Introduction
Liposarcoma is one of the most common soft tissue
sarcomas in adults, representing an estimated 17–25% of all
sarcomas (1,2). The tumor occurs at all ages, but is most
commonly identified in individuals between 40 and 60 years of age.
A total of four distinct histologic subtypes of liposarcoma are
recognized by the World Health Organization: i) Atypical lipomatous
tumor (ALT); ii) dedifferentiated liposarcoma (DLPS); iii) myxoid
liposarcoma (MLPS); and iv) pleomorphic liposarcoma (PLPS). ALT and
DLPS are genetically defined by a giant marker or ring chromosomes
with an amplification on chromosome 12 affecting, among others, the
genes mouse double minute 2 homolog and cyclin-dependent
kinase 4 (3). MLPS denotes one
such entity and is the second most common liposarcoma following ALT
(4). A significant proportion of MLPS
cases have the major cytogenetic hallmark of the t(12;16) (q13;p11)
chromosomal translocation. This translocation leads to fusion of
fused in liposarcoma (FUS; also termed
translocated in liposarcoma) and DNA damage-inducible
transcript 3 (DDIT3; also termed
CCAAT/enhancer-binding protein homologous protein) genes,
resulting in the production of the FUS-DDIT3 fusion protein
(5). In another subset of MLPS, a
minor chromosomal translocation, t(12;22)(q13;q12), results in
fusion of the Ewing's sarcoma (EWSR1) and
DDIT3 genes (6). PLPS is less
frequent and harbors a complex genomic profile with numerous gains
and losses similar to the genomic profile observed in poorly
differentiated sarcoma (7).
The histopathological findings of MLPS are typically
composed of well-circumscribed lobulated tumors. These contain a
mixture of uniform round to oval-shaped nonlipogenic mesenchymal
cells and signet ring lipoblasts in a prominent myxoid background,
rich in a delicate arborizing capillary vasculature (4). Although it is an extremely rare
phenomenon, 7 cases of MLPS with cartilaginous differentiation have
been reported to date (8–12). When this heterogeneous component is
present in MLPS, it may be difficult to distinguish MLPS from
malignant mesenchymoma or chondroid lipoma on histopathological
examination. Detection of the FUS-DDIT3 fusion gene is
useful for the differential diagnosis of these tumors. However,
only 3/7 of previous cases showed the FUS-DDIT3 fusion gene
in the typical liposarcomatous and cartilaginous components on
cytogenetic analysis (10–12). As there are few MLPS cases with this
heterogeneous component, whether the cartilaginous differentiation
in MLPS affects the clinical features and prognosis has not been
determined.
In the present study, an additional case of MLPS
with cartilaginous differentiation in which the FUS-DDIT3
fusion gene was detected by fluorescence in situ
hybridization (FISH) analysis is described, and the literature on
MLPS with cartilaginous differentiation is reviewed. The purpose of
this study was to clarify the clinical characteristics in MLPS with
cartilaginous differentiation.
Materials and methods
Patient characteristics
A 44-year-old woman had noted a painless mass in the
left thigh. The mass had been present for 4 years and had slowly
increased in size. The mass was elastic and soft, with no signs of
inflammation, but its borders were unclear. Radiological studies
showed an expansion of the soft tissue, but no bony changes or
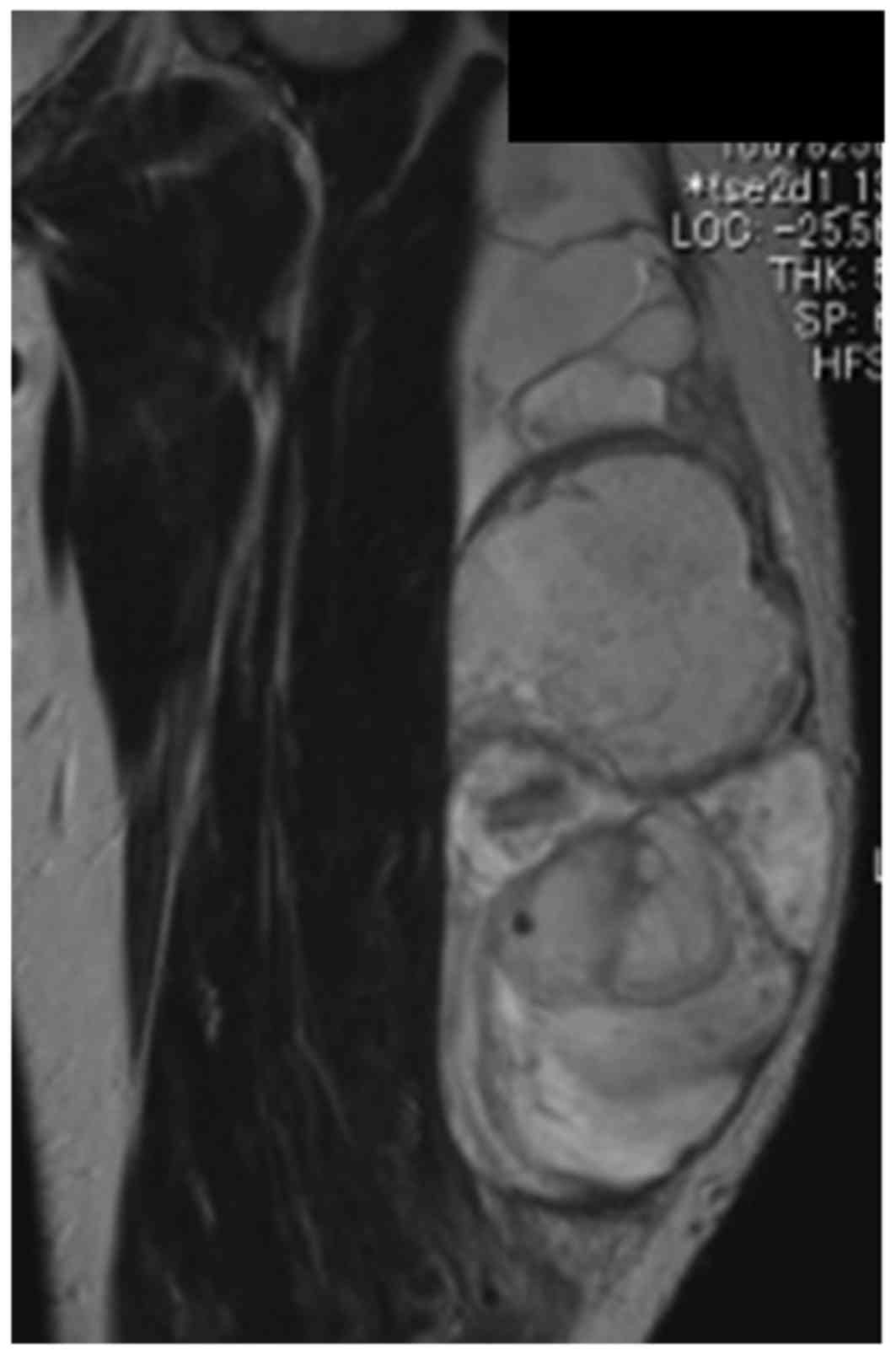
calcifications. Magnetic resonance imaging revealed a 21 cm mass
spreading on the lateral muscle component on the axial aspect of
the left thigh. The lesion appeared isointense to skeletal muscle
on T1-weighted images and heterogeneously hyperintense and
partially hypointense on T2-weighted images (Fig. 1). A needle biopsy was performed, and a
histologic diagnosis of MLPS was made based on the uniform round to
oval-shaped nonlipogenic mesenchymal cells and a number of small
lipoblasts within the myxoid stroma. The mass was excised with wide
margins, and a sterile portion was submitted for cytogenetic
analysis. The patient has had no recurrence or metastasis for 12
months subsequent to the surgery. The present report was approved
by the Ethics Committee of the Toyama University Hospital (Toyama,
Japan). The patient provided written consent for the report.
Immunohistochemical analysis
MIB-1 labeling index was evaluated by
immunohistochemical staining using Ki-67 antibody (1:100; catalog
no. M7240; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA).
Formalin-fixed paraffin-embedded tissues were cut into 4 µm
sections and mounted onto coated slides. Following
deparaffinization and rehydration, antigen retrieval was carried
out by heating with a microwave for 10 min in a citrate buffer
solution (10 mM) at pH 6.0. Following 30 min blocking with 5%
bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA) at room
temperature, the slide was incubated for 1 h at room temperature
with the Ki-67 antibody. Next, the slide section was incubated with
the biotinylated secondary antibody (polyclonal goat biotinylated
anti-mouse immunoglobulin; catalog no. E0433; 1:200; Dako, Agilent
Technologies) for 30 min at room temperature and subsequently
incubated with the avidin-biotin-peroxidase complex (Vector
Laboratories, Burlingame, CA, USA) for 30 min. Finally, staining
was visualized with 3,3′-diamino-benzidine-tetra hydrochloride. The
slide was counterstained with Mayer's hematoxylin. The MIB-1
labeling index was expressed as the percentage of positive cells
among 100 tumor cells in an area with high cell density.
Cytogenetic analysis
Representative fresh tissue from the surgical
resection sample was received for conventional cytogenetic
analysis. Culturing, harvesting, and preparation of slides were
performed as previously described (13). Briefly, the tissues were disassociated
mechanically and enzymatically and cultured at 37°C in RPMI 1640
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with
20% fetal bovine serum (ICN Biomedicals, Inc., Aurora, OH, USA) for
3–8 days. Cultured cells received overnight exposure to colcemid
(0.02 µg/ml). Following hypotonic treatment (0.8% sodium citrate
for 20 min at room temperature), the cells were fixed three times
with methanol:glacial acetic acid (3:1) at room temperature.
Chromosome analysis was performed on G-band by trypsin and Giemsa
(GTG) banding. GTG banding was performed by incubating the glass
slides in a 0.05% trypsin solution at 37°C for 15 sec, followed by
rinsing the slides in phosphate-buffered saline buffer and staining
in a 5% Giemsa stain for 8 min. The slides were rinsed with water
and air-dried. The karyotypes were expressed according to the
International System for Human Cytogenetic Nomenclature 2013
(14).
FISH analysis
An interphase FISH analysis was performed on
formalin-fixed, paraffin-embedded tissue sections of the tumor
using a commercially available DDIT3 (12q13) dual-color,
break-apart rearrangement probe (catalog no. 5J48-05; Abbott
Molecular Inc., Des Plaines, IL, USA). The probe consists of a
mixture of two FISH DNA probes. The first probe is a ~700 kb probe
(orange), which flanks the 3 side of the DDIT3 gene (12q13). The
second one is a ~663 kb probe (green), which flanks the 5 side of
the DDIT3 gene (12q13). The FISH analysis was performed according
to the manufacturer's protocol using Vysis Paraffin Pretreatment IV
and Post-Hybridization Wash buffer kit (Abbott Molecular Inc.), and
as described in a previous study (15). Hybridization signals were visualized
with an epi-fluorescence microscope, and images were captured on a
charge-coupled-device camera. A total of 50 nuclei showing both
green and orange signals were counted, and the percentage of the
fused signals in 50 nuclei was calculated by two different
observers. The fusion signal appeared yellow as orange and green
signals overlap partially or totally with oil immersed objective
lens. In cases where the nuclei overlapped and the complete area of
each nucleus was not visible, or the nuclei were too close together
to determine boundaries, the signals were not counted.
Results
Histology
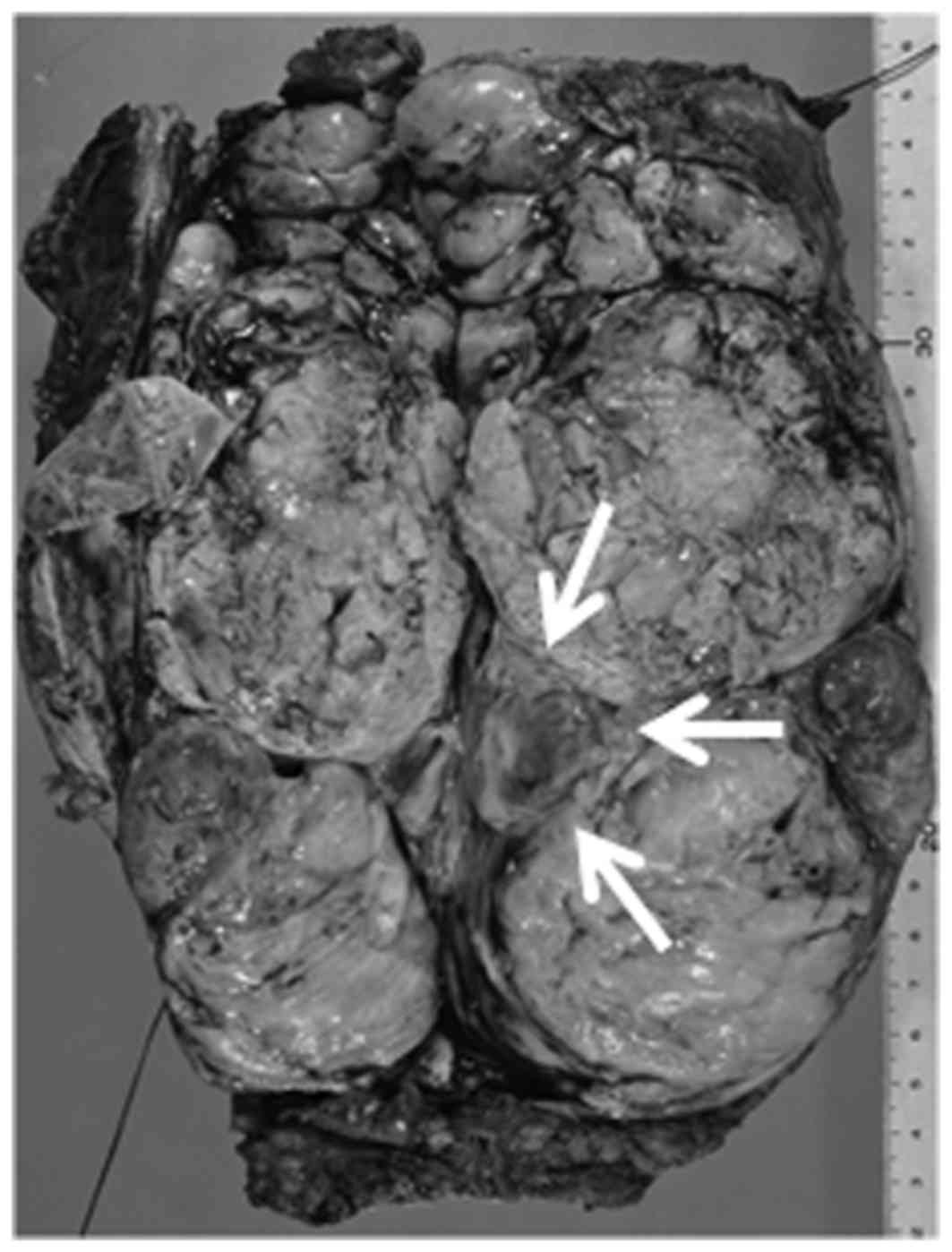
Grossly, the specimen measured 21×8×4 cm, and the
tumor had multinodular areas and a bluish cartilaginous spot
(indicated by the arrows in Fig. 2).
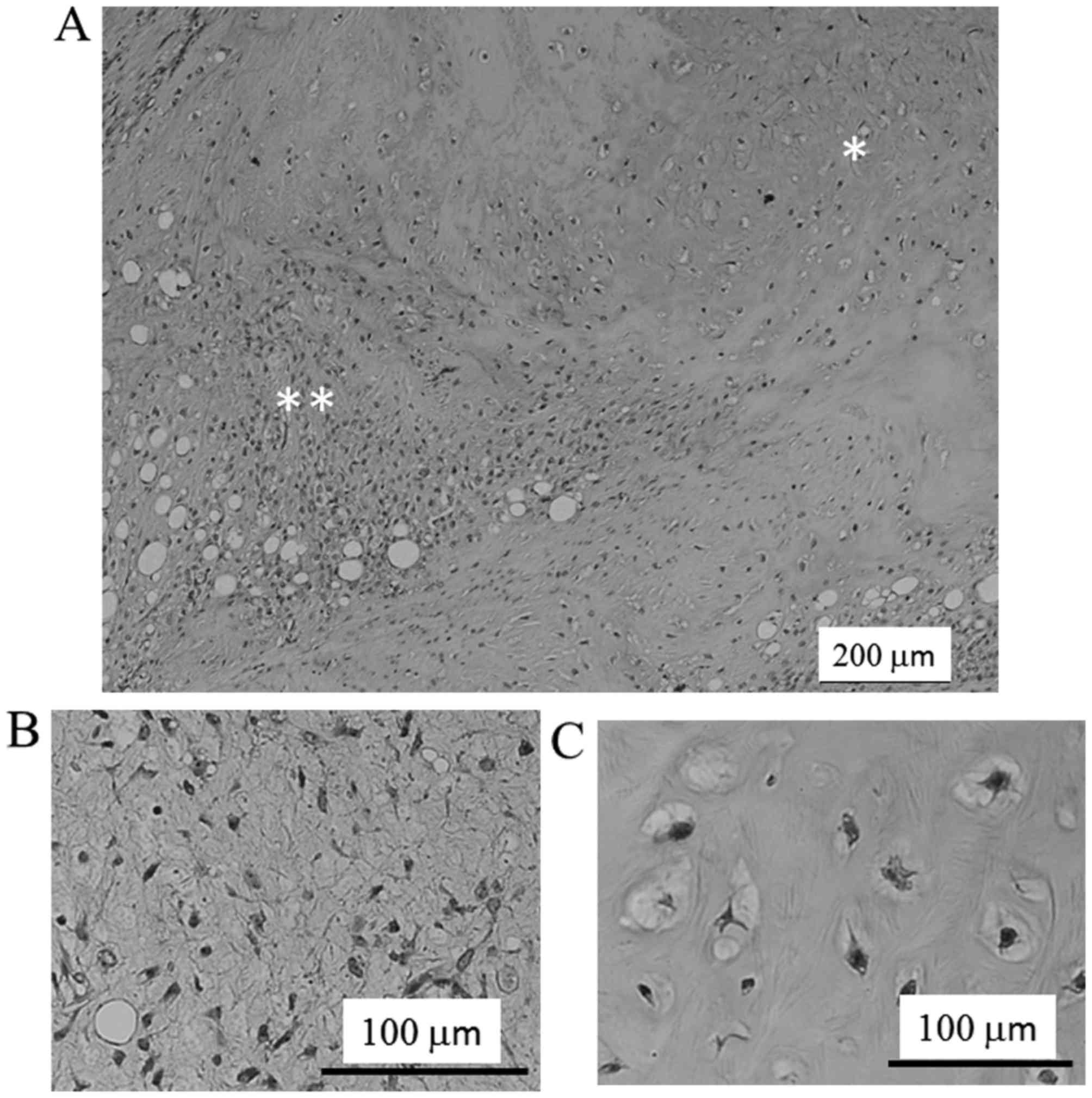
On low power microscopy, a liposarcomatous area was adjacent to the
cartilaginous area (Fig. 3A). The
lesion of the liposarcomatous component showed typical myxoid
liposarcoma, composed of the proliferation of spindle to
oval-shaped nonlipogenic cells and small lipoblasts in myxoid
stroma and a plexiform capillary vascular network. The tumor cell
was atypical with a high nuclear-cytoplasmic ratio and dense
chromatin. Additionally, the size and of the nuclei were moderately
or slightly enlarged, and the shape of the nuclei were irregular
compared with normal cells (Fig. 3B).
Significant mitotic figures were rare. It was found that the MIB-1
labeling index was ~1%. Small areas (~20%) of the tumor had a
cartilaginous component with mild cellularity of mature
chondrocytes and focally atypical chondrocytes (Fig. 3C). Based on these findings, the
diagnosis of MLPS with cartilaginous differentiation was made.
Cytogenetic analysis
In total, 18/20 analyzed metaphase cells were
karyotypically abnormal and characterized by the following
chromosomal complement: 46, XX, add (1) (p13), −2, der (4) t(1;4)(q11;p16), del (7) (p11.2), der (12) ?t(12;16) (q13;p11.2), −14, −16, der
(16) t(7;16)(p11.2;p11.2), −17, add
(17) (q25), add (21) (p11.2), +mar1, +mar2, +mar3, +mar4
(Fig. 4).
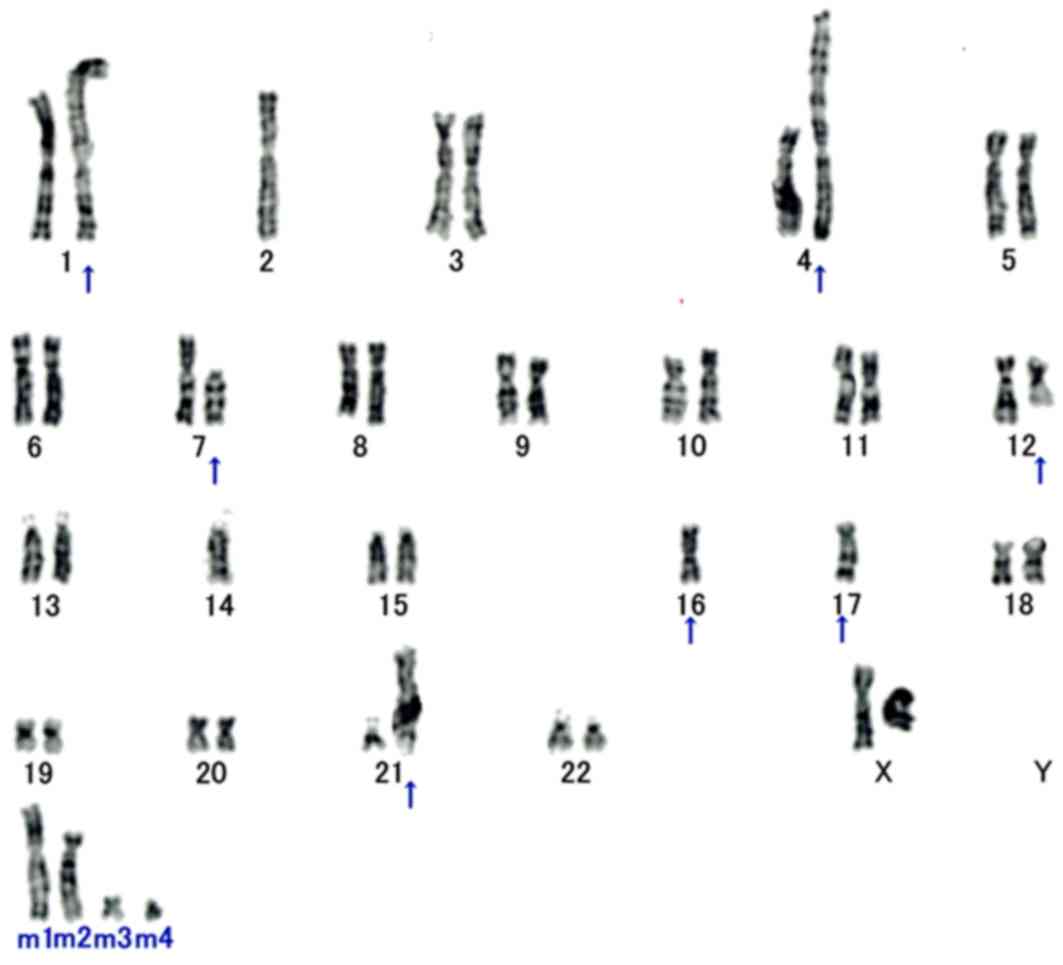 | Figure 4.Cytogenetic analysis. Karyotyping of
patient surgical specimen shows 46, XX, add(1)(p13), −2, der(4) t(1;4)(q11;p16), del(7) (p11.2), der(12) ?t(12;16)(q13;p11.2), −14, −16, der
(16)t(7;16)(p11.2;p11.2), −17,
add(17) (q25), add (21)(p11.2), +mar1, +mar2, +mar3, +mar4. All
aberrant chromosomes are indicated with arrows. The translocation
of t(12;16)(q13;p11.2) generates a FUS-DDIT3 fusion
transcript and is a cytogenetic hallmark of myxoid liposarcoma. |
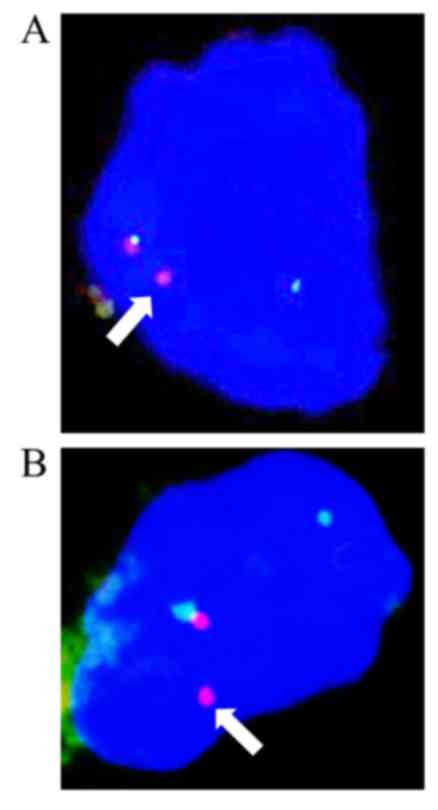
FISH
An interphase FISH analysis showed that >10% of
the cells from the typical MLPS area and the chondrocytes of the
cartilaginous component showed a split signal pattern of one green
and one orange, demonstrating a rearrangement in the DDIT3
gene (Fig. 5A and B).
Discussion
MLPS is the second most common histologic subtype
following ALT in liposarcoma and accounts for 15–20% of all
liposarcomas (4). MLPS usually occurs
in deep soft tissues of the extremities, particularly the thigh.
MLPS is histopathologically characterized by the mixture of uniform
round to oval-shaped non-lipogenic cells and small signet ring
lipoblasts in a prominent myxoid stroma. MLPS with cartilaginous
differentiation is extremely rare; to the best of our knowledge,
there have been only 7 previously reported cases (8–12). The
associations between clinical features and the cartilaginous
differentiation in MLPS are unclear, and the seven previous cases
and the present case are listed in Table
I. The range of ages was 26–63 years (median, 43 years), and
the site of involvement was the thigh in all cases, corresponding
to the most common site in MLPS (4).
The median size of the tumors was 11 cm (range, 8–21 cm). Nishida
et al (16) reported that MLPS
occurred in the extremities and trunk wall with a tumor size of
<10 cm in 64% of patients, while 36% exhibited a tumor size of
≥10 cm. In the previous largest study on MLPS, a tumor size of
>10 cm was demonstrated to be an independent prognostic factor
for disease-specific survival and metastasis-free survival
(17). However, all 7 cases of MLPS
with cartilaginous differentiation had a good prognosis, with no
evidence of disease at final follow-up. Furthermore, analysis of
the MIB-1 labeling index, a significant prognostic factor for
overall survival in MLPS (18),
demonstrated low values in 2/7 measurable cases (<5 and 1%).
From the finding of a low MIB-1 index, it is thought that MLPS with
cartilaginous differentiation has low malignancy and a good
prognosis. These findings indicate that the presence of
cartilaginous differentiation within MLPS may be a good prognostic
factor.
 | Table I.Characteristics of MLPS with
cartilaginous differentiation in previous studies. |
Table I.
Characteristics of MLPS with
cartilaginous differentiation in previous studies.
|
|
|
|
|
| Detection of
FUS-DDIT3 |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Case | Age/sex | Site | Duration, months | Size, cm | Analysis | Liposarco-matous
area | Cartilaginous
area | MIB-1 LI, % | Therapy | Prognosis
(months) | (Refs.) |
|---|
| 1 | 37/M | Thigh | 11 | 13 | NA | NA | NA | NA | CR, RT | NED (45) | (8) |
| 2 | 42/M | Thigh | 7 | 9 | NA | NA | NA | NA | CR, RT | NED (37) | (8) |
| 3 | 63/M | Thigh | 2 | 8 | NA | NA | NA | NA | CR, RT | NED (36) | (8) |
| 4 | 26/M | Thigh | NA | 11 | G band | NA | NA | NA | CR, RT | NED (36) | (9) |
| 5 | 47/M | Thigh | 12 | 13 | RT-PCR | + | + | NA | CR | NED (18) | (10) |
| 6 | 45/F | Thigh | 3 | 11 | RT-PCR | + | + | <5 | CR, RT | NED (6) | (11) |
| 7 | 37/M | Thigh | 12 | 8 | FISH | + | + | NA | CR, RT | NED (6) | (12) |
| Present | 44/F | Thigh | 48 | 21 | FISH | + | + | 1 | CR | NED (12) |
|
As MLPS with cartilaginous differentiation is a rare
condition, the differential diagnosis from other benign or
malignant soft tissue sarcomas, such as chondroid lipoma,
extraskeletal chondroma, extraskeletal myxoid chondrosarcoma and
malignant mesenchymoma, is difficult. Although chondroid lipoma and
extraskeletal chondroma have cells with a similar appearance to
lipoblasts (6), the presence of a
plexiform capillary vascular network, is a characteristic of MLPS.
Myxoid chondrosarcomas are different in that they show chondrocyte
atypia (19). The cartilaginous area
within MLPS is composed of mature hyaline cartilage without
chondrocyte atypia. Malignant mesenchymoma is defined as a
malignant soft tissue tumor that consists of two or more distinctly
different mesenchymal components in addition to fibrosarcomatous
elements (20). In the differential
diagnosis among these benign and malignant soft tissue tumors, the
detection of the fusion gene specific to MLPS, FUS-DDIT3 or
EWSR1-DDIT3, is extremely useful.
The FUS-DDIT3 fusion protein was reported to be
associated with the oncogenesis of MLPS by Riggi et al
(21). The expression of FUS-DDIT3
fusion protein in primary mesenchymal progenitor cells (MPCs)
induced the development of MLPS-like tumors in mice (21). The expression of FUS-DDIT3 may be the
initiating event in MLPS pathogenesis, and MPCs may constitute one
cell type from which MLPS originates. In addition, microarray-based
analysis of FUS-DDIT3-transformed adipose-derived mesenchymal stem
cells revealed downregulation of osteopontin (Opn) and the alpha 2
chain of type XI collagen (Col11a2) mRNA levels, with preservation
of peroxisome proliferator activated receptor-γ (PPAR-γ) gene
expression (22). We have previously
studied the EWSR1-DDIT3 mediated phenotypic selection of putative
target multipotent mesenchymal cells during MLPS development
through Opn and Col11a2 downregulation (23). These fusion genes as a hallmark of
MLPS are very useful for differential diagnosis from other soft
tissue sarcomas, and the associated protein FUS-DDIT3 or
EWSR1-DDIT3 performs an important role in the phenotypic selection
of targeted multipotent mesenchymal cells during MLPS development.
The rearrangement of the DDIT3 gene was shown by FISH
analysis, not only in the typical liposarcomatous area, but also in
the cartilaginous area. FUS-DDIT3 may participate in the process
producing cartilage differentiation. To date, there have been only
4 cases including the present case in which the hallmark fusion
gene was detected in lipomatous and cartilaginous components of
MLPS, as summarized in Table I. The
mechanism of cartilaginous differentiation induction within MLPS
remains unclear. Previous studies (21–23)
indicated that chondroid differentiation from mesenchymal
progenitor cells may be suppressed by fusion protein through
oncogenesis in typical MLPS. However, though FUS-DDIT3 is
detected in both the liposarcomatous and cartilaginous areas in
MLPS with cartilaginous differentiation, this tumor shows clinical
and pathological differences in prognosis and proliferation
activity from typical MLPS. Based on these findings, in addition to
the function of FUS-DDIT3 fusion gene, another molecular
mechanism or a modified mechanism in the production of
cartilaginous differentiation may be present. The mechanism of
cartilage differentiation within MLPS and its clinical significance
will become clear in the future with examination of additional
cases.
There were 3 limitations in the present study.
First, the number of MLPS cases with cartilaginous differentiation
was small, only 8 cases including the present case. The second
limitation was that there were only two cases in which the MIB-1
labeling index was reported; this is one of the important malignant
pathological features. Finally, in 4/8 cases the follow-up time
from the operation was <2 years. From the viewpoint of these
limitations, additional cases and long-term follow-up are necessary
to clarify whether the prognosis of MLPS with cartilaginous
differentiation is better compared with that of typical MLPS.
In conclusion, a rare case of MLPS with
cartilaginous differentiation was described, and DDIT3
rearrangement was demonstrated by FISH analysis in both lipomatous
and cartilaginous components. The mechanism of cartilaginous
differentiation in MLPS may be partially associated with the
FUS-DDIT3 fusion gene.
Acknowledgements
The present study was supported in part by the
Grants in Aid for Scientific Research (Japan Society for the
Promotion of Science; grant no. 24592227). The authors are grateful
to Professor Joji Imura, Department of Pathology, University of
Toyama (Toyama, Japan), for discussion on histopathological
diagnosis.
Glossary
Abbreviations
Abbreviations:
|
MLPS
|
myxoid liposarcoma
|
|
ALT
|
atypical lipomatous tumor
|
|
DLPS
|
dedifferentiated liposarcoma
|
|
PLPS
|
pleomorphic liposarcoma
|
|
FUS
|
fused in liposarcoma
|
|
DDIT3
|
DNA damage-inducible transcript 3
|
|
EWSR1
|
Ewing's sarcoma
|
|
FISH
|
fluorescence in situ
hybridization
|
|
MPCs
|
mesenchymal progenitor cells
|
|
Opn
|
osteopontin
|
|
Col11a2
|
alpha 2 chain of type XI collagen
|
|
PPAR-γ
|
peroxisome proliferator activated
receptor-γ
|
References
|
1
|
Weiss SW and Goldblum JR:
LiposarcomaEnzinger and Weiss's Soft Tissue Tumors. 5th. Weiss SW
Enzinger and Goldblum JR: Mosby Inc.; St. Louis: pp. 477–516.
2008
|
|
2
|
Dei Tos AP and Pedeutour F: Atypical
lipomatous tumorWHO Classification of Tumours of Soft Tissue and
Bone. Fletcher CDM, Bridge JA, Hogendoorn PCW and Mertens F: IARC;
Lyon: pp. 33–43. 2013
|
|
3
|
Coindre JM, Pédeutour F and Aurias A:
Well-differentiated and dedifferentiated liposarcomas. Virchows
Arch. 456:167–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Antonescu CR and Ladanyi M: Myxoid
liposarcomaWHO Classification of Tumours of Soft Tissue and Bone.
Fletcher CDM, Bridge JA, Hogendoorn PCW and Mertens F: IARC; Lyon:
pp. 39–41. 2013
|
|
5
|
Antonescu CR, Tschernyavsky SJ, Decuseara
R, Leung DH, Woodruff JM, Brennan MF, Bridge JA, Neff JR, Goldblum
JR and Ladanyi M: Prognostic impact of P53 status, TLS-CHOP fusion
transcript structure, and histological grade in myxoid liposarcoma:
A molecular and clinicopathologic study of 82 cases. Clin Cancer
Res. 7:3977–3987. 2001.PubMed/NCBI
|
|
6
|
Hosaka T, Nakashima Y, Kusuzaki K, Murata
H, Nakayama T, Nakamata T, Aoyama T, Okamoto T, Nishijo K, Araki N,
et al: A novel type of EWS-CHOP fusion gene in two cases of myxoid
liposarcoma. J Mol Diagn. 4:164–171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Idbaih A, Coindre JM, Derré J, Mariani O,
Terrier P, Ranchère D, Mairal A and Aurias A: Myxoid malignant
fibrous histiocytoma and pleomorphic liposarcoma share very similar
genomic imbalances. Lab Invest. 85:176–181. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siebert JD, Williams RP and Pulitzer DR:
Myxoid liposarcoma with cartilaginous differentiation. Mod Pathol.
9:249–252. 1996.PubMed/NCBI
|
|
9
|
Dijkhuizen T, Molenaar WM, Hoekstra HJ,
Wiersema J and van den Berg E: Cytogenetic analysis of a case of
myxoid liposarcoma with cartilaginous differentiation. Cancer Genet
Cytogenet. 92:141–143. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei YC, Li CF, Eng HL, Yeh MC, Lin CN and
Huang HY: Myxoid liposarcoma with cartilaginous differentiation:
Identification of the same type II TLS-CHOP fusion gene transcript
in both lipogenic and chondroid components. Appl Immunohistochem
Mol Morphol. 15:477–480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim H, Hwangbo W, Ahn S, Kim S, Kim I and
Kim CH: Myxoid liposarcoma with cartilaginous differentiation: A
case study with cytogenetical analysis. Korean J Pathol.
47:284–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ioannou MG, Kouvaras E, Papamichali R,
Karachalios T and Koukoulis G: Myxoid liposarcoma with
cartilaginous differentiation: A case study with fish analysis and
review of the literature. Pathol Res Pract. 209:666–669. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishio J, Althof PA, Bailey JM, Zhou M,
Neff JR, Barr FG, Parham DM, Teot L, Qualman SJ and Bridge JA: Use
of a novel FISH assay on paraffin-embedded tissues as an adjunct to
diagnosis of alveolar rhabdomyosarcoma. Lab Invest. 86:547–556.
2006.PubMed/NCBI
|
|
14
|
Simons A, Shaffer LG and Hastings RJ:
Cytogenetic nomenclature: Changes in the ISCN 2013 compared to the
2009 edition. Cytogenet Genome Res. 141:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamaguchi U, Hasegawa T, Morimoto Y,
Tateishi U, Endo M, Nakatani F, Kawai A, Chuman H, Beppu Y, Endo M,
et al: A practical approach to the clinical diagnosis of Ewing's
sarcoma/primitive neuroectodermal tumor and other small round cell
tumors sharing EWS rearrangement using new fluorescence in situ
hybridization probes for EWSR1 on formalin fixed, paraffin wax
embedded tissue. J Clin Pathol. 58:1051–1056. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nishida Y, Tsukushi S, Nakashima H and
Ishiguro N: Clinicopathologic prognostic factors of pure myxoid
liposarcoma of the extremities and trunk wall. Clin Orthop Relat
Res. 468:3041–3046. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fiore M, Grosso F, Lo Vullo S,
Pennacchioli E, Stacchiotti S, Ferrari A, Collini P, Lozza L,
Mariani L, Casali PG and Gronchi A: Myxoid/round cell and
pleomorphic liposarcomas: Prognostic factors and survival in a
series of patients treated at a single institution. Cancer.
109:2522–2531. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tateishi U, Hasegawa T, Beppu Y, Kawai A
and Moriyama N: Prognostic significance of grading (MIB-1 system)
in patients with myxoid liposarcoma. J Clin Pathol. 56:579–582.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Enzinger FM and Shiraki M: Extraskeletal
myxoid chondrosarcoma. An analysis of 34 cases. Hum Pathol.
3:421–435. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weiss SW and Goldblum JR: Malignant
mesenchymomaEnzinger and Weiss's Soft tissue tumors. 5th. Weiss SW
Enzinger and Goldblum JR: Mosby Inc.; St. Louis: pp. 1213–1214.
2008
|
|
21
|
Riggi N, Cironi L, Provero P, Suvà ML,
Stehle JC, Baumer K, Guillou L and Stamenkovic I: Expression of the
FUS-CHOP fusion protein in primary mesenchymal progenitor cells
gives rise to a model of myxoid liposarcoma. Cancer Res.
66:7016–7023. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rodriguez R, Rubio R, Gutierrez-Aranda I,
Melen GJ, Elosua C, García-Castro J and Menendez P: FUS-CHOP fusion
protein expression coupled to p53 deficiency induces liposarcoma in
mouse but not in human adipose-derived mesenchymal stem/stromal
cells. Stem Cells. 29:179–192. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki K, Matsui Y, Higashimoto M,
Kawaguchi Y, Seki S, Motomura H, Hori T, Yahara Y, Kanamori M and
Kimura T: Myxoid liposarcoma-associated EWSR1-DDIT3 selectively
represses osteoblastic and chondrocytic transcription in
multipotent mesenchymal cells. PLoS One. 7:e366822012. View Article : Google Scholar : PubMed/NCBI
|