Introduction
As one of the common malignant tumors in China,
hepatocarcinoma shows an increasing incidence rate and the age of
onset is also becoming increasingly younger. Surgical resection is
the most important treatment of early non-metastatic
hepatocarcinoma, but the 5-year recurrent rate is still higher than
60% after surgery (1). Resistance to
chemotherapy drugs is the main reason for poor prognosis of
hepatocarcinoma. As the first-line drug used in the treatment of
hepatocarcinoma, cisplatin has broad spectrum anticancer activity.
Cisplatin has been widely used in the treatment of various
malignant tumors including hepatocarcinoma, ovarian, prostate,
lung, esophageal, head and neck squamous cell carcinoma and thyroid
cancer. However, application of high doses of cisplatin can bring
adverse side effects on the nervous system, kidney and
gastrointestinal tract, treatment with cisplatin usually failed to
provide satisfactory outcomes in treatment of hepatocarcinoma
(2,3).
Therefore, it will be of significant clinical value for treatment
of hepatocarcinoma to identify drugs that can enhance the
chemosensitivity of hepatocarcinoma cells to cisplatin.
In previous years, combined chemotherapy has been
proved to be an import way in increasing the efficacy of the
treatment of hepatocarcinoma (4) and
cisplatin treatment combined with specific molecular targeted drug
has attracted increased attention. As an oral antidiabetic drug,
metformin is the first-line drug for the treatment of type 2
diabetes. Studies have shown that metformin has anticancer
function. Therefore, in this study, cisplatin was used to treat
hepatocarcinoma cells HepG2 and Huh-7 and effects of metformin on
chemosensitivity of hepatocarcinoma cells to cisplatin and the
specific molecular mechanism were studied. Our study provided new
ideas and theoretical basis for improving the chemosensitivity of
hepatocarcinoma cells to cisplatin.
Materials and methods
Main reagents
RPMI-1640 cell culture medium and fetal bovine serum
(FBS) were purchased from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Metformin, cisplatin, adenosine 5-monophosphate
(AMP)-activated protein kinase (AMPK) signaling pathway blocker
compound C and methyl thiazolyl tetrazolium assay (MTT) cell
proliferation assay kit were purchased from Sigma (Merck & Co.,
Inc., Whitehouse Station, NJ, USA). Annexin V-FITC apoptosis
detection kit was from Bogu Biological Science and Technology Co.,
Ltd. (Shanghai, China). Rabbit anti-human AMPK and p-AMPK
polyclonal antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA) (dilution, 1:1,000; cat. nos.
2532 and 4184). Rabbit anti-human β-actin polyclonal antibody and
goat anti-rabbit horseradish perox-idase-labeled secondary
polyclonal antibody were purchased from Boster Biological
Technology Co., Ltd. (Pleasanton, CA, USA) (dilution, 1:2,000; cat.
nos. BM0627 and BA1054). RIPA protein lysate was purchased from
Beijing Kangwei Century Biotechnology Co., Ltd. (Beijing,
China).
Cell culture
HepG2 and Huh-7 cell lines were purchased from
Guangzhou Cellcook Biotech Co., Ltd. (Guangzhou, China). HepG2 and
Huh-7 cells were cultured in RPMI-1640 medium containing 10% FBS,
100 U/ml penicillin and 100 U/ml streptomycin. Cells were kept in a
humidified incubator (37 °C, 5% CO2). Trypsin 0.25% was
added for digestion for 2 min and complete medium was added to
terminate the digestion before subculture. After centrifugation for
3 min at 1,000 rpm, cells were resuspended and transferred to a new
culture bottle. Culture medium was replaced every day and
subculture was performed every three days. Cells were collected at
logarithmic growth phase for follow-up experiments.
MTT assay to detect the proliferative
activity of hepatocarcinoma cells
HepG2 and Huh-7 hepatocarcinoma cells in good growth
conditions were inoculated into 96-well plates with 100 µl
(2×105/ml) per well, and were cultured in an incubator.
Twenty four hours later, culture medium was replaced with complete
culture medium containing different concentrations of cisplatin or
metformin. Three wells were set in each group and cells were
incubated for 48 h in the cell incubator. MTT reagent was added and
incubated for another 4 h. Optical density value of each well was
read at a wavelength of 480 nm using a multi-function microplate
reader.
Flow cytometry to detect cell
apoptosis
Cells treated with cisplatin (0, 2, 4, 6, 8 and 10
µM), metformin (10 mmol/l) and AMPK pathway blocker compound C (10
µmol/l) were digested to make single cell suspension.
Hepatocarcinoma cells (5×105 cells) were mixed with 100
µl of 1X binding buffer, and 5 µl of Annexin V-FITC and 5 µl of PI
reagent were also added, followed by incubation at room temperature
in the dark for 15 min. After that, 400 µl 1X binding buffer was
added to resuspend the cells and cell apoptosis rate was detected
by flow cytometry.
Western blot analysis to detect the
expression levels of AMPK and p-AMPK protein
Cells treated with cisplatin (0, 2, 4, 6, 8 and 10
µM), metformin (10 mmol/l) and AMPK pathway blocker compound C (10
µmol/l) were digested and havested. Cells were lysed to extract
total protein. Protein samples were mixed with loading buffer and
denatured at 98°C for 5 min. A total of 10 µg of protein from each
sample was subjected to sodium dodecyl sulphate-polyacrylamide gel
electrophoresis, followed by transmembrane to polyvinylidene
difluoride membrane. After blocking with 5% skimmed milk at room
temperature for 45 min, membranes were incubated with primary
antibody overnight at 4°C. Membranes were then rinsed with washing
solution 3 times, 10 min each time. Horseradish peroxidase labeled
secondary antibody (1:2,000) was used to incubate the membranes at
room temperature for 1 h. Membranes were then rinsed with washing
solution 3 times, 15 min each time. Color development was performed
using ECL chemiluminescence kit (Beijing Kangwei Century Biotech
Co. Ltd., Beijing, China). ImageJ software (NIH Image, Bethesda,
MD, USA) was used to quantitatively analyze the western blot
analysis.
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used to analyze the experimental data. Experimental data were
expressed as mean ± standard deviation. Comparisons between groups
were performed by t-test. A P<0.05 was considered to indicate a
statistically significant analysis.
Results
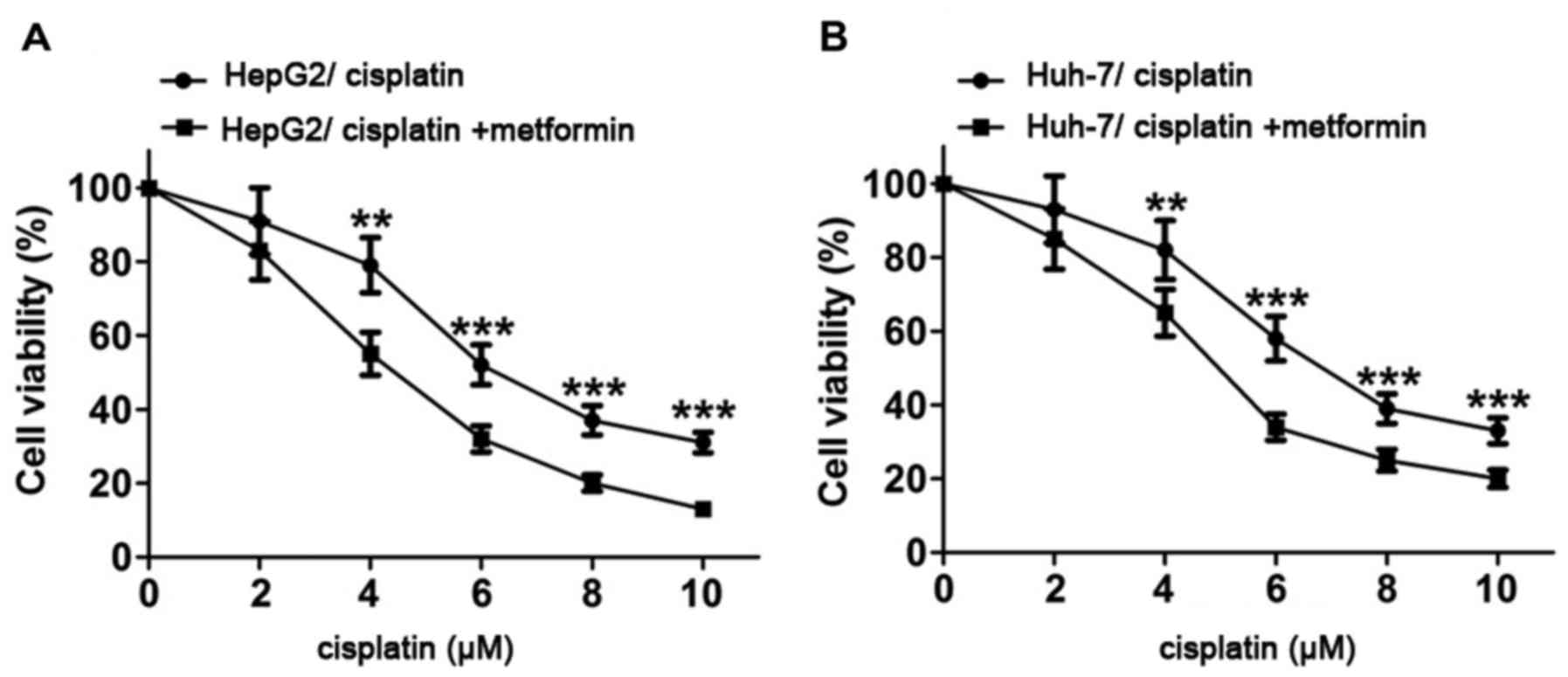
Metformin enhances the inhibitory
effect of cisplatin on proliferation of hepatocarcinoma cells
HepG2 and Huh-7 cells were treated with cisplatin at
0, 2, 4, 6, 8 and 10 µM for 48 h and proliferation activity of
HepG2 and Huh-7 cells was detected by MTT assay. Results showed
that proliferation activity of HepG2 and Huh-7 cells decreased with
the increase of cisplatin concentration. After treatment with
metformin (10 mmol/l) for 4 h, proliferation activity of HepG2 and
Huh-7 cells were significantly reduced compared with control group.
Results of this experiment suggest that metformin can enhance the
inhibitory effect of cisplatin on proliferation of HepG2 and Huh-7
cells (Fig. 1).
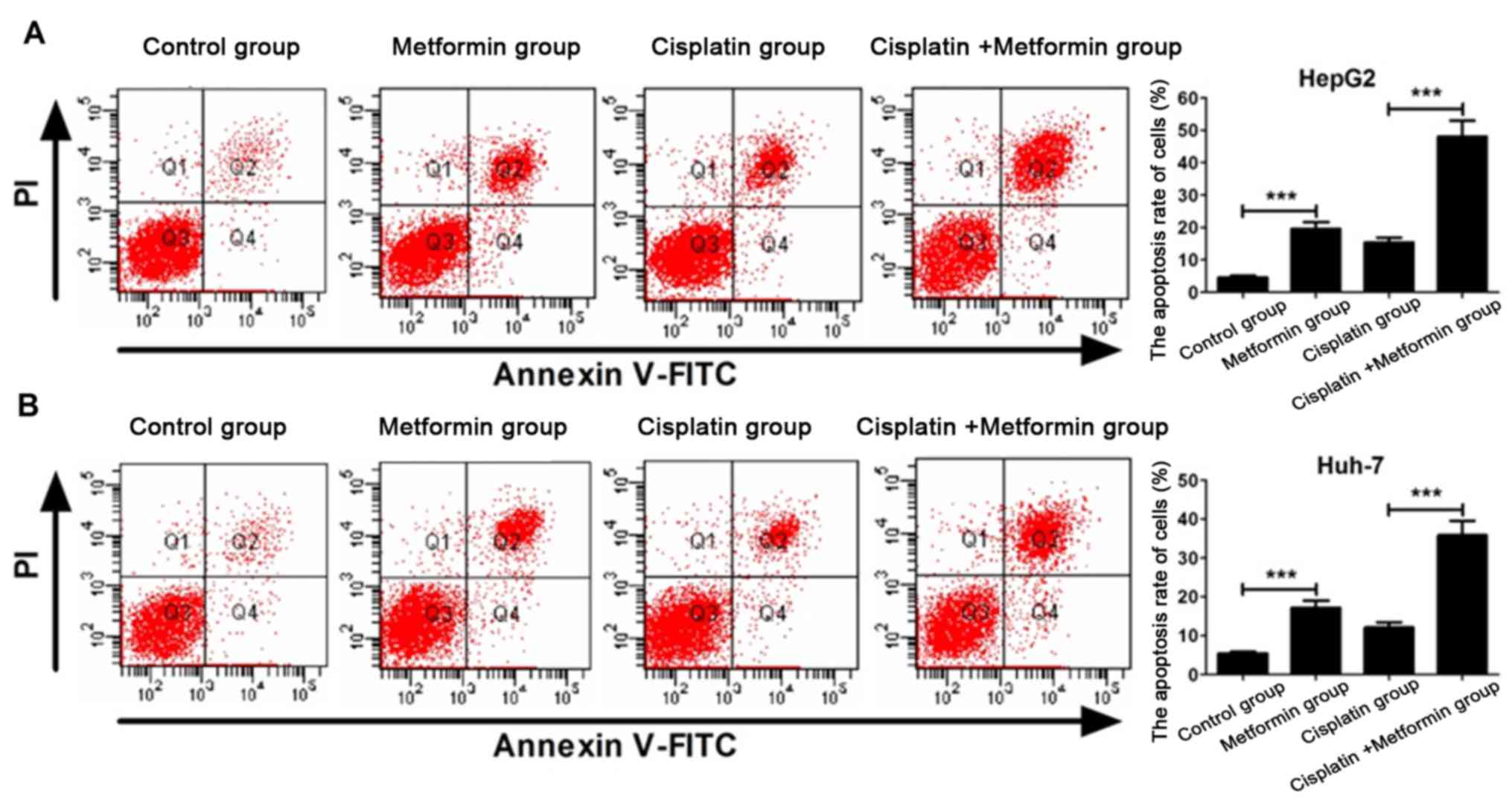
Metformin enhances cisplatin-induced
apoptosis of hepatocarcinoma cells
Flow cytometry experiment was carried out to
investigate whether metformin can enhance apoptosis of
hepatocarcinoma cells induced by cisplatin. Apoptosis rate of HepG2
hepatocarcinoma cells in metformin (10 mmol/l) group was
significantly higher than that in control group (P<0.05). After
incubation for 48 h, apoptosis rate of cisplatin (8 µM) + metformin
(10 mmol/l) was significantly higher than that of cisplatin group
(P<0.05) (Fig. 2A). Similar result
was found in Huh-7 hepatocarcinoma cells (Fig. 2B). These results indicate that
metformin can promote apoptosis of HepG2 and Huh-7 hepatocarcinoma
cells and enhance the ability of cisplatin in inducing apoptosis of
HepG2 and Huh-7 hepatocarcinoma cells.
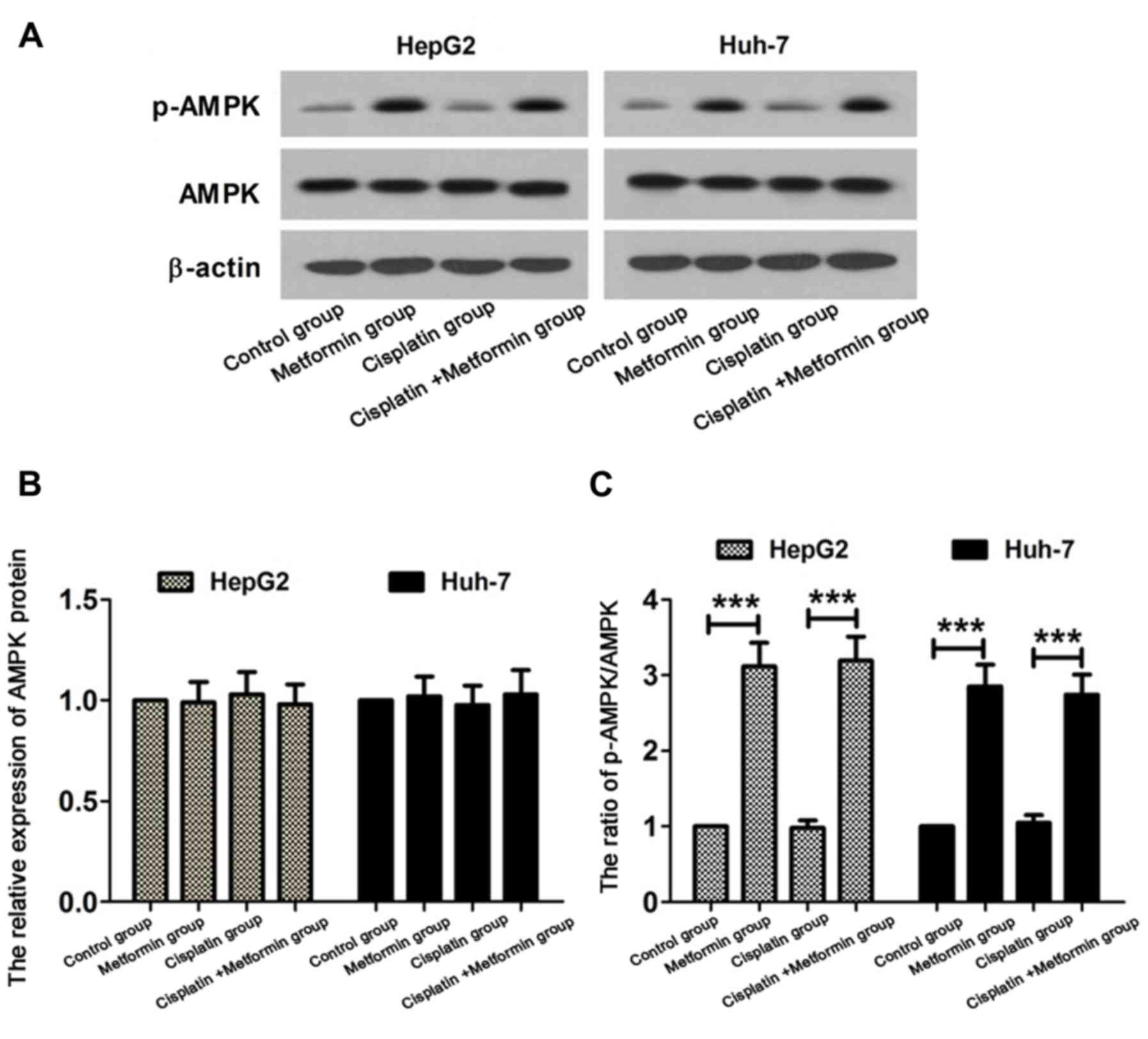
AMPK signaling pathway is activated in
hepatocarcinoma cells treated with metformin
In order to study the molecular mechanism of
metformin in enhancing the sensitivity of hepatocarcinoma cells to
cisplatin, the status of AMPK signaling pathway was detected by
western blot analysis. HepG2 and Huh-7 hepatocarcinoma cells were
treated with cisplatin (8 µM) and metformin (10 mmol/l) for 48 h.
Western blot analysis are shown in Fig.
3A. No significant difference in expression level of AMPK
protein was found among control, metformin, cisplatin and cisplatin
+ metformin group (Fig. 3B). Compared
with control, ratio of p-AMPK/AMPK was increased in metformin
group. Ratio of p-AMPK/AMPK in cisplatin + metformin was
significantly higher than that in cisplatin group (Fig. 3C). These results suggest that
metformin can activate the AMPK signaling pathway in
hepatocarcinoma cells.
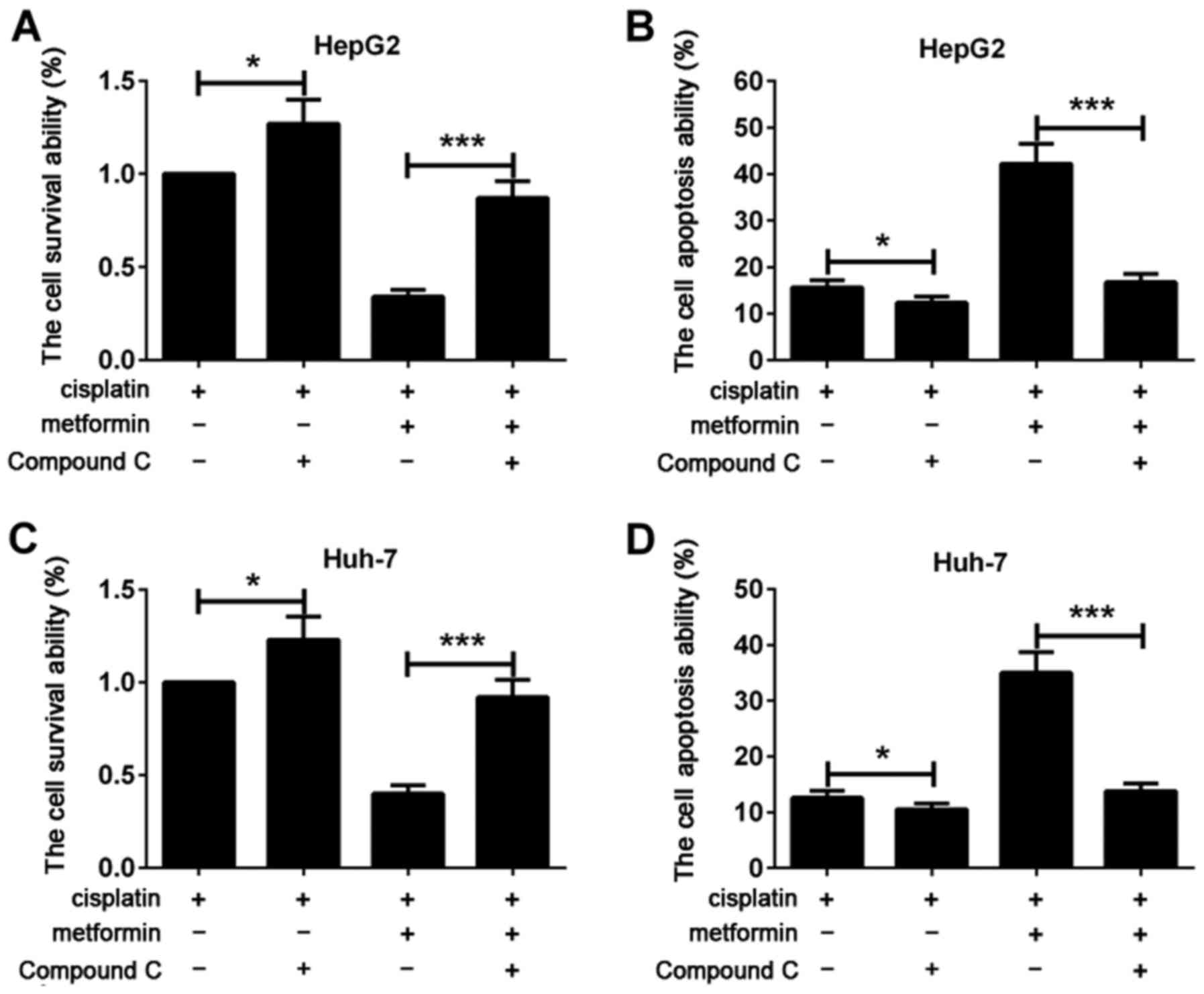
Compound C reverses the effect of
metformin in increasing the chemosensitivity of hepatocarcinoma
cells to cisplatin
In order to further confirm that metformin can
increase the chemosensitivity of hepatocarcinoma cells to cisplatin
by activating AMPK pathway, cells were treated with AMPK signal
blocking agent compound C (10 µmol/l) for 2 h. Changes of the
effect of metformin in inhibiting proliferation of hepatocarcinoma
cells and promoting apoptosis of hepatocarcinoma cells were
observed after the inhibition of AMPK signaling pathway. Flow
cytometry showed that, compared with cisplatin group, cell
viability of cisplatin + compound C group was increased and
apoptosis was decreased. Cell viability of cisplatin + metformin +
compound C was significantly higher than that of cisplatin +
metformin group. Apoptosis rate of cisplatin + metformin + compound
C was significantly lower than that of cisplatin + metformin group
(Fig. 4A and B). Similar results were
found in Huh-7 hepatocarcinoma cells (Fig. 4C and D). The results suggested that
effect of metformin in increasing the chemosensitivity of
hepatocarcinoma cells to cisplatin was reversed by compound C.
Therefore, we speculate that metformin may be an effective
activator of AMPK, and combined metformin treatment can enhance the
cytotoxic effect of cisplatin on tumor cells. This study showed
that AMPK signaling pathway was activated in metformin-treated
hepatocarcinoma cells, and compound C treatment reversed the effect
of metformin in increasing the chemosensitivity of hepatocarcinoma
cells to cisplatin, further confirming that activation of AMPK
signaling pathway is the key for metformin to improve the
chemosensitivity of hepatocarcinoma cells to cisplatin.
Discussion
As a non-specific cell cycle toxic drug, cisplatin
is one of the main drugs used in the clinical treatment of
hepatocarcinoma. Cisplatin can be hydrolyzed into platinum
diaminodichloride after entering cells to damage cell membrane
structure. Cisplatin can also bind to purine and pyrimidine bases
of DNA in nucleus to form cisplatin and DNA complex to cause DNA
breakage and error code, which in turn inhibit DNA replication and
transcription, thereby inducing tumor cell apoptosis to play a role
in treating tumors (5). However,
patients with hepatocarcinoma usually show varying degrees of
resistance to cisplatin, which in turn lead to the insensitivity to
cisplatin and unsatisfactory treatment outcomes, and this is the
main cause of failure of the treatment of hepatocarcinoma (6). Previous studies have shown that the
automatic DNA damage repair function is one of the main mechanisms
of cisplatin resistance (7). The
emergence of resistance to cisplatin can greatly reduce treatment
efficacy of hepatocarcinoma. Most researchers believe that
optimizing the combined drugs to enhance the antitumor effect of
cisplatin, and reduce the concentration of cisplatin to reduce its
side effects is the preferred strategy in treatment of
hepatocarcinoma (8). Therefore, the
identification of effective cisplatin resistance reversal agents is
the most effective method in increasing treatment efficacy of
hepatocarcinoma, it is also an active research topic and difficult
problem in chemotherapy of hepatocarcinoma.
Many studies have shown that metformin can reduce
the occurrence of oxidative phosphorylation and regulate energy
metabolism processes mainly through the inhibition of mitochondrial
respiratory chain complex I activity to reduce the energy state of
cells. Clinical study carried out by Evans et al showed
that, compared with type 2 diabetes patients treated with other
drugs, patients treated with metformin showed significantly reduced
incidence of cancer (9).
Meta-analysis showed that risk of hepatocarcinoma was reduced by
62% in type 2 diabetes patients treated with metformin (10). A prospective study on patients with
endometrial cancer showed a significant correlation between
metformin intake and increased recurrence-free survival and overall
survival (11). Wheaton et al
found that metformin could inhibit the proliferation and promote
apoptosis of A549 human lung adenocarcinoma cells by reducing the
activity of complex I in mitochondria of cancer cells (12). Overexpression of IL-6 can induce
tyrosine kinase inhibitor (TKI) resistance in TKI sensitive cells.
Metformin can enhance the sensitivity of human lung cancer cells to
epidermal growth factor (EGF) receptor TKI (EGF receptor TKI) by
inhibiting IL-6 signaling pathway. EGFR-TKI kinase inhibitors
combined with metformin therapy effectively prevented tumor growth
in nude mice transplanted with TKI-resistant tumor cells (13). Compared with the chemotherapy using
cisplatin alone, combination of cisplatin and metformin treatment
significantly increased the inhibitory effect on tumor growth
(14). This study found that
metformin can enhance the chemosensitivity of drug-resistant
hepatocarcinoma cell lines to chemotherapeutic drugs cisplatin, and
the possible molecular mechanism of the function of metformin was
also explored. Results showed that cisplatin could inhibit the
proliferation and promote apoptosis of HepG2 and Huh-7
hepatocarcinoma cells. HepG2 and Huh-7 hepatocarcinoma cells were
more sensitive to cisplatin after adding metformin.
AMPK is a serine/threonine protein kinase in
eukaryotic cells. As an important energy receptor kinase in the
cell, AMPK regulates intracellular production of adenosine
triphosphate (ATP) in a poor nutrient environment (15). Abnormal AMP/ATP ratio in the body can
be caused by various factors including ischemia, hypoxia, lack of
energy, and heat shock. Under such conditions, AMPK is activated,
resulting in a decrease in AMP/ATP ratio. Downstream substrate of
intracellular energy metabolism cycle will also be activated to
participate in the regulation of the synthesis of energy metabolism
related protein (16). Studies have
shown that metformin can activate AMPK, thereby inhibiting signal
transduction of mitogen-activated protein kinase and
phosphatidylinositol 3-kinase/protein kinase B and inhibiting the
growth of breast cancer, lymphoma and other cancers (17,18). Honjo
et al have found that metformin can inhibit oncogene
PI3K/Mammalian target of rapamycin (mTOR) signal pathway to reduce
survival rate of esophageal cancer cells and promote esophageal
cancer cell apoptosis, thereby enhancing the sensitivity of
esophageal cancer to chemotherapy (19). Metformin can inhibit phosphorylation
of mTOR downstream target gene p70-S6 and ribosomal S6 protein
kinase, and DEPTOR-related mTOR inhibition has been shown to be one
of the mechanisms of anti-hepatocarcinoma function of metformin
(20). These studies suggest that
AMPK can serve as a potential target for tumor therapy.
In conclusion, metformin enhanced the cytotoxicity
of cisplatin to hepatocarcinoma cells. This study revealed the
mechanism of metformin in increasing the chemosensitivity of
hepatocarcinoma cells to cisplatin. This study also proved that
metformin may potentially serve as an effective adjuvant drug of
cisplatin in the treatment of hepatocarcinoma. Our study provided
experimental basis for the development of chemotherapy in the
treatment of hepatocarcinoma.
References
|
1
|
Ma X, Yang Y, Li HL, Zheng W, Gao J, Zhang
W, Yang G, Shu XO and Xiang YB: Dietary trace element intake and
liver cancer risk: Results from two population-based cohorts in
China. Int J Cancer. 140:1050–1059. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heidari-Soreshjani S, Asadi-Samani M, Yang
Q and Saeedi-Boroujeni A: Phytotherapy of nephrotoxicity-induced by
cancer drugs: An updated review. J Nephropathol. 6:254–263. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yood Ulcickas M, Bortolini M, Casso D,
Beck JG, Oliveria SA, Wells KE, Woodcroft KJ and Wang LI: Incidence
of liver injury among cancer patients receiving chemotherapy in an
integrated health system. Pharmacoepidemiol Drug Saf. 24:427–434.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen HH and Kuo MT: Role of glutathione in
the regulation of Cisplatin resistance in cancer chemotherapy. Met
Based Drugs. 2010:4309392010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen P, Hu MD, Deng XF and Li B: Genistein
reinforces the inhibitory effect of cisplatin on liver cancer
recurrence and metastasis after curative hepatectomy. Asian Pac J
Cancer Prev. 14:759–764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cong Y, Wang L, Wang Z, He S, Zhou D, Jing
X and Huang Y: Enhancing therapeutic efficacy of cisplatin by
blocking DNA damage repair. ACS Med Chem Lett. 7:924–928. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Xu H, Dai X, Zhu Z, Liu B and Lu X:
Enhanced in vitro and in vivo therapeutic efficacy of codrug-loaded
nanoparticles against liver cancer. Int J Nanomed. 7:5183–5190.
2012. View Article : Google Scholar
|
|
9
|
Evans JM, Donnelly LA, Emslie-Smith AM,
Alessi DR and Morris AD: Metformin and reduced risk of cancer in
diabetic patients. BMJ. 330:1304–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q
and Kip KE: Metformin for liver cancer prevention in patients with
type 2 diabetes: A systematic review and meta-analysis. J Clin
Endocrinol Metab. 97:2347–2353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ko EM, Walter P, Jackson A, Clark L,
Franasiak J, Bolac C, Havrilesky LJ, Secord AA, Moore DT, Gehrig
PA, et al: Metformin is associated with improved survival in
endometrial cancer. Gynecol Oncol. 132:438–442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wheaton WW, Weinberg SE, Hamanaka RB,
Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu GM,
Budigner GS, et al: Metformin inhibits mitochondrial complex I of
cancer cells to reduce tumorigenesis. eLife. 3:e022422014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Han R, Xiao H, Lin C, Wang Y, Liu H,
Li K, Chen H, Sun F, Yang Z, et al: Metformin sensitizes
EGFR-TKI-resistant human lung cancer cells in vitro and in vivo
through inhibition of IL-6 signaling and EMT reversal. Clin Cancer
Res. 20:2714–2726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu G, Fang W, Xia T, Chen Y, Gao Y, Jiao
X, Huang S, Wang J, Li Z and Xie K: Metformin potentiates rapamycin
and cisplatin in gastric cancer in mice. Oncotarget. 6:12748–12762.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Chhipa RR, Pooya S, Wortman M,
Yachyshin S, Chow LM, Kumar A, Zhou X, Sun Y, Quinn B, et al:
Discrete mechanisms of mTOR and cell cycle regulation by AMPK
agonists independent of AMPK. Proc Natl Acad Sci USA. 111:pp.
435–444. 2014, View Article : Google Scholar
|
|
16
|
Vilà L, Roglans N, Perna V, Sánchez RM,
Vázquez-Carrera M, Alegret M and Laguna JC: Liver AMP/ATP ratio and
fructokinase expression are related to gender differences in AMPK
activity and glucose intolerance in rats ingesting liquid fructose.
J Nutr Biochem. 22:741–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hadad SM, Hardie DG, Appleyard V and
Thompson AM: Effects of metformin on breast cancer cell
proliferation, the AMPK pathway and the cell cycle. Clin Transl
Oncol. 16:746–752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi WY, Xiao D, Wang L, Dong LH, Yan ZX,
Shen ZX, Chen SJ, Chen Y and Zhao WL: Therapeutic metformin/AMPK
activation blocked lymphoma cell growth via inhibition of mTOR
pathway and induction of autophagy. Cell Death Dis. 3:e2752012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Honjo S, Ajani JA, Scott AW, Chen Q,
Skinner HD, Stroehlein J, Johnson RL and Song S: Metformin
sensitizes chemotherapy by targeting cancer stem cells and the mTOR
pathway in esophageal cancer. Int J Oncol. 45:567–574. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Obara A, Fujita Y, Abudukadier A,
Fukushima T, Oguri Y, Ogura M, Harashima S, Hosokawa M and Inagaki
N: DEPTOR-related mTOR suppression is involved in metformin's
anti-cancer action in human liver cancer cells. Biochem Biophys Res
Commun. 460:1047–1052. 2015. View Article : Google Scholar : PubMed/NCBI
|