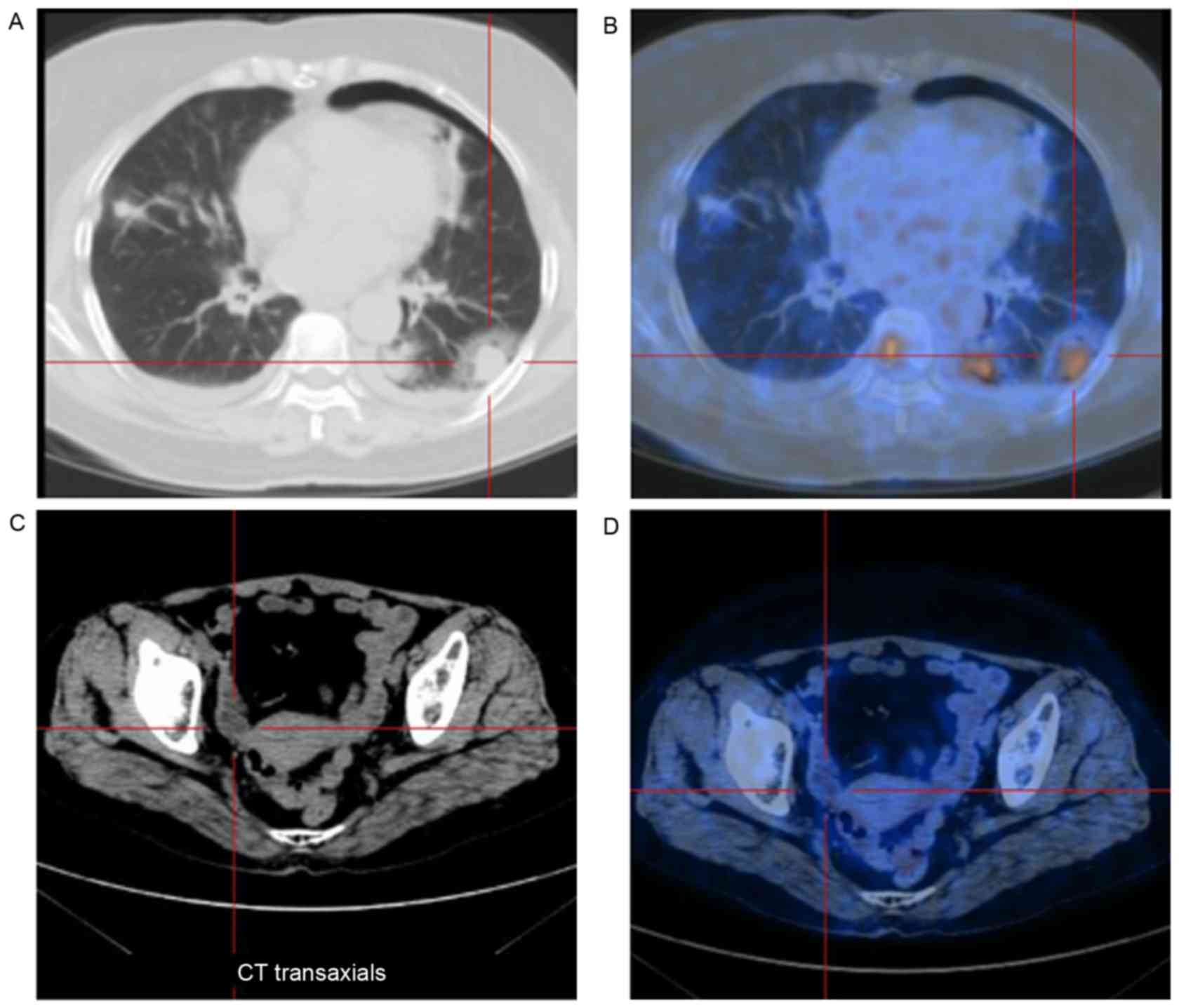
A whole-body fluorine-18 fluorodeoxyglucose positron
emission tomography (18F-FDG-PET) scan revealed mild
18F-FDG uptake in several nodular lesions in the lung;
the maximum standand uptake value (SUVmax) was within the range of
2.0–10.4 (normal value, <2.5). Furthermore, highly abnormal
accumulation was found in a number of bone lesions, including those
of the collarbone, ribs, femur and pelvis; the SUVmax was within
the range of 4.5–11.2, demonstrating multiple bony metastases.
However, no definite FDG uptake was found in the cervix and uterus,
which may have been diminished due to the diagnostic curettage of
the cervix and endometrium performed prior to the PET scan
(Fig. 2).
Bronchoscopic examination revealed only a small
volume of fresh blood in the right main bronchus. The washing fluid
from the alveoli was a reddish color and a cytological smear test
showed some epithelial cells, lymphocytes, granulocytes and a small
amount of other cells.
Uterine ultrasound showed a postmenopausal uterus
with endometrial hyperplasia. The initial cytological diagnosis of
the Pap smear was of cervical adenocarcinoma (data not shown).
Subsequently, biopsies of the cervix and endometrium were performed
via diagnostic curettage. The cervical biopsy at the 3, 6, 9 and 12
o'clock positions of the endometrium revealed chronic cervicitis
associated with coating squamous epithelial hyperplasia and focally
squamous metaplasia of the cervical gland (data not shown). The
genetic detection of human papillomavirus via the cervical swab was
negative.
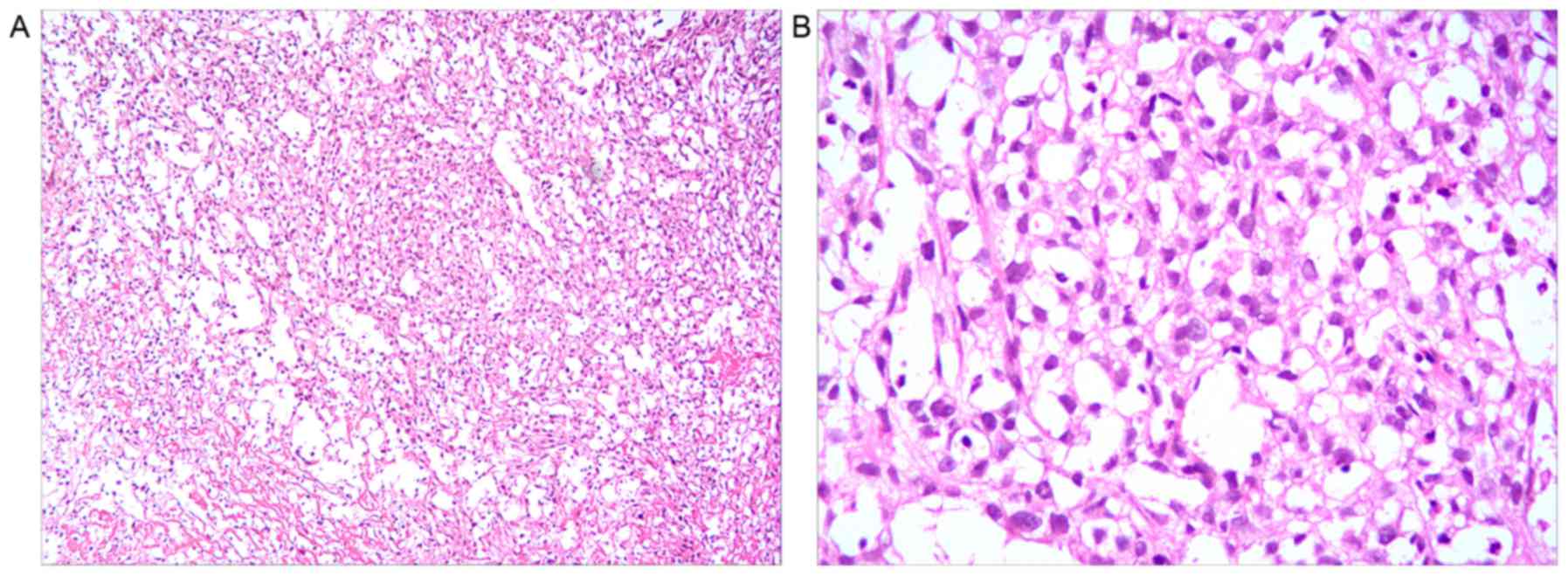
Cervical canals and uterus biopsies were fixed in
10% formalin for 6 h at 37°C, embedded with paraffin, cut into 5 µm
section and stained with hematoxylin-eosin for 20 min at room
temperature. Finally, slides were viewed using an imaging
microscope (magnification, ×100, Olympus BX51; Olympus, Tokyo,
Japan). The cells were observed to exhibit spindle nuclei, nuclear
pleomorphism, partial necrosis and scanty indistinct cytoplasm, and
the tissues showed biphasic tumors with epithelial and mesenchymal
components, which was consisitent with an MMMT (Fig. 3).
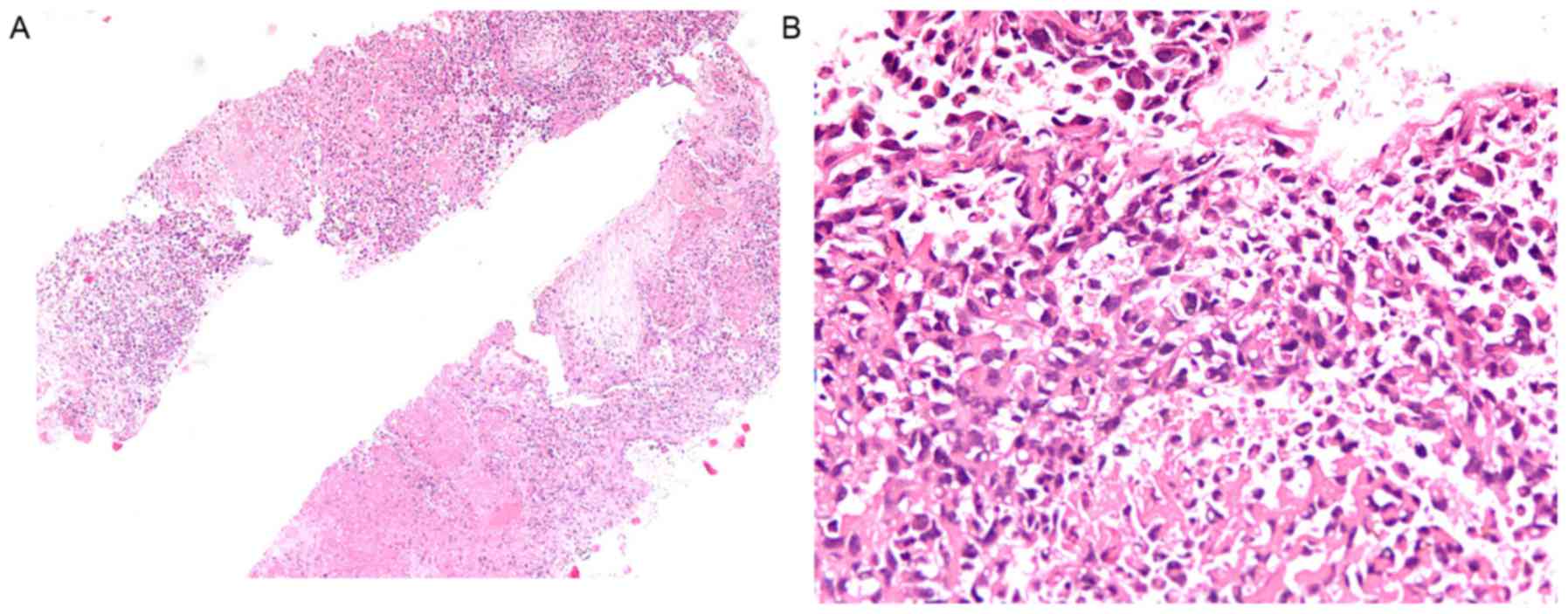
Percutaneous lung sampling by CT-guided biopsy was
conducted for the pathological diagnosis. Histological examination
of the lung biopsy sample revealed an infiltrating tumor of
predominantly sarcoma morphology composed of multiple atypical
spindle cells with hyperchromatic nuclei and multinucleation, and
partial necrosis (Fig. 4). Serial
sections of the lung specimen were used for immunohistochemical
analysis. Primary antibodies used for staining were
anti-human-vimetin mAb (cat. no. LAB040Hu71; Biocompare, San
Antonio, TX, USA), anti-human-p16 (cat. no. MBS9386591;
MyBioSource, San Diego, CA, USA), anti-human-napsin (cat. no.
ab73021; Abcam, Cambrige, UK), anti-human-cytokeratin 7 (CK7) (cat.
no. ZA103; Zomanbio, Beijing, China), anti-human-CK20 (cat. no.
ab76126; Abcam), anti-human-CEA (cat. no. ab133633; Abcam),
anti-human CA125 (cat. no. 119–13259; RayBiotech Inc., Atlanta, GA,
USA), anti-human-CDX2 (cat. no. MAB3665; R&D Systems,
Minneapolis, MN, USA), and anti-human-villin (cat. no. sc-58897;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), all diluted
to 1:100. Secondary antibodies used for staining included
horseradish peroxidase (HRP)-conjugated goat anti-mouse
immunoglobulin G (IgG) (cat. no. 31430) or HRP-conjugated goat
anti-rabbit IgG antibodies (cat. no. 31460; Thermo Fisher
Scientific, Inc.). Slides were de-waxed with xylene and rehydrated
using a graded ethanol series (100, 95, 80, 70 and 50%) into water.
For antigen retrieval, sections were heated in citrate buffer (pH
6.0) for 10 min at 95°C in a microwave oven. After cooling to room
temperature, the sections were digested with 0.05% trypsin for 10
min at 37°C. Endogenous peroxidase activity was quenched using 0.3%
H2O2 in methanol for 30 min at room
temperature. Following PBS washes, non-specific antibody binding
was blocked by incubating slides with 10% normal goat non-immune
serum (cat. no. 50062Z; Invitrogen; Thermo Fisher Scientific, Inc.)
at 37°C for 30 min. Subsequent to washing with PBS, sections were
incubated with primary antibodies at 4°C overnight with 1:100
diluitions. Following further PBS washes, sections were incubated
with the secondary antibodies (1:100) for 30 min at room
temperature. Finally, after incubating with diaminobenzidine for 30
min at room temperature, slides revealed immunoreactivity for
vimentin, and were focally positive for p16 and negative for
napsin, CK7, CK20, CEA, CA125, homeobox protein CDX2 and villin
(Fig. 5).
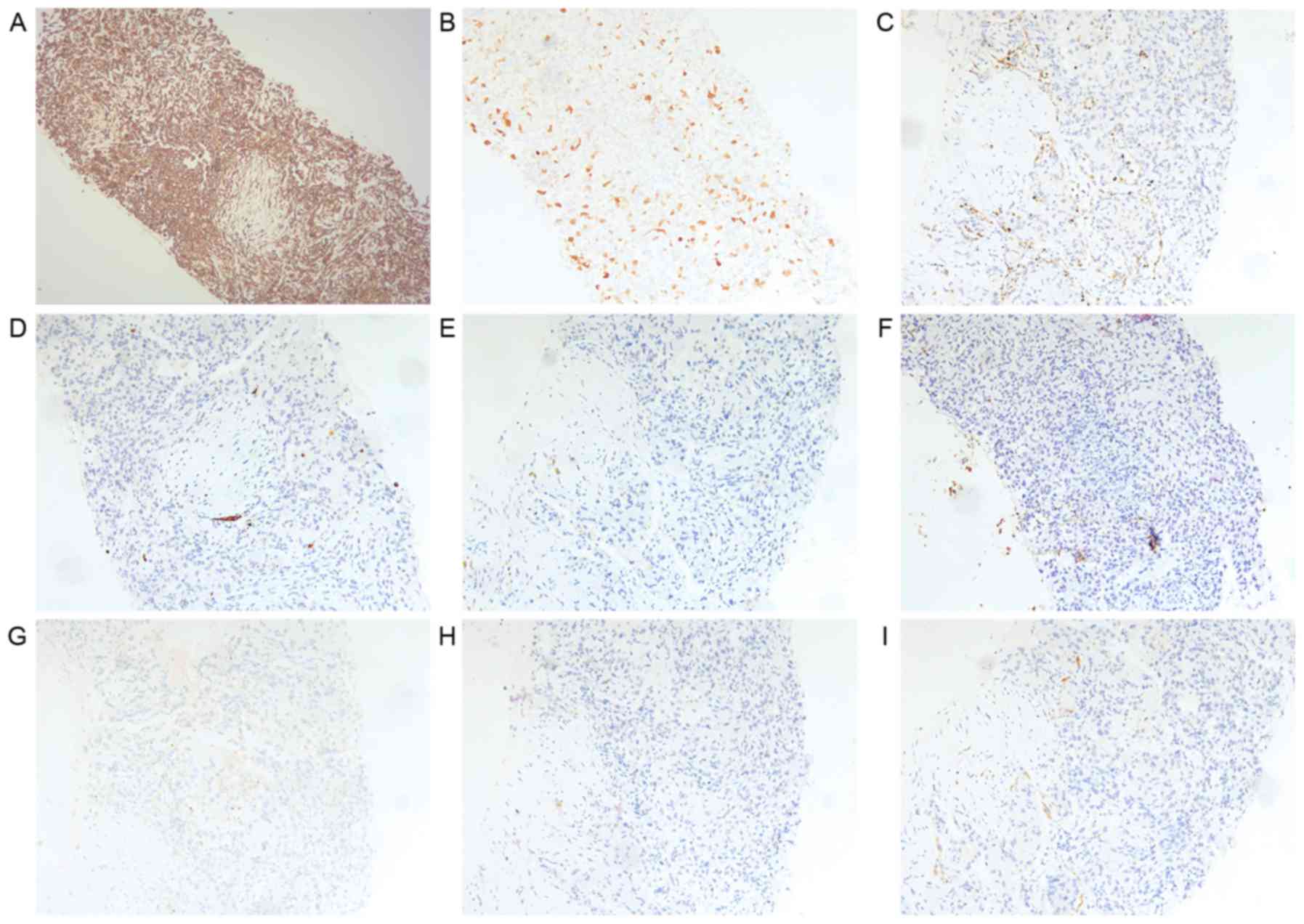 | Figure 5.Hispathological examination of the
pulmonary lesion sampled by percutaneous computed tomography-guided
lung biopsy. Immunohistochemical staining of the lung specimen
revealing (A) immunoreativity for vimentin (original magnification,
×100), (B) focal positivity for p16 (original magnification, ×100)
and negative results for (C) napsin, (D) CK7, (E) CK20, (F)
carcinoembryonic antigen, (G) carbohydrate antigen 125, (H)
homeobox protein CDX2 and (I) villin. Original magnification, ×100.
CK, cytokeratin. |
Histopathological examination of specimens from the
uterus, cervix and lung nodular tissues showed the same features as
each other. These immunohistochemical features, particularly
vimentin positivity, were identical to the findings of tissues
originating from the uterus. Based on these histopathological and
immunohistochemical findings, the diagnosis of MMMT with pulmonary
metastasis was confirmed.
The patient's treatment was initiated by
administration of intravenous voriconazole at a dose of 200 mg
twice a day for 7 days. However, the state of the lung field was
not improved and was aggravated further, we discontinued anti-fungi
therapy and performed serial examinations mentioned above.
Unfortunately, the patient ended treatment and was discharged due
to financial difficulties. She had a highly aggressive course of
disease and died only 4 months after diagnosis.
Written informed consent was obtained from the
patient for publication of the present study.
Discussion
MMMTs, also known as carcinosarcomas, are composed
of malignant epithelial and mesenchymal elements (carcinomatous and
sarcomatous components), and have an estimated incidence of <2
cases/100,000 women per year (7,8). MMMTs can
also originate in the vagina, ovary, uterine cervix or female
peritoneum, but these forms are even rarer (9–12). The
clinical initial presentation of MMMTs has been recorded as pain
and abdominal expansion in 62.5% of cases, and as a palpable mass
and vaginal bleeding in 25% of cases (13).
Although the most common site of distant metastasis
is the lungs (14), uterine cancer
metastasis to the lungs is rare (5.41%); according to the Chinese
Academy of Medical Sciences Cancer Hospital analysis of patients
admitted over a period of 10 years (1999–2009), ~3569 (40%) cancer
patients developed lung metastases. Metastasis to the lungs is
usually found through routine examinations, including lung CT and
whole body PET. Patients with metastatic lung cancer present with
primary tumor-related complications as the main clinical symptom
and have no clear lung-related symptoms at an early stage (5). In the present case, hemoptysis that was
reported as the only early clinical symptoms, and followed by
vaginal bleeding are rare.
CT scans of metastatic lung cancer frequently reveal
single or multiple spherical nodules with a smooth periphery
(5). In the present case, CT revealed
the characteristic halo sign, a solid nodule surrounded by a
ground-glass attenuation halo. In patients suffering from
immunosuppression, the halo sign is highly indicative of an
angioinvasive fungal infection, most typically aspergillosis
(15). Other infections, including
mycobacterial and certain viral infections, also present with the
halo sign on CT (16). In the present
study, the female patient was initially suspected of having
pulmonary invasive aspergillosis due to the typical halo-sign of
the pulmonary lesion, however, β-D-glucan and galactomannan tests
were negative. The radiology findings demonstrated well demarcated
nodules without marked lymph node enlargement in the mediastinum
and hilum (17,18). Accounting for these features, a
metastatic lung tumor of extrathoracic origin was also a possible
diagnosis. Finally, the nodular opacities surrounded by a
ground-glass attenuation halo (halo-sign) in this case were
presumed to be due to the proliferation of metastatic lung tumor
cells, followed by the destruction of peripheral vessels, which
accounted for the continuous hemoptysis (19).
In conclusion, cases with lung metastases from MMMTs
with sustained hemoptysis as the initial only symptom, and multiple
nodular opacities surrounded by a ground-glass attenuation halo
(halo-sign) are rare. Physician must be aware of metastatic
pulmonary tumors that closely resemble aspergillomas infection and
show hemoptysis as the initial symptom, not only when considering
infectious diseases, but also in oncological practice.
Acknowledgements
The authors would like to thank Dr Li Peng
(Department of Pathology, Union Hospital) for performing and
assisting with the hispathological examination. This study was
supported by grants from the National Natural Science Foundation of
China (nos. 81470274 and 81770090) and the 12th Five-Year National
Science and Technology Program of Social Development, Ministry of
Science and Technology, China (no. 2012BAI05B02).
Glossary
Abbreviations
Abbreviations:
|
MMMTs
|
malignant mixed Müllerian tumors
|
|
CT
|
computed tomography
|
References
|
1
|
Bhoil A, Kashyap R, Bhattacharya A and
Mittal BR: F-18 fluorodeoxyglucose positron emission
tomography/computed tomography in a rare case of recurrent
malignant mixed mullerian tumor. World J Nucl Med. 13:64–66. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davidson RS, Nwogu CE, Brentjens MJ and
Anderson TM: The surgical management of pulmonary metastasis:
Current concepts. Surg Oncol. 10:35–42. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suresh K and Shimoda LA: Lung Circulation.
Compr Physiol. 6:897–943. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Mehdi AB, Tozawa K, Fisher AB, Shientag
L, Lee A and Muschel RJ: Intravascular origin of metastasis from
the proliferation of endothelium-attached tumor cells: A new model
for metastasis. Nat Med. 6:100–102. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Margaritora S, Porziella V, D'Andrilli A,
Cesario A, Galetta D, Macis G and Granone P: Pulmonary metastases:
Can accurate radiological evaluation avoid thoracotomic approach?
Eur J Cardiothorac Surg. 21:1111–1114. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greene RE, Schlamm HT, Oestmann JW, Stark
P, Durand C, Lortholary O, Wingard JR, Herbrecht R, Ribaud P,
Patterson TF, et al: Imaging findings in acute invasive pulmonary
aspergillosis: Clinical significance of the halo sign. Clin Infect
Dis. 44:373–379. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanthan R and Senger JL: Uterine
carcinosarcomas (malignant mixed müllerian tumours): A review with
special emphasis on the controversies in management. Obstet Gynecol
Int 2011. 4707952011.
|
|
8
|
D'Angelo E and Prat J: Pathology of mixed
Müllerian tumours. Best Pract Res Clin Obstet Gynaecol. 25:705–718.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahuja A, Safaya R, Prakash G, Kumar L and
Shukla NK: Primary mixed mullerian tumor of the vagina-a case
report with review of the literature. Pathol Res Pract.
207:253–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McCluggage WG: Mullerian adenosarcoma of
the female genital tract. Adv Anat Pathol. 17:122–129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee SH, Kim J, Kim JH, Lee KH, Park JS and
Hur SY: Malignant mixed mullerian tumor of the cervix including
components of a rhabdomyosarcoma: Case report and literature
review. Eur J Gynaecol Oncol. 31:462–466. 2010.PubMed/NCBI
|
|
12
|
Kuyumcuoğlu U and Kale A: Homologous type
of malignant mixed Mullerian tumor of the uterus presenting as a
cervical mass. J Chin Med Assoc. 72:533–535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Montalvo-Esquivel G, Chanona-Vilchis JG,
Herrera-Gómez A, Meneses-García AA and Isla-Ortiz D: Primary
ovarian carcinosarcoma. Report of eight cases. Ginecol Obstet Mex.
82:483–489. 2014.(In Spanish).
|
|
14
|
Spanos WJ Jr, Peters LJ and Oswald MJ:
Oswald Patterns of recurrence in malignant mixed müllerian tumors
of the uterus. Cancer. 57:155–159. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greene RE, Schlamm HT, Oestmann JW, Stark
P, Durand C, Lortholary O, Wingard JR, Herbrecht R, Ribaud P,
Patterson TF, et al: Imaging findings in acute invasive pulmonary
aspergillosis: Clinical significance of the halo sign. Clin Infect
Dis. 44:373–379. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee YR, Choi YW, Lee KJ, Jeon SC, Park CK
and Heo JN: CT halo sign: The spectrum of pulmonary diseases. Br J
Radiol. 78:862–865. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Internullo E, Cassivi SD, Van Raemdonck D,
Friedel G and Treasure T; ESTS Pulmonary Metastasectomy Working
Group, : Pulmonary metastasectomy: A survey of current practice
amongst members of the European Society of Thoracic Surgeons. J
Thorac Oncol. 3:1257–1266. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saito Y, Omiya H, Kohno K, Kobayashi T,
Itoi K, Teramachi M, Sasaki M, Suzuki H, Takao H and Nakade M:
Pulmonary metastasectomy for 165 patients with colorectal
carcinoma: A prognostic assessment. J Thorac Cardiovasc Surg.
124:1007–1013. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim Y, Lee KS, Jung KJ, Han J, Kim JS and
Suh JS: Halo sign on high resolution CT: Findings in spectrum of
pulmonary diseases with pathologic correlation. J Comput Assist
Tomogr. 23:622–626. 1999. View Article : Google Scholar : PubMed/NCBI
|