Introduction
Ovarian cancer has become a common malignant tumor
in the reproductive system of women, and the mortality rate is
higher than other diseases (1).
Statistics revealed that there are ~210,172 cases of ovarian cancer
and ~104,683 deaths of women due to this disease in 2008 (2). A study by Tamura (3) showed that most of women in the first
diagnosis of ovarian cancer already have different degrees of
metastasis in that the early symptoms of ovarian cancer were not
obvious. It is believed that the key reason for this was that most
patients could not obtain an early diagnosis (4). At present, it is confirmed that early
diagnosis of stage I can be cured in 90% and stage II in 70% of the
patients (5). Therefore, promoting
studies on the pathogenesis of ovarian cancer may help us to
identify the disease in the early or curable stage, and to provide
a new and effective treatment.
Calcium channel protein is essential for maintaining
calcium homeostasis inside and outside the cell (6). Previous evidence showed that calcium
ions in the cells acted as intracellular messengers, and played a
regulatory role in many metabolism and physiological activities of
cells, such as mediation of cell metabolism rate, controlling the
contraction of muscle cells, regulating cell secretion and
division, as previously suggested (7–10). It was
found that calcium ions may be involved in some crucial death
processes of cells, and too many calcium ions may cause different
degrees of cell death, which may be the main mechanism of
cardiomyopathy caused by ischemia (11). Researchers also suggested that the
destruction of intracellular calcium homeostasis may be closely
associated with the occurrence of certain cancers (12–14).
Although the increase of intracellular calcium concentration can
result in cell apoptosis and even death, there is scarce research
on the calcium channel protein in ovarian cancer cells (15).
Therefore, in this study, we investigated the role
of calcium channel protein in ovarian cancer cells, in order to
provide some theoretical and experimental basis for follow-up study
and clinical ovarian cancer treatment.
Materials and methods
Experimental reagents
Calcium channel protein activator (nicardipine) was
purchased from Sigma Chemical Co. (St. Louis, MO, USA), and methyl
thiazolyl tetrazolium (MTT) assay from Shanghai Biological
Engineering Co., Ltd. (Shanghai, China), while fluorescence
quantitative polymerase chain reaction (PCR) reagents were from
Takara (Tokyo, Japan).
Ovarian cancer cell line and its
culture
Human ovarian cells were purchased from CICC and
preserved in liquid nitrogen. The culture condition was 37°C, 5%
CO2, and 10% fetal bovine serum (Roche, Basel,
Switzerland) was added in the culture medium, and 0.25% trypsin was
used for digestion in each passage.
Extraction and fluorescence
quantitative PCR of ovarian cancer cell RNA
Frozen tissue samples (0,1 g) were taken from liquid
nitrogen and thawed on ice. Subsequently, 0.45 ml of RNA Plus was
added, followed by homogenization with mortar in an Eppendorf tube.
The contents were later transferred into the centrifuge tube after
washing. Next, 200 µl of chloroform was added and mixed with
vortexing for 15 sec. The tube contents were centrifuged at 10,500
× g, 4°C for 15 min. After that the supernatant was transferred to
an EP tube (RNase removed) with an equal volume of isopropanol, and
it was reversed, and mixed while keeping on ice for 10 min. The
tube was centrifuged again as previously and the supernatant was
removed, then 750 µl of 75% ethanol was added and mixed gently,
followed by centrifugation at 10,500 × g, 4°C, for 10 min. The
supernatant and ethanol that remained were removed. Finally,
appropriate amount of RNase-free water was added to the RNA pellet.
The extracted RNA was assessed for quality and quantity, before
proceeding for reverse transcription. The operation referred to the
fluorescence quantitative PCR instructions of Takara with a slight
change.
Determination of intracellular calcium
concentration
The calcium concentration in ovarian cancer cells
and normal ovarian cells was determined as described elsewhere
(16), to detect the intracellular
free calcium concentration in human erythrocytes.
MTT assay used for cell activity
One hundred microliters of ovarian cancer cells with
a concentration of 2×104/ml were cultured in 96-well
plates. After 12 h, calcium protein activator of different
concentrations (0, 1, 4, 8, 12, 16 and 20 mmol/1) was added into
each well with 3 replicates in each group. After culturing under
conditions of 37°C, 5% and CO2 for 48 h, 20 µl of MTT
reagent was added into each well. After 4 h, the medium was removed
and 100 µl of DMSO detection reagent was added, followed by
agitation. Light absorption values (A) were detected at 490 nm. The
cell inhibition rate was calculated as: (the value of A in the
control group - A in the calcium channel activator group)/the value
of A in the control group × 100%.
Flow cytometry used to detect
apoptosis
Trypsin was used to digest ovarian cancer cells
grown in different activators for 48 h, followed by gentle washing
3–5 times with sterile phosphate-buffered saline (PBS) (pH 7.2) and
then fixed with 80% cold ethanol. After the treatment, ovarian
cancer cells were placed at −20°C overnight, and were rinsed with
PBS 3–5 times the next day to remove remaining ethanol. After cell
counting, the cell concentration was set to 1× 106/ml.
PI dye (50 µl/ml) was added at room temperature and out of direct
sunlight for 30 min. Flow cytometry was then used to detect each
sample for 2×105 cells. Analysis was carried out by
CellQuest software.
Hoechst 33258 staining
For detection of cell morphology, Hoechst 33258
staining was performed according to the manufacturer's
instructions.
Statistical analysis
SPSS 20.0 software (Chicago, IL, USA)was used for
analysis of data. Related measurement results are expressed as mean
± standard deviation and measurement data were detected by
χ2 test.
Results
Expression of calcium channel protein
in different cells
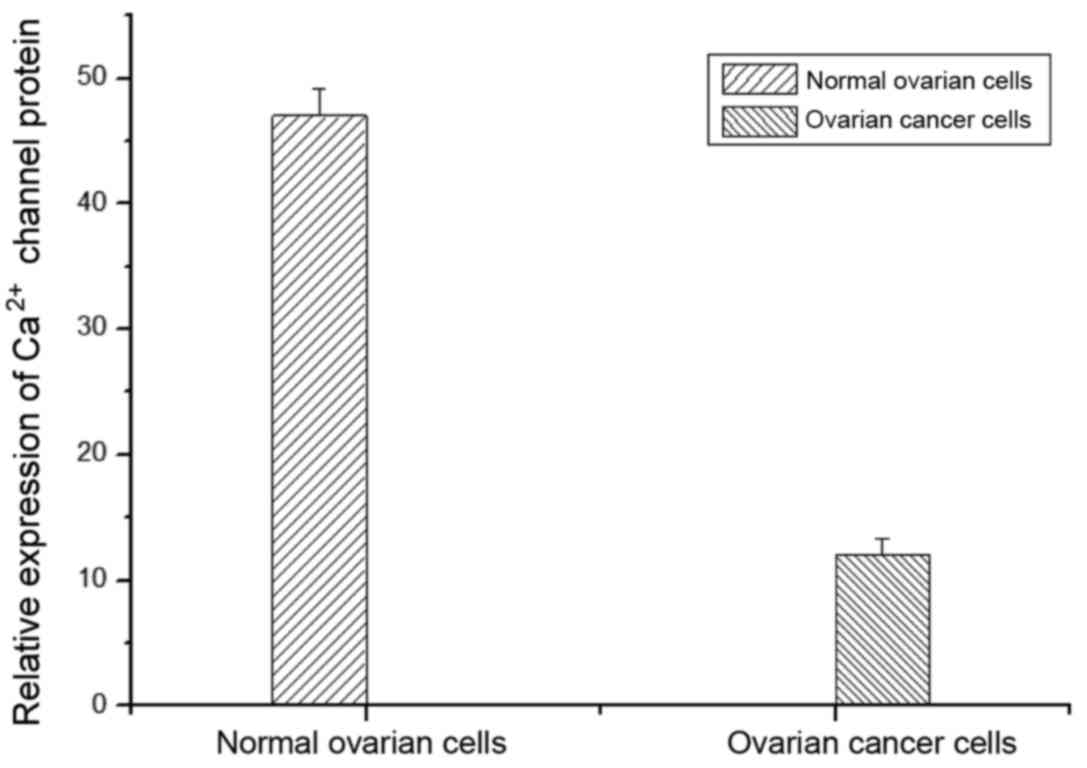
In this study, fluorescent quantitative PCR was
applied to detect the expression of calcium channel protein in
normal ovarian cells and ovarian cancer cells. As shown in Fig. 1, the expression of calcium channel
protein in normal cells was significantly higher than that of
ovarian cancer cells.
Calcium content in ovarian cancer
cells after treatment with different calcium channel protein
activators
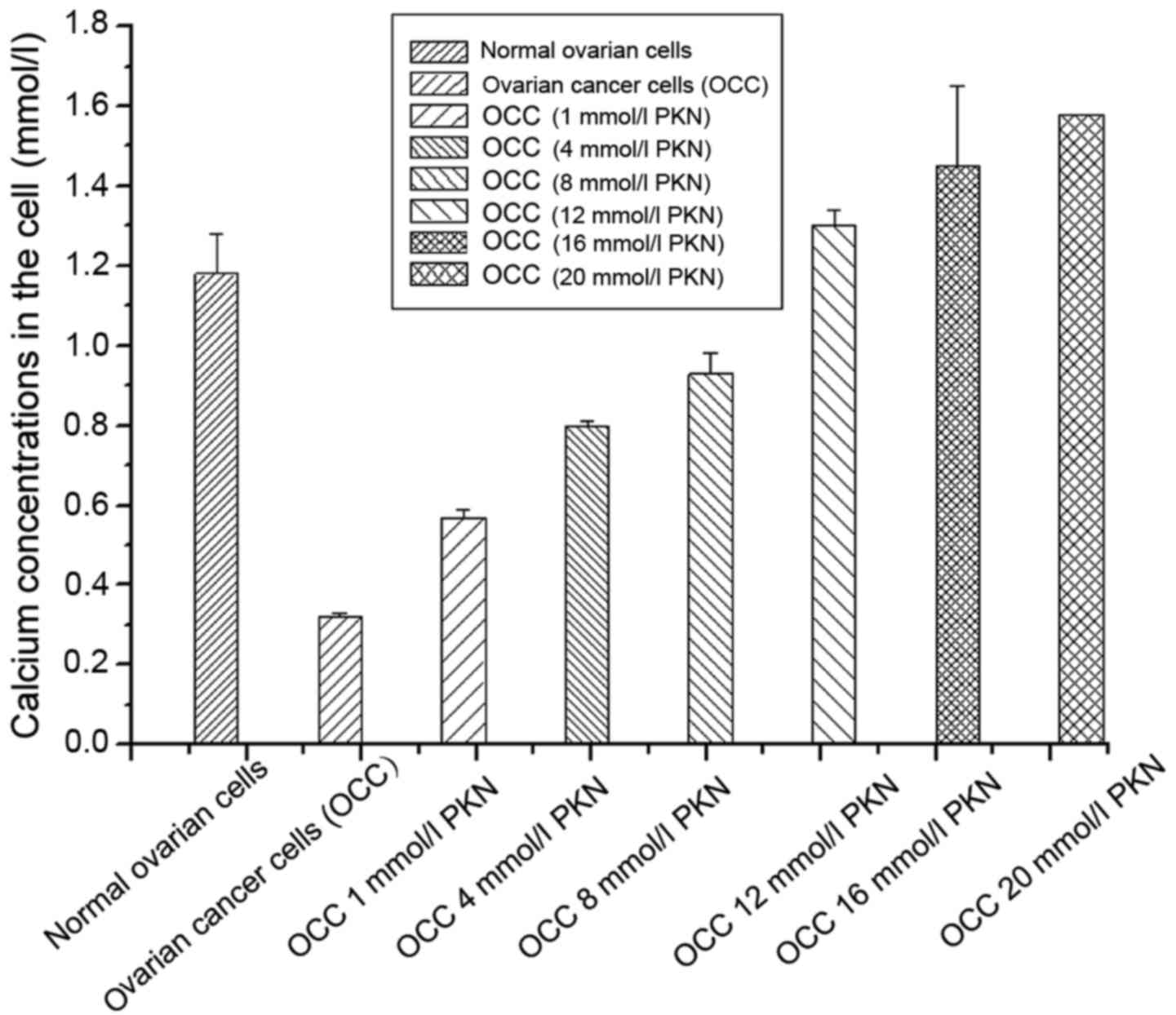
Calcium concentrations in ovarian cancer cells were
detected after treatment with different calcium channel protein
activators. It was found that calcium concentration in normal
ovarian cells (the control group) was significantly higher than
untreated ovarian cancer cells with calcium channel protein
activator. With increase of the concentration of the activator, the
intracellular calcium concentration showed a downward trend after
the first increase (Fig. 2).
Inhibitory effects of different
calcium channel protein activators on ovarian cancer cells
MTT assay showed that when the concentrations of
calcium channel protein activator were 1, 4, 8, 12, 16 and 20
mmol/l, the proliferation inhibition rates of ovarian cancer cells
were 4.6, 21.3, 48.3, 67.9, 52.8 and 31.8%, respectively. It was
indicated that the inhibitory effects of calcium channel protein
activator on the proliferation of ovarian cancer cells applied in a
dose-dependent manner, which was more obvious when the activator
concentration was at 12 mmol/l (Table
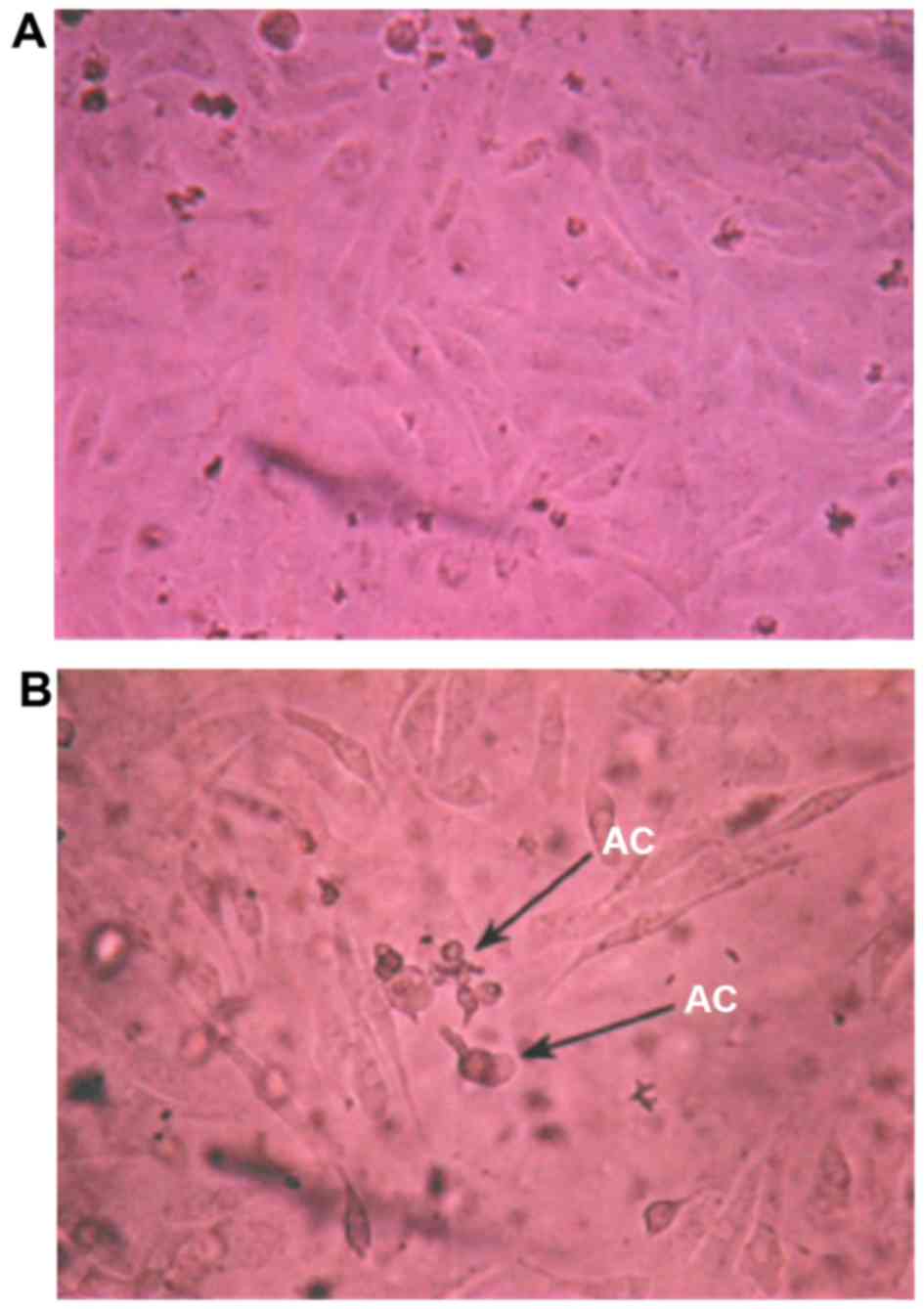
I). When the concentration of calcium channel protein activator
was 12 mmol/l at 48 h, ovarian cancer cells became round, the cell
membrane showed blebbing, refractive index decreased and apoptotic
bodies emerged (Fig. 3).
 | Table I.Effects of different concentrations of
calcium channel protein activator on ovarian cancer cell
proliferation. |
Table I.
Effects of different concentrations of
calcium channel protein activator on ovarian cancer cell
proliferation.
| Group | A450 | Cell proliferation
inhibitory rate (%) |
|---|
| Control (normal
ovarian cells) | 0.796±0.0328 |
|
| Treatment (calcium
channel protein activator) (mmol/l) |
|
|
|
1 | 0.736±0.0348 | 4.6a |
|
4 | 0.648±0.0213 | 21.3a |
|
8 | 0.547±0.041 | 48.3a |
| 12 | 0.376±0.0027 | 67.9b |
| 16 | 0.485±0.016 | 52.8a |
| 20 | 0.591±0.02 | 31.8a |
Observation of apoptosis of ovarian
cancer cells induced by calcium channel protein activator
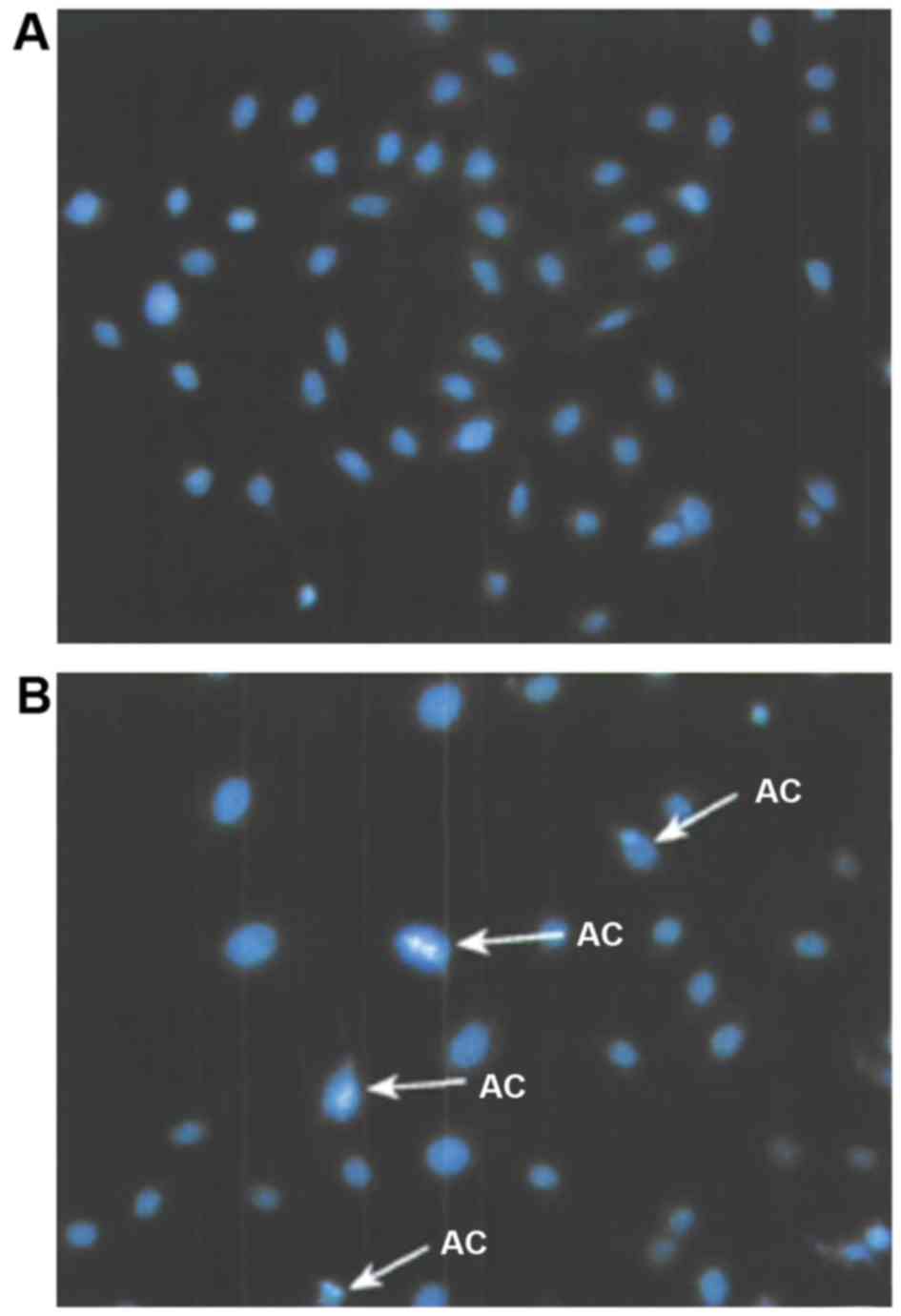
Ovarian cancer cells were treated with calcium
channel protein activator with a concentration of 12 mmol/l for 48
h, and followed by PBS washing and Hoechst 33258 staining. The
cells were then observed through a fluorescent microscope, as shown
in Fig. 4. In the treatment group,
apoptosis of nucleus chromatin showed a condensed state, which
turned highly condensed and marginalized in late apoptosis along
with cell division.
Flow cytometry for apoptosis of
ovarian cancer cells induced by calcium channel protein
activator
It was found that for apoptosis in ovarian cancer
cells induced by calcium channel protein activator (Table II), the apoptosis rates (48 h later)
were 5.4, 23.8, 51.2, 68.4, 53.8 and 36.7% with calcium channel
protein activators at 1, 4, 8, 12, 16 and 20 mmol/l, respectively.
There was a significant difference compared with the control group
(1.73%) (P<0.05). It indicates that calcium channel protein can
promote apoptosis of ovarian cancer cells by increasing the
intracellular calcium concentration, which was consistent with the
MTT results.
 | Table II.Effects of calcium channel protein
activator on apoptosis of ovarian cancer cells (mean ± SD,
n=12). |
Table II.
Effects of calcium channel protein
activator on apoptosis of ovarian cancer cells (mean ± SD,
n=12).
| Group | Cell apoptosis rate
(%) |
|---|
| Control | 2.13±0.017 |
| Treatment (calcium
channel protein activator) (mmol/l) |
|
|
1 |
54.8±0.042a |
|
4 |
65.7±0.012a |
|
8 |
74.2±0.068a |
| 12 |
83.4±0.037b |
| 16 |
79.6±0.024a |
| 20 |
67.3±0.042a |
Discussion
In this study, we proved that compared with normal
ovarian cells, calcium concentration was significantly lower in the
ovarian cancer cells and calcium channel protein activator can
induce apoptosis in ovarian cancer cells by increasing the
intracellular calcium concentration. It showed that calcium ions
can participate in regulating apoptosis of ovarian cells to a
certain extent. Previous findings showed that the lack of
intracellular calcium can lead to redox imbalance in the cells,
followed by the damage of intracellular membrane (17–19). Braga
et al proved that in ovarian cells, calcium ions can
interact with other intracellular factors such as AMP to regulate
the early cell apoptosis (20). Other
studies have shown that the intracellular calcium-regulating enzyme
can regulate the cell apoptosis by acting downstream of cytosolic
calcium; however, the mechanism of action remains unclear (21).
Li et al suggested that the calcium ions in
the cell may be associated with some tumor suppressor genes to
regulate the apoptosis of malignant cells; however, the mechanism
of action is still unknown (22).
After studying the relevant research, we found that there are
theories demonstrating that calcium ions are involved in the
regulation of apoptosis in late apoptosis as intracellular signals.
However, there is no related experiment on the interactions between
calcium ions and ovarian cancer cells. Therefore, in this study, to
the best of our knowledge, we identified for the first time that
calcium ion can regulate cell apoptosis through its intracellular
content in a dose dependent manner.
References
|
1
|
Guppy AE, Nathan PD and Rustin GJ:
Epithelial ovarian cancer: a review of current management. Clin
Oncol (R Coll Radiol). 17:399–411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yan XD: Comparative proteomics analysis on
resistant proteins of ovarian cancer platinum drugs and the
analysis of functions of drug resistant proteins Annexin A3
(unpublished PhD dissertation). Peking Union Medical University;
2008, http://cdmd.cnki.com.cn/article/cdmd-10023-2009063673.htm
|
|
3
|
Tamura G: Hypermethylation of tumor
suppressor and tumor-related genes in neoplastic and non-neoplastic
gastric epithelia. World J Gastrointest Oncol. 1:41–46. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boente MP, Godwin AK and Hogan WM:
Screening, imaging, and early diagnosis of ovarian cancer. Clin
Obstet Gynecol. 37:377–391. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Verhagen AM and Vaux DL: Cell death
regulation by the mammalian IAP antagonist Diablo/Smac. Apoptosis.
7:163–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Antonsson B: Bax and other pro-apoptotic
Bcl-2 family ‘killer-proteins’ and their victim the mitochondrion.
Cell Tissue Res. 306:347–361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang M, Li Y, Zhang H and Xue S: BAPTA
blocks DNA fragmentation and chromatin condensation downstream of
caspase-3 and DFF activation in HT-induced apoptosis in HL-60
cells. Apoptosis. 6:291–297. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Veldhuis JD and Klase PA: Mechanisms by
which calcium ions regulate the steroidogenic actions of
luteinizing hormone in isolated ovarian cells in vitro.
Endocrinology. 111:1–6. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Veldhuis JD and Klase PA: Calcium ions
modulate hormonally stimulated progesterone production in isolated
ovarian cells. Biochem J. 202:381–386. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao SY, Wang QJ and Ji YB: Effect of
solanine on the membrane potential of mitochondria in HepG2 cells
and [Ca2+]i in the cells. World J Gastroenterol.
12:3359–3367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Monteiro P, Oliveira PJ, Gonçalves L and
Providência LA: Mitochondria: role in ischemia, reperfusion and
cell death. Rev Port Cardiol. 22:233–254. 2003.PubMed/NCBI
|
|
12
|
Wang JY, Chen BK, Wang YS, Tsai YT, Chen
WC and Chang WC, Hou MF, Wu YC and Chang WC: Involvement of
store-operated calcium signaling in EGF-mediated COX-2 gene
activation in cancer cells. Cell Signal. 24:162–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hajnóczky G, Davies E and Madesh M:
Calcium signaling and apoptosis. Biochem Biophys Res Commun.
304:445–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu ZM, Chen GG, Vlantis AC, Tse GM, Shum
CK and van Hasselt CA: Calcium-mediated activation of PI3K and p53
leads to apoptosis in thyroid carcinoma cells. Cell Mol Life Sci.
64:1428–1436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Naziroğlu M and Lückhoff A: A calcium
influx pathway regulated separately by oxidative stress and
ADP-Ribose in TRPM2 channels: single channel events. Neurochem Res.
33:1256–1262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yorek MA, Davidson EP, Dunlap JA and
Stefani MR: Effect of bradykinin on cytosolic calcium in
neuroblastoma cells using the fluorescent indicator fluo-3. Biochim
Biophys Acta. 1177:215–220. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Ng WL, Wang P, Tian L, Werner E,
Wang H, Doetsch P and Wang Y: MicroRNA-21 modulates the levels of
reactive oxygen species by targeting SOD3 and TNFα. Cancer Res.
72:4707–4713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walter L and Hajnóczky G: Mitochondria and
endoplasmic reticulum: The lethal interorganelle cross-talk. J
Bioenerg Biomembr. 37:191–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dulce RA, Yiginer O, Gonzalez DR, Goss G,
Feng N, Zheng M and Hare JM: Hydralazine and organic nitrates
restore impaired excitation-contraction coupling by reducing
calcium leak associated with nitroso-redox imbalance. J Biol Chem.
288:6522–6533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Braga EA, Loginov VI, Klimov EA,
Kilosanidze G, Khodyrev DS, Kaganova NL, Kazybskaia TP, Ermilova
VD, Gar'kavtseva RF, Pronina IV, et al: Activation of RHOA gene
transcription in epithelial tumors may be caused by gene
amplification and/or demethylation of its promotor region. Mol Biol
(Mosk). 40:865–877. 2006.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pani G, Galeotti T and Chiarugi P:
Metastasis: cancer cell's escape from oxidative stress. Cancer
Metastasis Rev. 29:351–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Lin G and Zhou K: Research Progress
on the relationship between tumor suppressor gene PTEN and cell
apoptosis. J Guangdong Medical College. 23:676–678. 6862005.(In
Chinese).
|