Introduction
Gastric cancer (GC) is one of the common malignant
tumors in human, ranking as the fourth most common cancer worldwide
and the second highest cause of cancer-related death (1,2).
Currently, a combination of surgical resection and postoperative
chemotherapy is the most common method of GC treatment. However,
the majority of patients that manifest clinical symptoms are
terminal as they already have metastasis and therefore, the overall
survival rate of patients with GC is low. When GC is diagnosed at
an early stage, its 5-year survival rate can be as high as 90%.
When diagnosed at a later stage, however, the 5-year survival rate
of GC patients can be as low as 10% (3,4).
Biological markers have the potential to aid in
early diagnosis, treatment and prognosis of tumors (5). In recent years, there have been some
potential biomarkers that have emerged for the clinical diagnosis
of GC, such as p27, cyclin E, E-cadherin, HER2, c-myc and p53.
However, results have shown that there are no biomarkers that have
been identified up-to-date that can be independently used for the
occurrence and development of GC (6).
Thus, developing molecular tag-based, sensitive and specific
biomarkers for GC can have an important significance on the early
diagnosis rate, effective treatment and reduced mortality of
patients with GC.
When a tumor is present, cytokines and chemokines
are produced by infiltrating inflammatory or tumor cells, which
result in the change of the tumor microenvironment, and further
promote tumor angiogenesis, proliferation, diffusion and metastasis
(7). As there has been a flux of
protein analysis technology, antibody microarray-based assays has
been used for the research of disease proteomics, and it can
comprehensively and accurately reflect the changes in protein
expression levels that occur during the development of diseases
(8,9).
Based on the changes of some upregulated or downregulated
expression of proteins, antibody microarray-based technology can be
used to discover biomarkers for early diagnosis, the evaluation of
treatment effects and choice of new targets for treatment (10).
In this study, proteomic chip-based analysis was
performed to simultaneously identify 507 cytokines using a cytokine
antibody array in gastric tissues to screen for cytokines that were
differentially expressed in cases of GC. We identified 105
cytokines with significant differences between GC tissues and
normal gastric mucosa. Our results suggest that these
differentially expressed cytokines could be associated with GC.
Materials and methods
Patients and tissue samples
Pairs of GC and adjacent non-cancerous mucosa
tissues were first diagnosed and confirmed by clinical and
pathological examination. These samples were then obtained from
patients (n=8) who underwent D2 gastrectomy (radical gastrectomy
with level 2 extended lymphadenectomy) between February, 2014 and
June, 2015 at the Henan Provincial People's Hospital, Zhengzhou,
China. The cancerous and normal gastric tissues were washed with
physiological saline and subsequently frozen within 30 min of
removal in a liquid nitrogen tank after immediate pathological
examination. The senior pathologist routinely conducted the
diagnosis for GC based on hematoxylin and eosin (H&E) staining.
The tumor-node-metastasis (TNM) stage of these tumors were assigned
according to the American Joint Committee on Cancer guidelines.
This study was conducted in accordance with the declaration of
Helsinki. Written informed consent was obtained from all
participants.
Inclusion criteria
This study was reviewed and approved by the
Institutional Review Board and Ethics Committee of Zhengzhou
University. The prospective subject cohort consisted of matched
pairs of tumor/normal gastric tissues. The inclusion criteria are
as follows: i) histological diagnosis of GC, ii) the tumor can be
at any TNM stage, iii) gastric resection must have been performed
with a curative/radical intention, and iv) signed informed consent
was obtained; and v) no chemotherapy or radiotherapy treatment
prior to surgery.
Proteomic chip-based cytokine antibody
assay in GC tissues
All samples were used to assess the expression
levels of 507 cytokines using 16 antibody arrays
(RayBio® L-Series human antibody array L-507 membrane
kit; RayBiotech, Norcross, GA, USA) according to the manufacturer's
instructions. Briefly, all protein samples were extracted from the
tissues, followed by quantification and biotinylation. Incubation
of the array of membranes with biological samples overnight at 4°C
resulted in the binding of cytokines to corresponding antibodies.
Signals were visualized using HRP-conjugated streptavidin and
imaged by ImageQuant LAS 4000 Scanner (GE Healthcare Corp., Logan,
UT, USA). The final spot intensities were measured as the original
intensities, subtracting the background. The data were normalized
to the positive controls in the individual slide and intensity
ratios between GC and adjacent noncancerous mucosa tissues of each
experiment were compared.
Bioinformatics analysis of
differentially expressed protein factors associated with GC
The significant differentially expressed protein
factors in the GC samples were analyzed using bioinformatics
(http://www.expasy.org/vg/index/protein). Gene Ontology
(GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment
analysis of these protein factors were further conducted, aiming to
screen the functions of the candidate cytokines associated with the
development of GC.
Statistical analysis
All statistical and data analyses were performed
using version 19.0 of the SPSS software (SPSS, Inc., Chicago, IL,
USA). The P-values were calculated using a two sample t-test. In
addition, fold change values of cytokines were calculated to
indicate their relative expression levels normalized to the control
samples. Any fold change >2 or <0.5 in signal intensity among
the groups was considered relevant. P<0.05 was considered
statistically significant.
Results
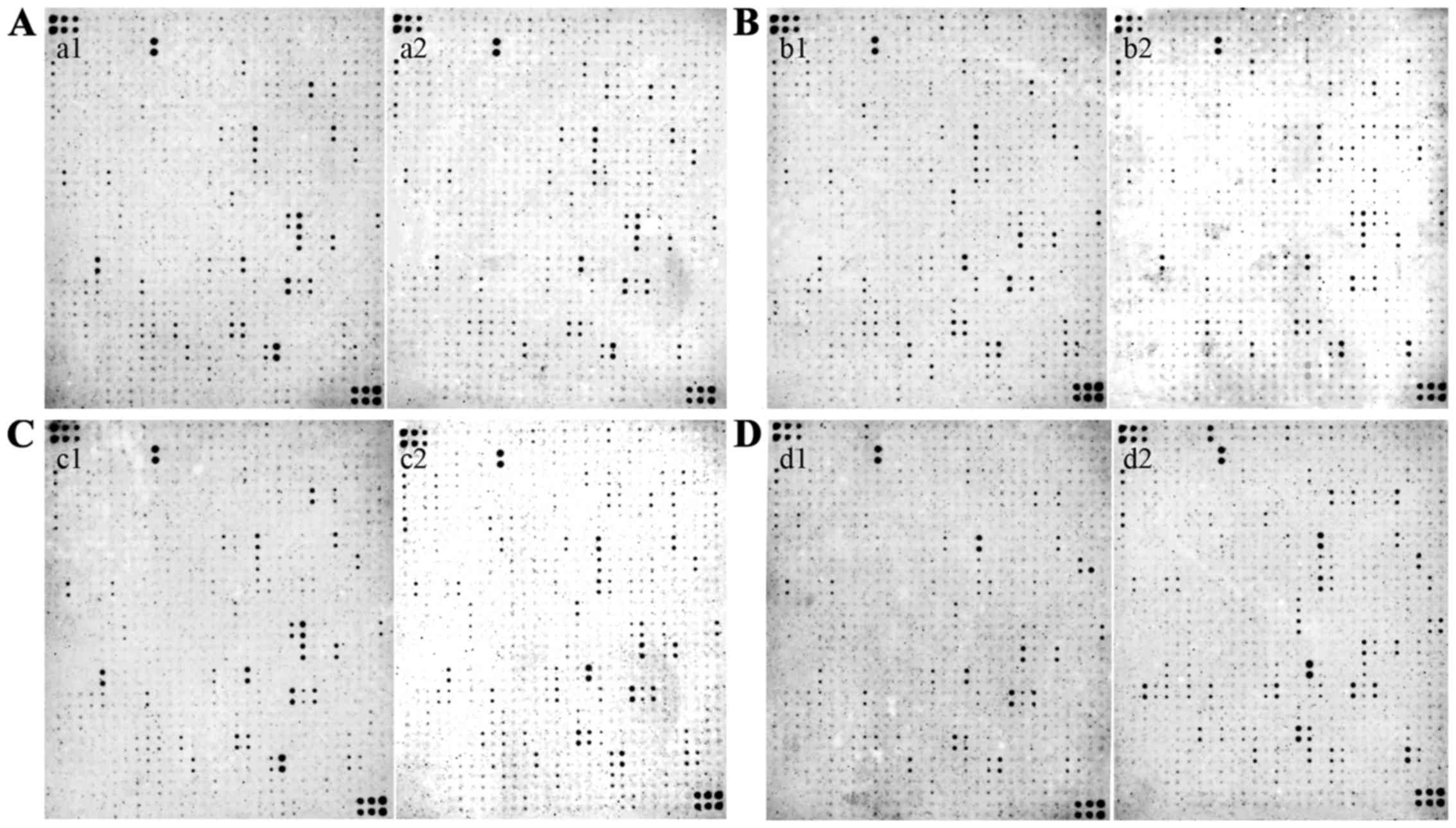
Analysis of cytokines in GC
Analyses of 507 cytokines were made by the Tool
Software for RayBio (RayBiotech, Inc., Norcross, GA, USA) human
biotin-label based antibody arrays. All of the cytokines that were
expressed in a given GC tissue were placed into the following
groups based on their intensities relative to the non-GC tissue
(Fig. 1): High abundance
(>2.0-fold), no-change (between 2-fold and >-2-fold), and low
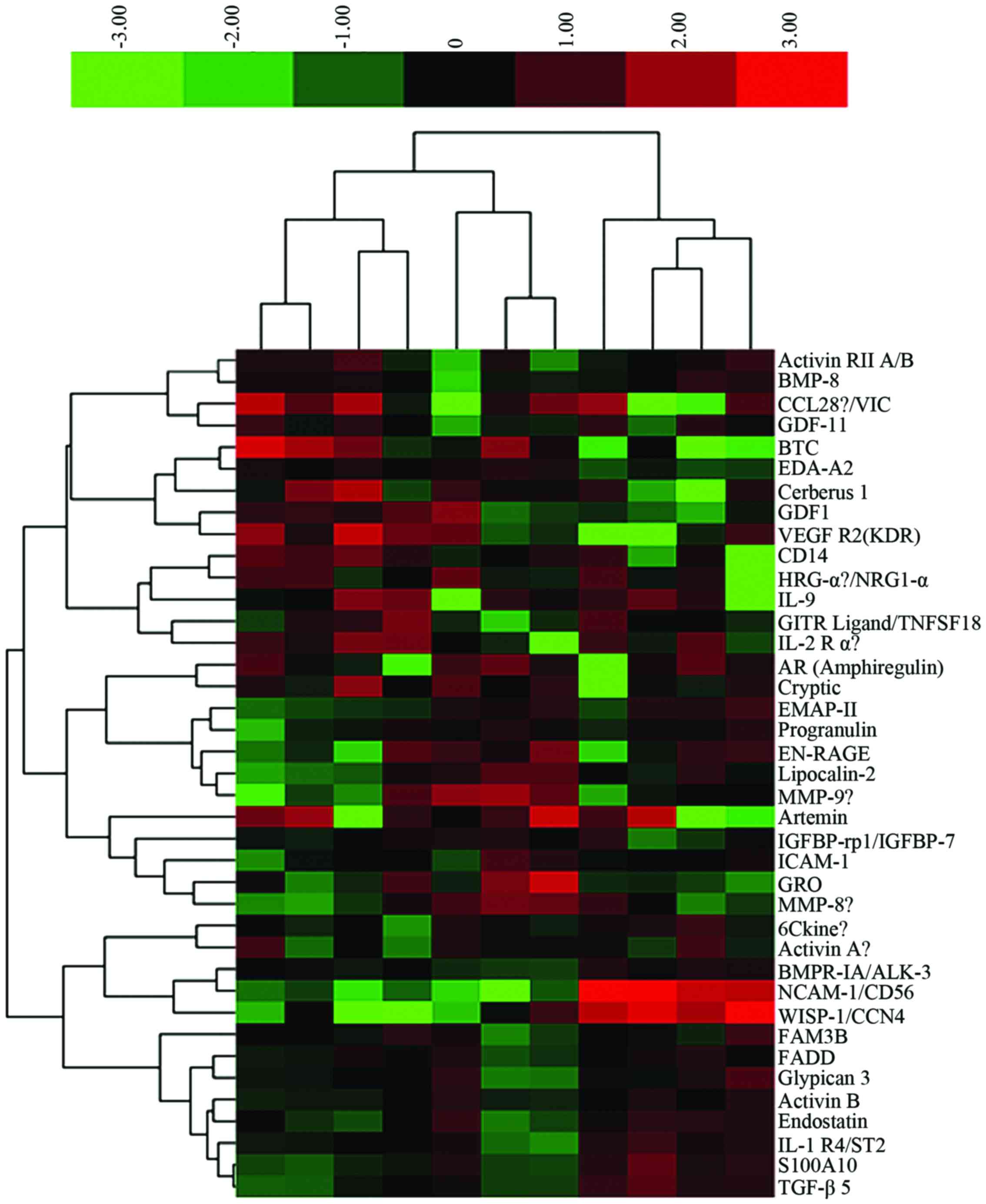
abundance (<-2-fold). These identifications were grouped in the
heat map is shown in Fig. 2. One
hundred and five differentially expressed proteins were identified
in the samples (Table I).
 | Table I.One hundred and five differentially
expressed proteins associated with GC. |
Table I.
One hundred and five differentially
expressed proteins associated with GC.
| Protein factors | Fold | P-value | Protein factors | Fold | P-value | Protein factors | Fold | P-value | Protein factors | Fold | P-value |
|---|
| XEDAR | 2.65 | 0.047 | Activin RIIA | 0.45 | 0.011 | FGF-5 | 0.11 | 0.013 | GDF8 | 0.09 | 0.014 |
| GFRα-3 | 0.45 | 0.024 | FADD | 0.38 | 0.008 | G-CSF R/CD114 | 0.10 | 0.023 | IL-3 | 0.18 | 0.019 |
| BMPR-II | 0.21 | 0.008 | M-CSFR | 0.39 | 0.039 | IL-7 | 0.09 | 0.013 | M-CSF | 0.42 | 0.030 |
| MMP-12 | 0.24 | 0.021 | IL-12 p70 | 0.48 | 0.048 | ICAM-1 | 0.26 | 0.025 | GDF11 | 0.29 | 0.045 |
| IL-26 | 0.40 | 0.046 | AgRP | 0.46 | 0.044 | CXCR3 | 0.08 | 0.029 | BMP-3 | 0.19 | 0.041 |
| NCAM-1/CD56 | 4.83 | 0.000 | GDF1 | 0.10 | 0.004 | TMEFF1 | 2.07 | 0.046 | GRO | 0.09 | 0.027 |
| Dkk-4 | 0.27 | 0.011 | IL-2 | 0.04 | 0.019 | BMP-4 | 0.50 | 0.014 | IL-20 Rα | 0.17 | 0.034 |
| MMP-1 | 0.25 | 0.024 | BD-1 | 0.49 | 0.016 | LRP-6 | 0.08 | 0.027 | IL-1 F8/FIL1β | 0.36 | 0.039 |
| CCR5 | 0.32 | 0.041 | HCR/CRAM-A/B | 0.35 | 0.032 | MIP2 | 0.12 | 0.032 | BMP-7 | 0.50 | 0.043 |
| IL-1 R6/IL-1
Rrp2 | 0.36 | 0.023 | Kremen-2 | 0.33 | 0.018 | GCSF | 0.04 | 0.024 | EGF | 0.29 | 0.019 |
| TPX | 0.50 | 0.034 | 6Ckine | 0.40 | 0.033 | MMP-8 | 0.21 | 0.044 | CCR4 | 0.43 | 0.012 |
| BMP-8 | 0.32 | 0.028 | IL-12 p40 | 0.13 | 0.021 | CD163 | 0.17 | 0.023 |
IGFBP-rp1/IGFBP-7 | 0.25 | 0.003 |
| Glypican 5 | 0.48 | 0.020 | IL-2 Rα | 0.23 | 0.034 | GRO-a | 0.14 | 0.028 |
Thrombospondin-1 | 0.17 | 0.044 |
| BMPR-IA/ALK-3 | 0.43 | 0.043 | BAX | 0.37 | 0.012 | IL-17C | 0.24 | 0.033 | S100 A8/A9 | 0.47 | 0.013 |
| Epiregulin | 0.25 | 0.015 | E-Selectin | 0.03 | 0.024 | IL-18 Rα/IL-1
R5 | 0.35 | 0.020 | Lipocalin-2 | 0.27 | 0.038 |
|
MMP-11/Stromelysin-3 | 0.40 | 0.035 | Activin B | 0.41 | 0.002 | TGF-β2 | 0.44 | 0.046 | BMP-5 | 0.31 | 0.030 |
| IL-2 Rβ/CD122 | 0.10 | 0.019 | IP-10 | 0.50 | 0.046 | Growth hormone
(GH) | 0.17 | 0.012 | TLR4 | 0.21 | 0.016 |
| IL-17 | 0.07 | 0.025 | Fas/TNFRSF6 | 0.09 | 0.029 | Frizzled-6 | 0.28 | 0.033 | TIMP-1 | 0.34 | 0.047 |
| VE-Cadherin | 0.31 | 0.023 | IL-8 | 0.05 | 0.048 | IL-18 BPa | 0.17 | 0.023 | SLPI | 0.46 | 0.032 |
| IL-10 Rα | 0.32 | 0.019 | Activin RII
A/B | 0.25 | 0.036 | IGF-II R | 0.38 | 0.029 | EDA-A2 | 0.12 | 0.001 |
| Fas ligand | 0.35 | 0.021 | TRAIL R1/DR4 | 0.14 | 0.015 | SAA | 0.09 | 0.021 | EN-RAGE | 0.29 | 0.037 |
| Coagulation factor
III | 0.48 | 0.013 | CXCR5/BLR-1 | 0.42 | 0.025 | MIG | 0.10 | 0.030 | CCR2 | 0.37 | 0.043 |
| MMP-10 | 0.42 | 0.031 | EDG-1 | 0.32 | 0.046 | Progranulin | 0.34 | 0.027 | GDF5 | 0.31 | 0.016 |
| IL-10 | 0.09 | 0.024 | Endothelin | 0.47 | 0.035 | IL-1α | 0.24 | 0.017 | Activin
RIA/ALK-2 | 0.25 | 0.040 |
| APJ | 0.35 | 0.012 | Activin C | 0.19 | 0.048 | MMP-9 | 0.14 | 0.050 | IL-22 R | 0.33 | 0.039 |
| CXCR4 (fusin) | 0.46 | 0.041 | IL-15 Rα | 0.09 | 0.043 | IGFBP-2 | 0.28 | 0.015 | EMAP-II | 0.44 | 0.038 |
| Glypican 3 | 0.46 | 0.037 |
|
|
|
|
|
|
|
|
|
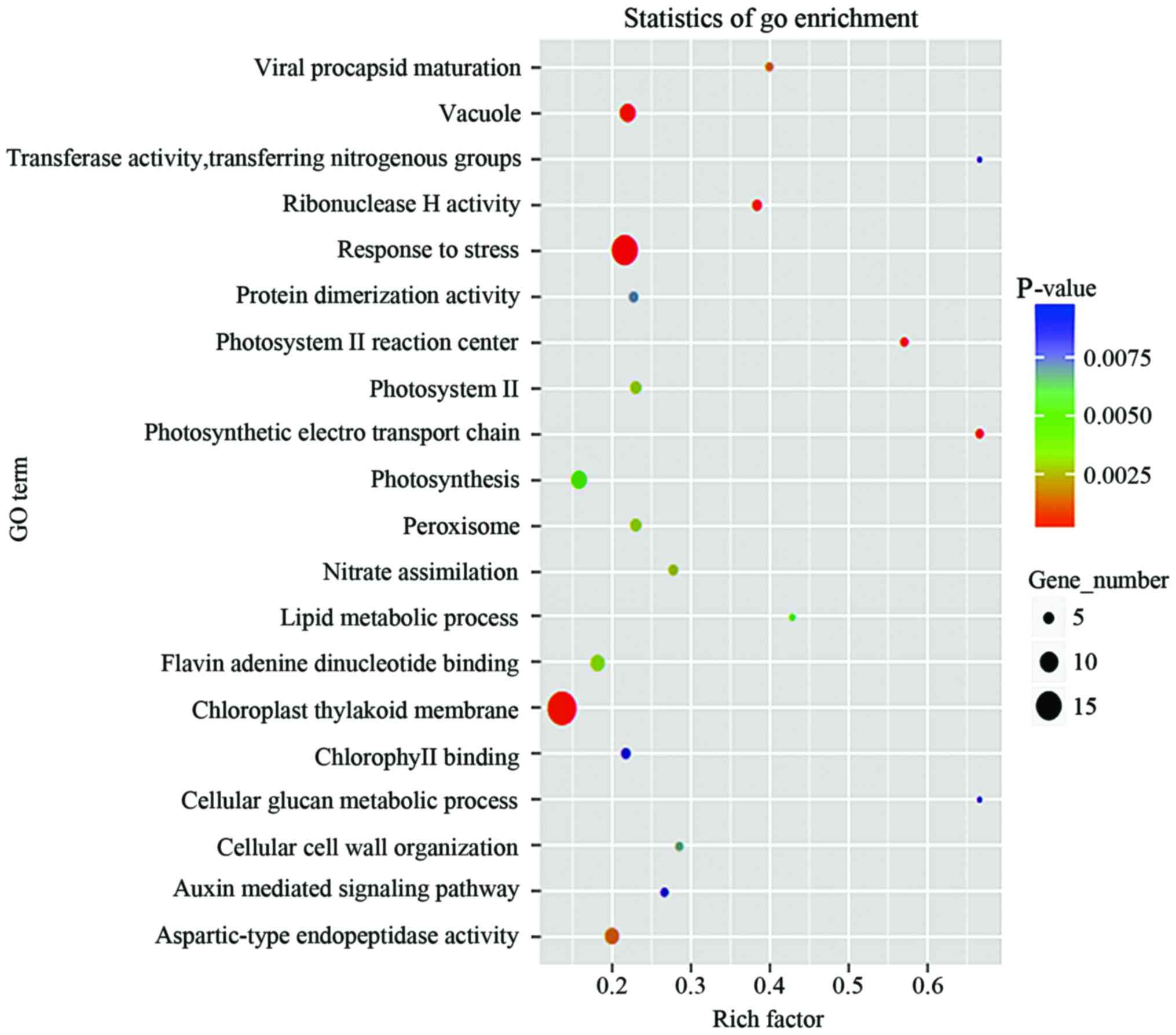
Bioinformatics analysis of identified
differentially expressed proteins in GC
In order to make high-throughput annotations of each
protein and to determine the biological or functional distributions
of differentially expressed cytokines, the GO and KEGG enrichment
analysis of significantly differentially expressed proteins of each
group were analyzed. As shown in Figs.
3–5, the specific biological
processes or molecular functions that the candidate cytokines are
involved in were determined, the degree of concordance between the
differentially expressed cytokines and the expected functions are
shown. Some proteins were found associated with certain functions
(Table I).
Discussion
In recent years, despite some advancement of GC
diagnosis, patients with GC still need to be diagnosed through
invasive procedures such as endoscopy or surgery pathological
diagnosis. Due to a low early diagnosis rate, most GC patients that
are eventually diagnosed have already entered into the late stage
with metastasis of the cancer, resulting a in low survival rate.
The traditional biomarkers of GC, such as CEA, CA19-9 and XA74-4,
are usually not specific and sensitive, as their sensitivity is
only 18–57% (11). Therefore, looking
for highly specific and sensitive GC biomarkers can contribute to
early diagnosis, targeted therapy and a better prognosis of
patients with GC (12).
Antibody microarray-based technology, which can
simultaneously detect the expression levels of multiple proteins
and has the combined advantages of the specificity of enzyme-linked
immunosorbent assay (ELISA), sensitivity of
enhanced-chemiluminescence (ECL) and high-throughput capacity of
microspot, represents a promising tool for the field of
onco-proteomics (10,13). This assay can be used to compare and
analyze proteins at various stages in the occurrence and
development of tumors in order to screen for biomarkers for early
diagnosis of tumors, specialized drug therapy and prognostic
evaluation. In addition, this technology utilizes proteomics
through the antibody microarray-based technology, which plays an
important role in the research of clinical, pharmacology, signal
transduction, cell cycle regulation, cell structure and neural
biology (14,15). Therefore, in the present study, we
performed a proteomic chip-based analysis to investigate the
differentially expressed cytokines that are associated with the
development of GC. This assay is capable of rapidly and
specifically detecting the expression levels of numerous cytokines,
growth factors, soluble receptors of growth factors, angiogenic
factors, metalloproteinases and other proteins using small amounts
of experimental sample in a single experiment.
Chemokines and their receptors have shown a variety
of biological functions in many processes, including the regulation
of tumor cell proliferation, angiogenesis, invasion and metastasis
(16–18). Many cancer-related cytokines,
chemokines, metalloproteinases, growth factors and angiogenic
factors are produced not only by the tumor cells themselves but
also by the activated stroma and immune cells that are associated
with tumors (16). The inflammatory
mediators that are produced by immunocompetent cells and cancer
cells can directly stimulate carcinogenesis (19–23).
As inflammation is a characteristic feature of the
development and progression of GC, we hypothesized that cytokines
released by the tumor microenvironment or by the cancer cells could
represent novel diagnosis and predictive biomarkers. The
simultaneous detection of multiple cytokines, which is afforded by
this technology, is an important tool for biomarker discovery and
can help us identify the key molecules that are important in cancer
development (24). GO enrichment
analysis showed that these significantly differentially expressed
proteins in GC samples are involved in many biological and
immunological processes, mainly in response to stress, chloroplast
thylakoid membrane, vacuole, photosynthesis, aspartic-type
endopeptidase activity and flavin-adenine dinucleotide binding.
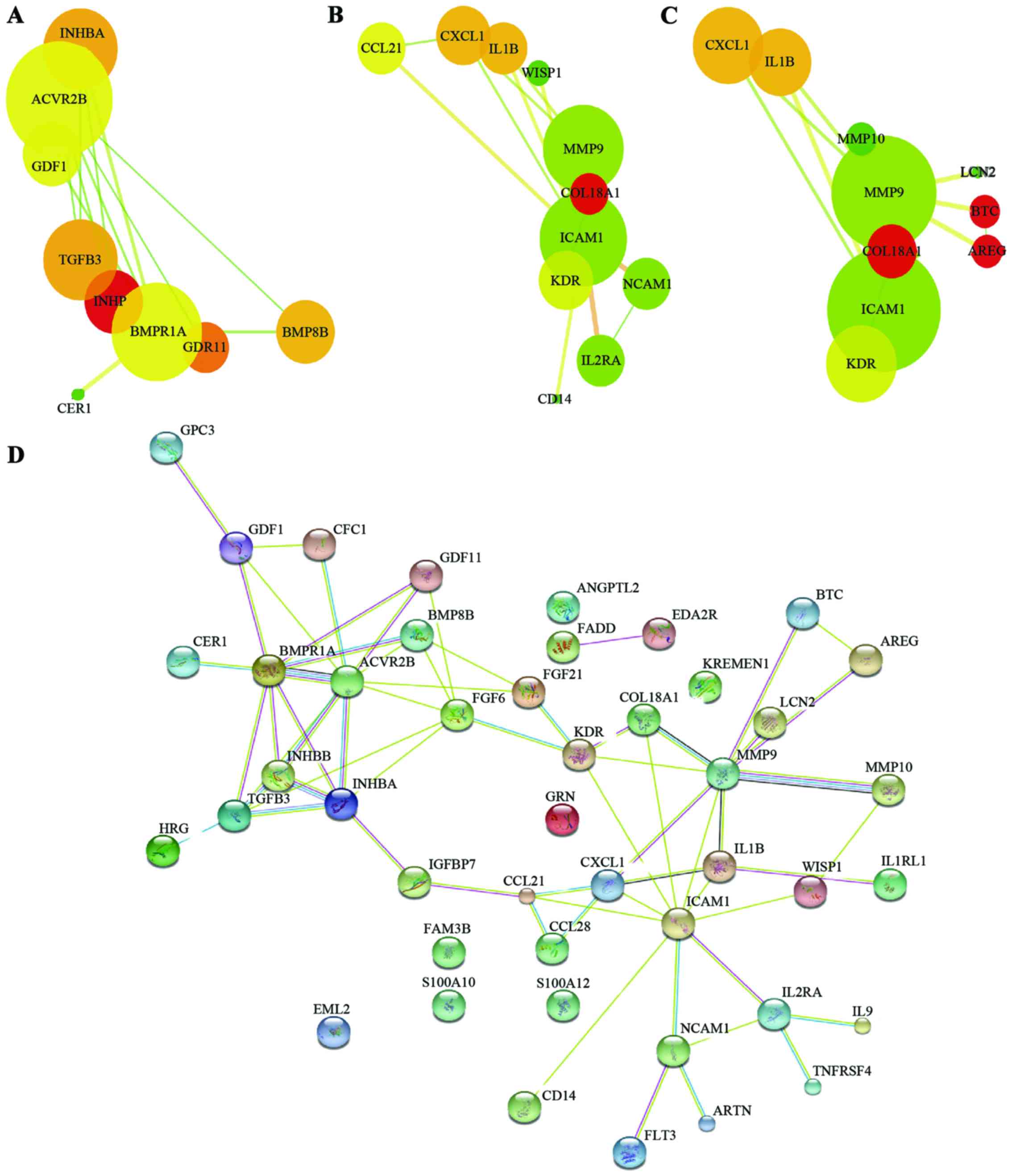
KEGG enrichment analysis demonstrated that these differentially
expressed proteins are mainly involved in the process of
cytokine-cytokine receptor interaction, transforming growth
factor-β (TGF-β) signaling pathway, tumor necrosis factor (TNF)
signaling pathway, and mitogen-activated protein kinase (MAPK)
signaling pathway. Moreover, our analysis revealed the key
signaling pathways or networks that are related to a set of
biomarkers identified in the training set. We imported the list of
these 39 proteins into the IPA software.
In conclusion, our results suggest that 105
cytokines are frequently expressed in GC tissues and may be
involved in occurrence and development of GC. While promising, our
results are based on a relatively small sample of patients. A
larger patient cohort is needed to validate the association of the
candidate cytokines we identified and their involvement in GC.
Further functional study of these cytokines may provide a promising
approach for diagnostic and predictive biomarkers for GC.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li NM, Liu F, Lv FY and Zhang QW:
Influencing factors and interventional strategies for early enteral
nutrition after gastric carcinoma surgery. J Cancer Res Ther.
12:689–692. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fu G, Niu Z, Zhou Y, Zhou X, Wang H and Su
Z: Influence of visceral fat area on laparoscopic radical
gastrectomy in patients with gastric carcinoma. Zhonghua Wei Chang
Wai Ke Za Zhi. 18:804–807. 2015.(In Chinese). PubMed/NCBI
|
|
4
|
Peddanna N, Holt S and Verma RS: Genetics
of gastric cancer. Anticancer Res. 15:2055–2064. 1995.PubMed/NCBI
|
|
5
|
Sakai N, Yoshidome H, Shida T, Kimura F,
Shimizu H, Ohtsuka M, Takeuchi D, Sakakibara M and Miyazaki M:
CXCR4/CXCL12 expression profile is associated with tumor
microenvironment and clinical outcome of liver metastases of
colorectal cancer. Clin Exp Metastasis. 29:101–110. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matboli M, El-Nakeep S, Hossam N, Habieb
A, Azazy AE, Ebrahim AE, Nagy Z and Abdel-Rahman O: Exploring the
role of molecular biomarkers as a potential weapon against gastric
cancer: A review of the literature. World J Gastroenterol.
22:5896–5908. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amedei A, Prisco DD and Elios MM: The use
of cytokines and chemokines in the cancer immunotherapy. Recent Pat
Anticancer Drug Discov. 8:126–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdiche YN, Miles A, Eckman J, Foletti D,
Van Blarcom TJ, Yeung YA, Pons J and Rajpal A: High-throughput
epitope binning assays on label-free array-based biosensors can
yield exquisite epitope discrimination that facilitates the
selection of monoclonal antibodies with functional activity. PLoS
One. 9:e924512014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perlee LT, Christiansen J, Dondero R,
Grimwade B, Lejnine S, Mullenix M, Shao W, Sorette M, Tchernev VT,
Patel DD, et al: Development and standardization of multiplexed
antibody microarrays for use in quantitative proteomics. Proteome
Sci. 2:92004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kloppot P, Selle M, Kohler C, Stentzel S,
Fuchs S, Liebscher V, Müller E, Kale D, Ohlsen K, Bröker BM, et al:
Microarray-based identification of human antibodies against
Staphylococcus aureus antigens. Proteomics Clin Appl. 9:1003–1011.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ebert MP and Röcken C: Molecular screening
of gastric cancer by proteome analysis. Eur J Gastroenterol
Hepatol. 18:847–853. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Erkan M, Reiser-Erkan C, Michalski CW,
Kong B, Esposito I, Friess H and Kleeff J: The impact of the
activated stroma on pancreatic ductal adenocarcinoma biology and
therapy resistance. Curr Mol Med. 12:288–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Puig-Costa M, Codina-Cazador A,
Cortés-Pastoret E, Oliveras-Ferraros C, Cufí S, Flaquer S,
Llopis-Puigmarti F, Pujol-Amado E, Corominas-Faja B, Cuyàs E, et
al: Discovery and validation of an INflammatory PROtein-driven
GAstric cancer Signature (INPROGAS) using antibody microarray-based
oncoproteomics. Oncotarget. 5:1942–1954. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y: Serum proteomic pattern analysis
for early cancer detection. Technol Cancer Res Treat. 5:61–66.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ménard C, Johann D, Lowenthal M, Muanza T,
Sproull M, Ross S, Gulley J, Petricoin E, Coleman CN, Whiteley G,
et al: Discovering clinical biomarkers of ionizing radiation
exposure with serum proteomic analysis. Cancer Res. 66:1844–1850.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caronni N, Savino B, Recordati C, Villa A,
Locati M and Bonecchi R: Cancer and chemokines. Methods Mol Biol.
1393:87–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong J, Chen Y and Wang LJ: Emerging
molecular basis of hematogenous metastasis in gastric cancer. World
J Gastroenterol. 22:2434–2440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Verbeke H, Geboes K, Van Damme J and
Struyf S: The role of CXC chemokines in the transition of chronic
inflammation to esophageal and gastric cancer. Biochim Biophys
Acta. 1825:117–129. 2012.PubMed/NCBI
|
|
19
|
Biswas SK and Mantovani A: Macrophage
plasticity and interaction with lymphocyte subsets: Cancer as a
paradigm. Nat Immunol. 11:889–896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mariani F and Roncucci L: Chemerin/chemR23
axis in inflammation onset and resolution. Inflamm Res. 64:85–95.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Galdiero MR, Garlanda C, Jaillon S, Marone
G and Mantovani A: Tumor associated macrophages and neutrophils in
tumor progression. J Cell Physiol. 228:1404–1412. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung M, Ören B, Mora J, Mertens C,
Dziumbla S, Popp R, Weigert A, Grossmann N, Fleming I and Brüne B:
Lipocalin 2 from macrophages stimulated by tumor cell-derived
sphingosine 1-phosphate promotes lymphangiogenesis and tumor
metastasis. Sci Signal. 9:ra642016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Natesan M and Ulrich RG: Protein
microarrays and biomarkers of infectious disease. Int J Mol Sci.
11:5165–5183. 2010. View Article : Google Scholar : PubMed/NCBI
|