Introduction
Psoriasis (PS) is one of the most frequent
dermatological diseases, shows polygenic inheritance, and occurs in
approximately 2% of the population (1,2). The
etiology and pathogenesis is not yet clear, and may be associated
with virulence genes, environmental factors and immunological
derangement. Recently, increasing evidence suggests that changes of
skin microbiota, especially Firmicutes, may contribute to the
pathogenesis of PS. However, it is not known whether these
alterations in the microbiome are a consequence of PS itself.
Nevertheless, consideration of the microbiome provides a new
outlook on PS; the microbiota may be associated with the
inflammatory reaction and therefore should be considered in the
treatment of PS (3).
PS is believed to be an autoimmune disease (AID)
with an inherited genetic predisposition, and is potentially
mediated by multiple immune cells, such as dendritic cells, T cells
and spongiocytes, as well as pro-inflammatory cytokines produced by
these cells (4,5). CD8+ T cells, which represent
the autoimmune core of the disease, drive the initial phase of PS
and act as a repository of specific disease memory (4,6). Several
recent studies have demonstrated that interleukin (IL)-17-producing
CD4+ and CD8+ T cells may act as critical
factors in driving the autoimmune reaction during both the initial
phase of PS and the subsequent proinflammatory loop (6). Furthermore, it has been suggested that
other cytokines such as IL-23/9/22, tumor necrosis factor α (TNF-α)
and interferon α (IFN-α) may also play important roles in the
pathogenesis of PS (6–8). Recently, more attention has been given
to the IL-23/Th17 axis, which is believed mainly to play an
important role in controlling the proinflammatory loop in psoriatic
plaques (7). TNF-α and IFN-α have
been recognized as exacerbation factors in PS progression, and play
important roles in the inflammatory cascade that leads to different
symptoms, ranging from skin lesions to arthritis (8,9). The
concept of T cell involvement in the pathogenesis of PS is
supported by the beneficial effect of using cyclosporine (CsA) and
tacrolimus (FK506), both of which suppress T cell activation
(10). Recently, it has been
demonstrated that adalimumab therapy is also beneficial in the
control of PS (11). It acts by
suppressing T cells and decreasing plasma IL-17 levels and
IL-17-positive cells in psoriatic lesional skin (11).
Infection is believed to be a key risk factor for
inducing or aggravating the disease (12). In addition, the younger the patient,
the more serious the illness is, and the more prone the patient is
to relapse (13). Provoking
infections have been reported to appear in more than 40% of PS
patients (14). There is increasing
evidence indicating that both bacterial and viral infection could
play important roles in the pathophysiology of PS (15,16). In
addition, as PS has an inherited genetic predisposition,
immunological derangement, AID status and other factors may also
play a crucial role in its pathogenesis. The co-existence of PS
with AID, primary biliary cirrhosis (PBC), primary sclerosing
cholangitis, and autoimmune hepatitis have been reported frequently
(17–19). A total of 13% of patients with PBC
have co-existing PS, which is more than 6 times greater than the
incidence in the general population (18).
PS is a type of immune inflammatory disease that
involves multiple factors, which contributes to the lack of
effective drugs and therapeutic approaches (20). Although there are literature reports
that long-term application of an immunosuppressant after liver
transplantation (LT) is helpful in the treatment of PS and can
restrain disease progress and relapse to a certain extent, there
are still patients that undergo PS recurrence, progression, and
delayed healing after LT, which seriously affect the liver graft's
and patient's survival. The evidence associated with the treatment
for co-existing PS after LT is scarce and mostly consists of case
reports (21,22). Furthermore, if the primary disease is
preoperatively differentiated, there is no clear guidance on the
selection of postoperative immunosuppression treatment. Therefore,
the aim of this study was to analyze the therapeutic experience of
LT patients with different pathologies [posthepatic cirrhosis (PCs)
or hepatocellular carcinoma (HCC)] co-occurring with PS, thereby
increasing the evidential support for different treatment
options.
Materials and methods
Basic data
Five LT patients with accompanying PS were selected
for this study from a total of 617 LT patients. All 5 patients were
preoperatively positive for hepatitis B virus (HBV). Their average
age was 51±3 years (male:female, 3:2). In terms of primary disease,
two were diagnosed with PCs and the other three had hepatitis B
cirrhosis complicated with HCC, with no accompanying autoimmune
liver disease (ALD). All 5 patients were diagnosed with PS before
LT, and no de novo PS after LT was reported. This study
approved by the Ethics Committee of Human Experimentation of the
PLA 309th Hospital (Beijing, China). Written informed consent was
obtained in accordance with the Declaration of Helsinki of the
World Medical Association.
Patients' history and physical
findings
All 5 patients had a 6–10 years PS history (mean,
8.10±1.52 years) and a long chronic hepatitis history beyond 10
years (median, 24.80±2.86 years). All were preoperatively confirmed
to be HBV-positive (Table I).
Anti-HBV therapy included lamivudine or entecavir when the patients
exhibited PCs.
 | Table I.General conditions in recipients of
liver transplantation with psoriasis. |
Table I.
General conditions in recipients of
liver transplantation with psoriasis.
|
| Gender | Age | Protopathy | LT time | PS history
(years) | MELD (scores) | AFP (µg/l) | PASI scores | Time to PS relieve
after LT (months) | Time to PS relapse
after LT (months) | Induction IS
project | Initial IS
project | Maintain IS
plan |
|---|
| 1 | M | 52 | HCC, CHB, LC | 2006 | 9 | 11.0 | 1,240 | 43 | 3.5 | – | Mel+OTC | Ster+FK506+MMF | SRL |
| 2 | M | 51 | CHB, LC | 2008 | 8 | 10.5 | – | 46 | 4.8 | 24 | Mel+OTC | Ster+FK506+MMF | FK506+MMF |
| 3 | F | 49 | HCC, CHB, LC | 2008 | 10 | 12.0 | 1,000 | 40 | 3 | – | Mel+OTC | Ster+FK506+MMF | SRL+MMF |
| 4 | F | 55 | CHB, LC | 2010 | 7.5 | 13.0 | – | 41 | 4.5 | 22 | Mel+OTC | Ster+FK506+MMF | FK506 |
| 5 | M | 48 | HCC, CHB, LC | 2009 | 6 | 16.0 | 1,240 | 39 | 2 | – | Mel+OTC | Ster+FK506+MMF | SRL |
The PS lesions were multiple, bright-colored, red
alternating with white, diffuse and flushing. They gradually
emerged from the head and face to the neck and trunk with no
obvious inducement. Other lesion features were furfuraceous scales,
and obsolete scales attached to the surface of lesions, with
different degrees of desquamation, not involving the mucosa and
submucosa. The PS lesions in all patients were mainly distributed
on the trunk, and without regular treatment, there were no cured,
old, or new lesion alternations caused by the long, protracted
course of the disease.
The mean psoriatic area and severity index (PASI)
score was 41.4±6.9 (PASI scores are the most commonly used clinical
measure of PS, with values ranging from 0 to 72) (Table I). Patients with PCs presented liver
function and blood coagulation dysfunction along with ascites and
malnutrition or hypoproteinemia. Moreover, patients with PCs and
HCC also showed high levels of α fetoprotein and hepatic mass
signals in abdominal computed tomography (CT) and magnetic
resonance imaging (MRI) images.
As the confirmation of a PS diagnosis is usually
based on clinical features, including skin rashes, predilection
sites and seasonal onset, most diagnosis is not difficult. The
cultivation of the dander which performed by the methods of
real-time quantitative PCR and immunohistochemistry usually used to
detect the expression of HBV-DNA or other viruses (16,23–25) and
the expression trends of IL-17/23 (7,11) or TNF-α
and INF-γ (6–8). So, when these patients were treated in
our center, they all had a lengthy PS history and had been treated
in hospital, our diagnosis was based on the earlier diagnosis and
treatment of patients by a specialist. Meanwhile, combined with the
patient's history including the PASI score, we confirmed the
diagnosis and excluded other nonspecific infections with the
laboratory (hematological examination, cultivation of the dander
(11,23,24,26,27)
and imaging (CT and MRI scanning) diagnosis methodology.
Operation and therapeutic
approaches
The 5 selected patients were well-prepared for LT.
Methylprednisolone plus basiliximab was used as the preoperative
induction treatment. FK506 plus mycophenolate mofetil (MMF) and
hormone was used as the initial scheme in the early-stage
postoperative period, with hormone withdrawal 1 week after LT
(Table I).
The classification and selection standard of the
therapeutic approach was based on current,
internationally-recognized treatment strategies for patients with
HCC or not, when performed following LT (the HCC patients accepted
sirolimus (SRL)-based immunosuppressive therapy after LT, and the
non-HCC patients were treated with tacrolimus-based
immunosuppressive therapy). Therefore, the 2 patients with HBV and
PCs had been using FK506 with or without a postoperative MMF
program. The others patients, with HBV cirrhosis and HCC, had been
switched to a SRL-based replacement therapy within 1 month after
LT. Moreover, FK506 was gradually reduced and withdrawn at six
months after LT (Table I).
In addition, all 5 patients were given anti-HBV
therapy to prevent hepatitis B recurrence, via intravenous
injection of human hepatitis B immunoglobulin (HBIG) to maintain
effective blood antibody titers.
Index analysis
Basic data including liver function, rejection, and
survival time were analyzed in LT recipients with PS. PASI scores
for the 5 cases were compared before and after LT. Meanwhile, the
adjustment of immunosuppressant was summarized for different
patients with PS.
Statistical analysis
The data were analyzed with SPSS 19.0 software (IBM
SPSS, Armonk, NY, USA). The measurement data are shown as mean ±
standard deviation, and were compared with Student's t-tests; in
addition, count data were compared with Chi-square tests.
Differences with P<0.05 were considered statistically
significant.
Results
Basic information
All patients were regularly followed up
postoperatively, and had survival periods of 8.3±1.5 years. There
were no obvious acute rejections during the follow-up time, nor
were there signs of hepatitis B or tumor recurrence. The general
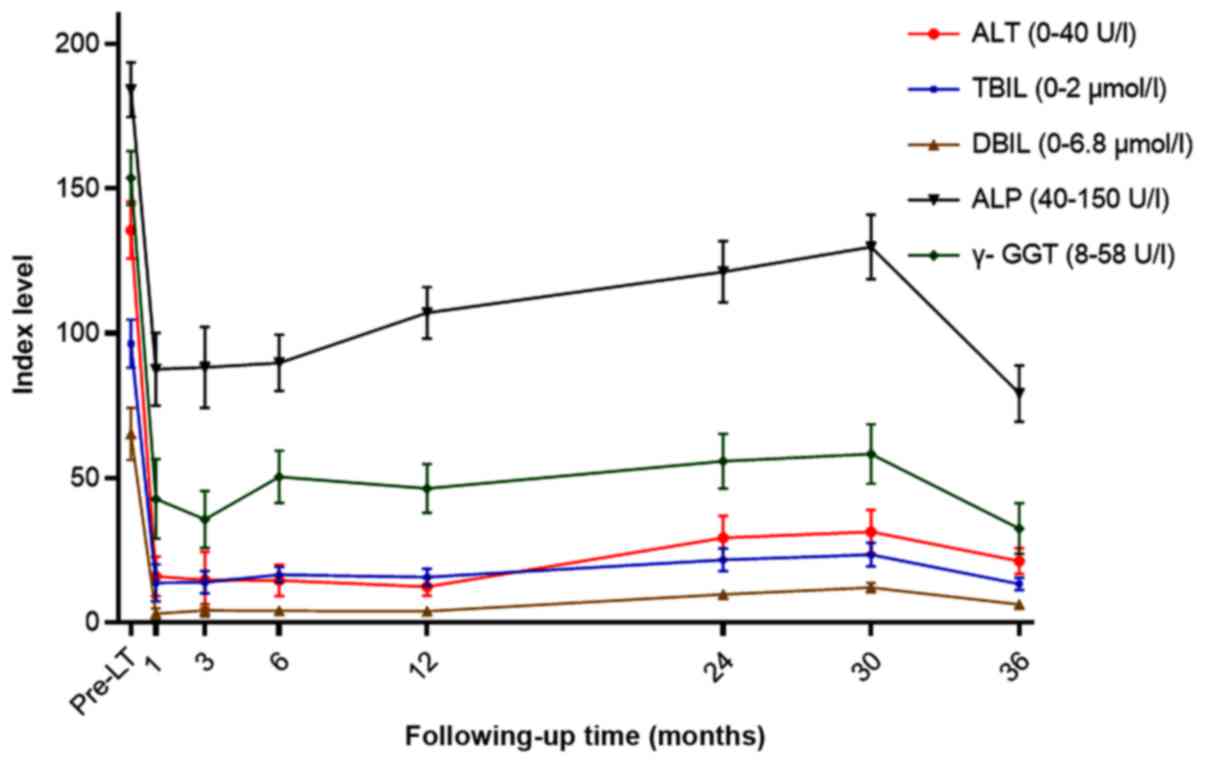
information and pathological findings are shown in Table I. The main liver function indexes,
alanine aminotransferase (ALT), direct bilirubin (DBIL), total
bilirubin (TBIL), γ-gltamyltranspeptidase (γ-GGT), and alkaline
phosphatase (ALP), were recorded for 1 month (Table II; Fig.
1).
 | Table II.The liver function changes of the
recipients of liver transplantation with psoriasis at different
time points. |
Table II.
The liver function changes of the
recipients of liver transplantation with psoriasis at different
time points.
|
| Pre-LT | 1 month | 3 months | 6 months | 12 months | 24 months | 30 months | 36 months |
|---|
| ALT (0–40 U/l) |
135.63±9.78 |
15.97±6.79 |
14.74±9.69 |
14.62±5.35 |
12.41±3.11 |
29.36±7.56 |
31.35±7.59 |
21.32±4.46 |
| TBIL (0–21
µmol/l) |
96.45±8.41 |
13.84±6.48 |
13.91±3.96 |
16.58±2.67 |
15.76±2.97 |
21.66±3.89 |
23.47±4.01 |
13.34±2.17 |
| DBIL (0–6.8
µmol/l) |
65.16±9.13 |
3.18±1.89 |
4.20±2.18 |
4.06±1.49 |
3.98±0.98 |
9.78±1.43 |
12.11±1.62 |
6.31±1.19 |
| ALP (40–150
U/l) |
184.21±9.47 |
87.57±12.66 |
88.18±13.99 |
89.82±9.83 |
107.12±8.96 |
121.23±10.56 |
129.78±11.14 |
79.25±9.76 |
| γ-GGT (8–58
U/l) |
153.64±9.33 |
42.79±13.79 |
35.63±9.92 |
50.40±9.02 |
46.32±8.55 |
55.77±9.43 |
58.26±10.23 |
32.54±8.83 |
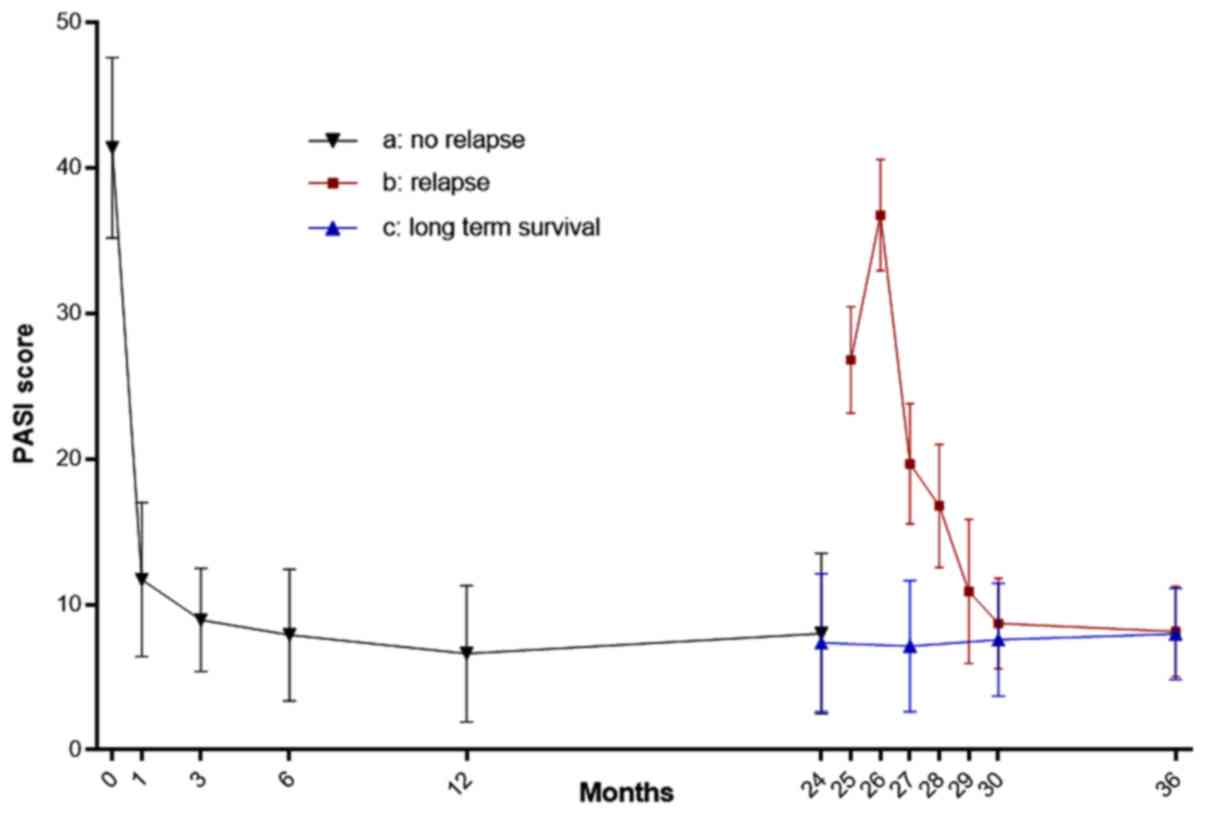
PASI changes
Compared with their preoperative condition, the PASI
scores of patients reduced significantly during the first 6 months
after LT (P<0.05). However, the two HBV and PCs patients
underwent PS recurrence after 2 years, resulting in PASI score
increases. After switching to an SRL-based treatment, the PASI
scores were gradually decreased and maintained at a steady level
from the third year onwards, with no increases to date. The PASI
score changes of the 5patients before and after LT are shown in
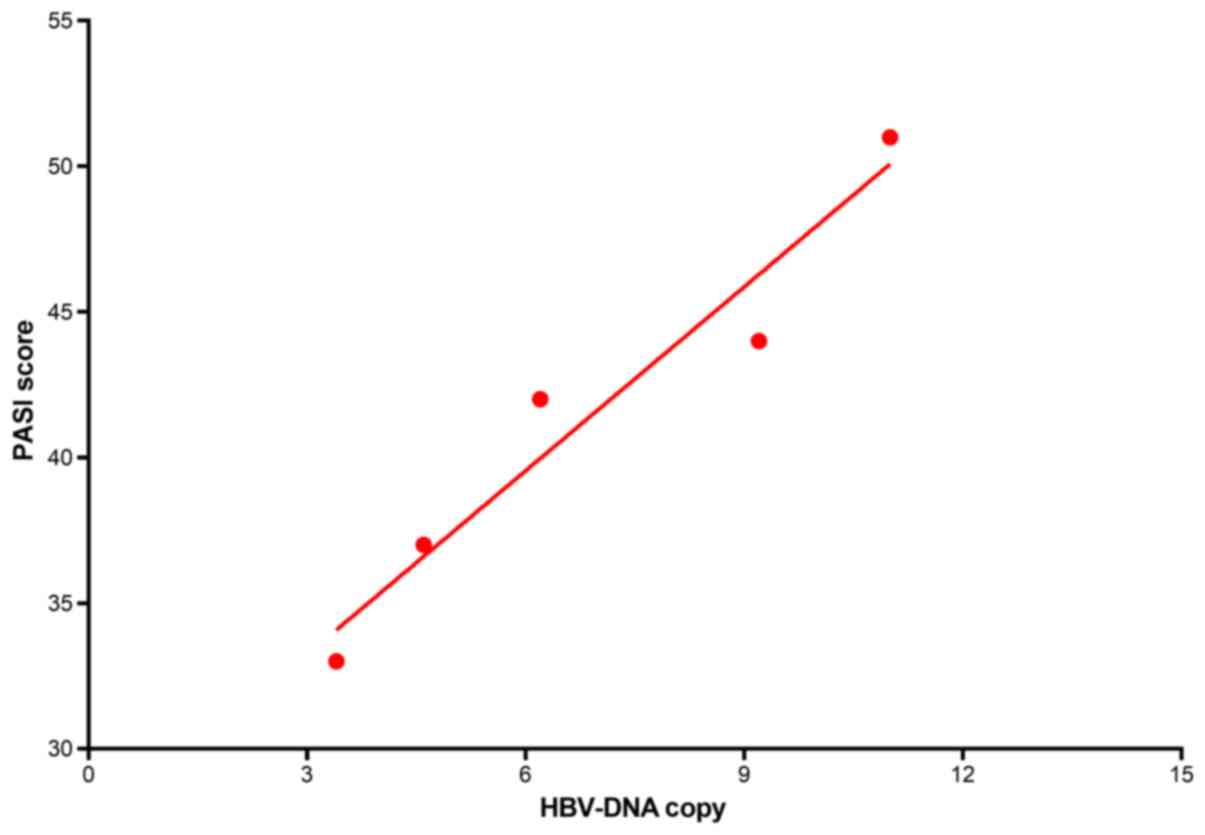
Fig. 2. Furthermore, the preoperative
HBV-DNA copy numbers were (6.9±3.2) ×107, and showed a
positive correlation with the PASI scores (r=0.97, P=0.006)
(Fig.3).
Adjustment of immunosuppressive
regimens
Non-recurrent cases
The three patients without PS recurrence were all
diagnosed with HCC and treated with SRL plus or minus MMF therapy.
The initial treatment regimen was FK506+MMF+steroid for 1 month
(the steroid was stopped within a week), subsequently switched to
SRL with the FK506 decreased gradually and withdrawn at 6 months
postoperation. The concentration of SRL did not go beyond 10 ng/ml,
in accordance with liver and renal functions, as well as the
presence or absence of rejection and drug toxicity reactions. PS
was controlled, and the scope of skin lesions reduced gradually
within 6 months after surgery. The patients were fully stable and
cured within 12 months, with no rejection or disease relapse (PS or
HCC) (Fig. 4).
Recurrent cases
The recurrent cases were the two patients with
hepatitis B and PCs. The initial treatment, employed for the first
6 months, was FK506 plus MMF, with the blood concentration of FK506
kept at 8–12 ng/ml and the dosage maintained at 2.0–3.5 mg per
treatment, twice a day, which was in line with the primary disease
and the patients' weight. The graft survival and liver and renal
functions remained stable at normal levels, while no obvious
adverse drug reactions or rejections occurred. Furthermore, the PS
was gradually controlled, and kept in remission and at stationary
stages. During this period, the PS lesion areas became smaller, the
color became weak, the inflammation reduced, and the scales
gradually decreased. After the first 6 months, the
immunosuppressive treatment was gradually switched to FK506 alone,
with blood concentrations at 5.0–8.0 ng/ml within 12 months, and
remaining at around 5.0 ng/ml after 12 months. This was gradually
reduced, and stayed at 3.5–5.0 ng/ml after 18 months, with regular
detection of liver and kidney function. PS was well-controlled,
with no progression.
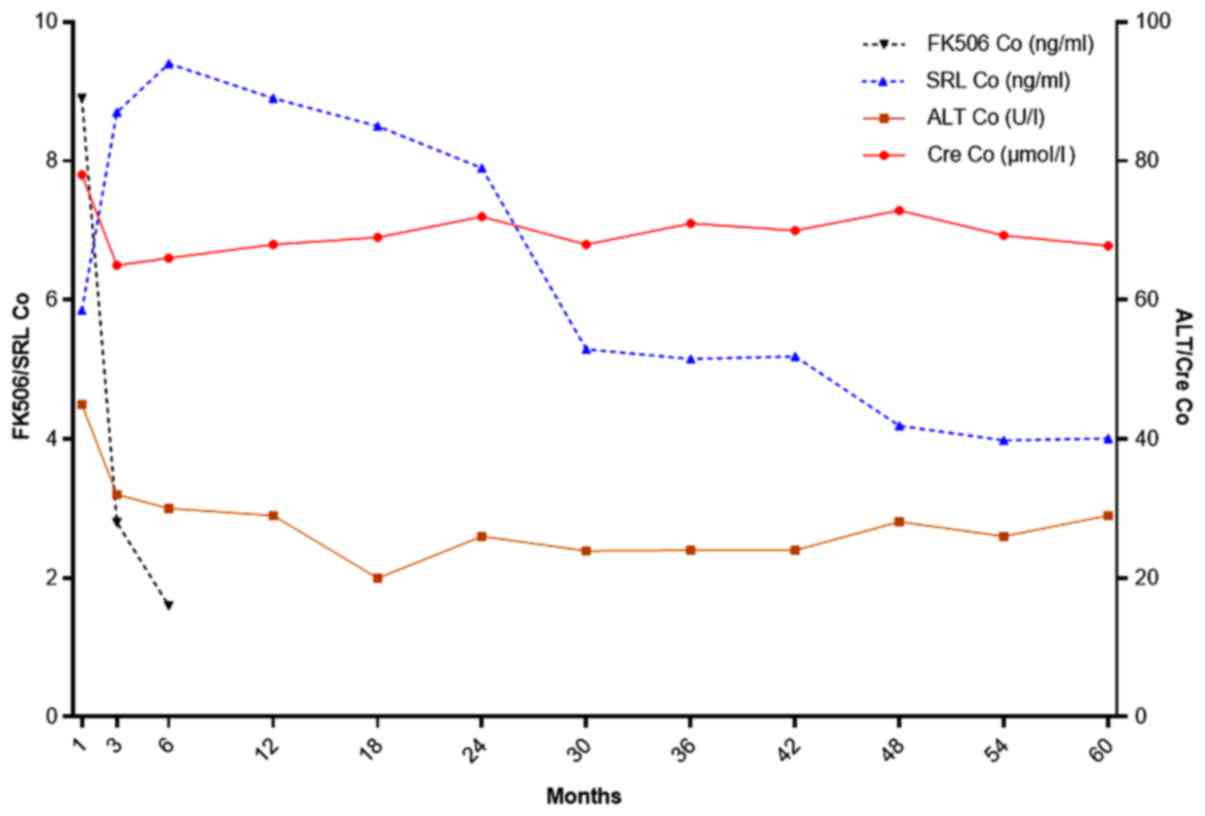
These two patients underwent disease relapse with no
obvious inducement at 22 and 24 months after LT. This may have been
a result of the low FK506 concentration, so the dosage of FK506 was
adjusted and maintained at almost 10 ng/ml. However, the PS was not
controlled well and became exacerbated. Another unexpected aspect
was obvious nephrotoxicity and adverse drug reactions, but with no
rejection. Therefore, based on our experience with HCC co-existing
with PS, therapy was switched to SRL plus or minus MMF.
Interestingly, the patients achieved remission and PS symptoms were
gradually controlled after being treated for 3 months. The drug
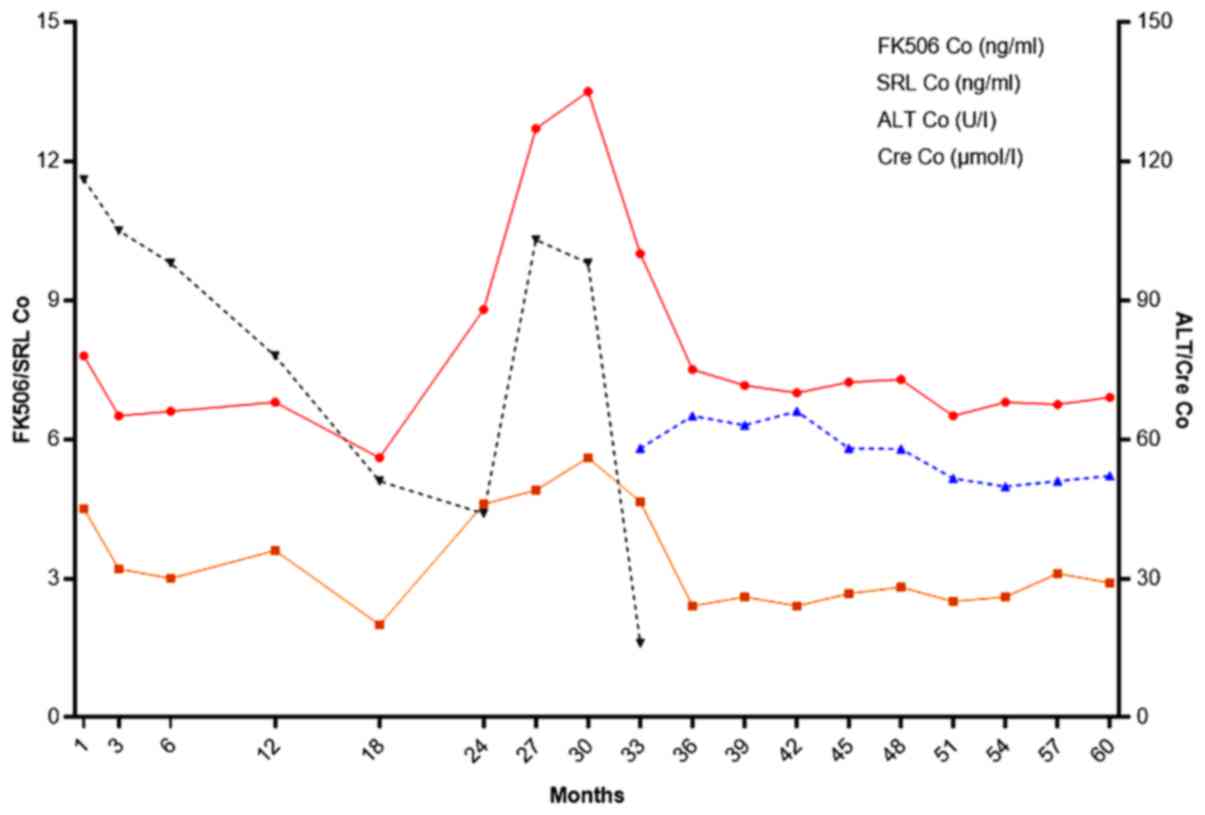
changes and monitoring index of one patient are shown in Fig 5.
Discussion
PS is a refractory dermatological disease with
polygenic inheritance. It may be associated with virulence genes,
environmental factors, infections, and immunologic derangement
(1,3–5). The main
pathological characteristics are excessive keratinocyte
proliferation and parakeratosis, with recurring and repeated
proliferation, desquamation and inflammation in the epidermis
(5,8,13). To the
best of our knowledge, current treatments for PS are still limited
in effect, which makes it difficult to control disease progression.
Guidelines of care for the management of PS and psoriatic arthritis
from the American Academy of Dermatology (AAD) (2) suggest that the main treatment methods
include topical medications, immunosuppression therapy (CsA and
FK506), physical therapy and others, for example biotherapy.
Traditional Chinese medicine is also used in China. The AAD
Guidelines (2) also indicate that the
purpose of therapy is to control the advance of PS, delay
progression to whole body involvement, alleviate symptoms such as
erythema, desquamation and local patch thickening, stabilize
illness, avoid recurrence, avoid adverse effects, and improve
patients' quality of life. Therefore, exploring effective
treatments for PS has become the subject of intensive research and
remains a difficult problem in immunology.
LT may cause patients to acquire a distinct and
unique immune status, with a need for lengthy or lifelong
immunosuppressive treatment, and also a requirement for the
combination of at least two immunosuppressive agents (28). However, PS in the general population
is rarely treated with more than one immunosuppressive agent, so LT
patients with co-existing PS may undergo many more infections after
the transplant operation. Another issue is that, if FK506 or CsA
are used after LT, especially with long-term survival, the large
dosages required for PS treatment often go beyond the maximum
needed for the prevention of rejection, and may lead to drug
toxicity reactions (infection and de novo cancer) and poor
treatment effects. It has been reported that the immunosuppressive
therapy for PS vulgaris mainly focuses on FK506 (29–32), but
the evidence for co-existing PS after LT is relatively scarce
(33–35). The limited literature describing FK506
treatment supplies some evidence, and shows that the long-term
application of FK506 may lead to disease progression and repetition
(33,35). Therefore, combinational or single
application immunosuppressive agents for these patients should be
considered, taking into account their therapeutic effects,
including PS control and rejection prevention, and their side
effects.
Case reports from Hoover (22) and Collazo et al (21) indicate that etanercept can control
post-transplantation PS progress effectively in patients with or
without co-existing hepatitis C virus (HCV). The study by
Foroncewicz et al (12)
addressed the problem of PS recurrence when AID is associated with
PS after LT, with a mean follow-up time of 7.80±1.48 years. It
indicated that, although long-term immunosuppressive therapy was
applied after LT, there was still a possibility of PS recurrence.
Foroncewicz et al (12) also
suggested that the therapeutic effects of CsA are better than those
of FK506 for patients with recurrence. Although, ten cases of
recurrent PS were included to compare the therapeutic efficiency of
CsA and tacrolimus after LT, but only two patients exhibited
HBV/HCV infection. So, the safety and efficacy of CsA still need to
be further proved.
Research on the treatment of PCs or HCC co-existing
with PS after LT is rarely reported. Therefore, the emphasis of our
study lay on supplying different drug selections for different
patients and reducing the toxic and side-effects of combinational
drug applications. Two of five PS patients were preoperatively
diagnosed with PCs and chronic hepatitis B. These patients were
treated with FK506 plus a small dosage of MMF, for an extended
period of time. When the FK506 dosage was reduced to prolong
survival time, there was a recurrence of PS. In contrast, when the
FK506 dosage was increased, PS was controlled but the
nephrotoxicity of FK506 increased severely day by day. Instead of
increasing FK506, switching to SRL for the relapsing patients
controlled PS effectively. Three of five PS patients were
preoperatively diagnosed with PCs and HCC. We used an SRL-based
immunosuppressive treatment protocol for the purposes of
prevention, treatment of postoperative tumor recurrence, and
avoiding adverse drug reactions from lengthy application of FK506.
The initial FK506+MMF treatment was switched to SRL 1 month after
LT, and the MMF was reduced or withdrawn in accordance with patient
and graft survival. The three patients underwent remission within 6
months, with a mean period 2.83±0.76 months. During follow-up
within 8.3±1.5 years, no PS recurrence or progress occurred; in
addition, no rejection and nephrotoxicity events emerged. Moreover,
the SRL dosage was gradually stabilized by 2 years after LT.
Overall, our analysis of the treatment of PCs and HCC co-existing
with PS suggested that switching to SRL therapy not only prevented
tumor relapse, but also effectively controlled PS pathogenesis.
Moreover, it has been shown that dysregulation of the mechanistic
target of rapamycin (mTOR) pathway plays a role in PS pathogenesis.
mTOR is significantly increased in lesional skin and non-lesional
skin, both at the gene and protein level (26,27), and
these alterations can be modulated by anti-TNF-α treatment
(27). Therefore, we suggest that the
SRL therapy could be used for ordinary PS patients as well as LT
patients with PS, although its safety and efficacy for ordinary PS
patients still remains to be proven.
Unlike FK506, the therapeutic concentration of SRL
did not rely on drug dosage, but mainly on liver and renal
function, as well as rejection status. Therefore, we suggest that
SRL levels are maintained at or below 10 ng/ml to avoid
interstitial pneumonia, thrombocytopenia and other severe adverse
reactions. For the two patients with co-existing PCs and PS,
because there was no evidence of HCC, we used the recommended
immunosuppressive treatment of FK506 plus or minus MMF. As the
immune tolerance of recipients to grafts following FK506 treatment
displays an obvious time-dependence, the dosage was gradually
decreased as time progressed. This may be the main reason that
these two patients underwent postoperative PS relapse.
We discovered that, despite the fact that an FK506
concentration lower than 5 ng/ml at 1 year postoperation can
benefit patients' survival, this concentration cannot effectively
meet the need for PS control and inhibition to effect a cure and
maintain a stable state. Moreover, the long-term lowering of drug
concentration can lead to PS progression and recurrence. Previous
case reports have indicated that postoperative blood concentrations
of FK506 maintained at more than 10 ng/ml for extended times
contributed to PS therapy; however, the patients were prone to
adverse reactions which they could not tolerate, leading to
withdrawal or reduction, which may lead to further PS progression.
Therefore, considering our experience with previous cases of HCC
with or without PS after LT, we tried SRL therapy for patients who
had accepted FK506 therapy after LT, but without obvious effect,
even after adjusting the dosage when undergoing PS relapse. The
results proved to be satisfactory, the PS of these two patients
were controlled at the resting stage 3 months later, and eventually
reached cure levels. All of the above evidence suggests that
immunosuppressive treatment based on SRL had a good curative effect
on patients with co-exiting PS after LT, whether or not there is
preoperative HCC, and that SRL may be used to replace FK506 for
invalid patients or those repeated recurrence with no more therapy
effect of FK506. For the small samples, when application with SRL
therapy, it needs to be under strict surveillance of the drug
concentration and adverse events.
That viral infections play important roles in PS
pathophysiology in the general population is well recognized
(12,15,16). It
has been reported that human immunodeficiency virus, HCV, and
herpes simplex virus 1 infections may trigger or even induce PS
onset (15,16), and it has also been observed that a
direct relationship exists between PS activity and cytomegalovirus
activation. Nevertheless, there is little research on HBV
involvement in PS pathogenesis. Previous literature indicates that
HBV infection may induce or aggravate PS progress (23–25) and
HBV can be detected in PS skin lesions (24,25). The
patients included in this study were all diagnosed preoperatively
as HBV-positive with lengthy chronic hepatitis B histories.
Furthermore, their preoperative HBV copy numbers were positively
correlated with PS severity. Therefore, HBIG was regularly injected
postoperatively for all cases, to prevent hepatitis B recurrence.
Although there is no evidence-based proof demonstrating a causal
relationship between HBV and PS, the majority of PS patients are
HBV positive. Therefore, anti-hepatitis B therapy may contribute to
PS treatment; however, much remains to be done to explore the
functional mechanism HBV in the occurrence of PS.
As a refractory dermatosis, PS co-exists with AIDs,
especially ALD and scleroderma, so large dosages of
immunosuppressant are often required to maintain effective
treatment. Some experts contend that CsA is superior to FK506.
However, we suggest here that PS therapy should follow a
personalized medicine principle because of the few researches with
rather small samples. There may be a better choice to combine CsA
for selected patients with AID. Selection of an immunosuppressant
should be in accordance with the severity of PS and the types of
comorbidities. Application of an SRL-based immunosuppressant
treatment may be a promising and beneficial approach for PS
patients, regardless of LT status.
In summary, this study shows that SRL therapy can
provide a superior therapeutic effect compared with FK506
treatment, for selected patients with co-existing PS after LT. This
study also demonstrated that SRL therapy, even though given at
lower doses and concentrations, could maintain PS in a cured or
stable state in LT patients. Therefore, we conclude that SRL
therapy may provide a promising new therapeutic approach for PS
that may be superior to tacrolimus treatment. When co-existing HBV
is found preoperatively, regularly injection of HBIG should be used
to prevent HBV from relapsing or aggravating PS. There remains a
need for further clinical studies with large samples to confirm the
safety and efficacy of SRL therapy for co-existing PS after LT.
Moreover, any application of SRL therapy for ordinary PS patients
should be under strict surveillance, as further study is also
needed to test and verify its benefit for these patients.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (nos. 81502376 and 81270509).
Glossary
Abbreviations
Abbreviations:
|
AAD
|
American Academy of Dermatology
|
|
AID
|
autoimmune disease
|
|
CsA
|
cyclosporine
|
|
CT
|
computed tomography
|
|
FK506
|
tacrolimus
|
|
HBIG
|
human hepatitis B immunoglobulin
|
|
HBV
|
hepatitis B virus
|
|
HCC
|
hepatocellular carcinoma
|
|
HCV
|
hepatitis C virus
|
|
IFN-α
|
interferon α
|
|
IL
|
interleukin
|
|
LT
|
liver transplantation
|
|
MMF
|
mycophenolate mofetil
|
|
MRI
|
magnetic resonance imaging
|
|
mTOR
|
mechanistic target of rapamycin
|
|
PASI
|
psoriatic area and severity index
|
|
PBC
|
primary biliary cirrhosis
|
|
PCs
|
posthepatic cirrhosis
|
|
PS
|
psoriasis
|
|
SRL
|
sirolimus
|
|
TNF-α
|
tumor necrosis factor α
|
|
ALD
|
autoimmune liver disease
|
|
ALT
|
alanine aminotransferase
|
|
DBIL
|
direct bilirubin
|
|
TBIL
|
total bilirubin
|
|
γ-GGT
|
γ-gltamyltranspeptidase
|
|
ALP
|
alkaline phosphatase
|
|
AFP
|
alpha fetoprotein
|
|
IS
|
immunosuppressant.
|
References
|
1
|
Lowes MA, Suárez-Fariñas M and Krueger JG:
Immunology of psoriasis. Annu Rev Immunol. 32:227–255. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Menter A, Korman NJ, Elmets CA, Feldman
SR, Gelfand JM, Gordon KB, Gottlieb A, Koo JY, Lebwohl M, Lim HW,
et al: Guidelines of care for the management of psoriasis and
psoriatic arthritis. Section 3. Guidelines of care for the
management and treatment of psoriasis with topical therapies. J Am
Acad Dermatol. 60:643–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schommer NN and Gallo RL: Structure and
function of the human skin microbiome. Trends Microbiol.
21:660–668. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diani M, Altomare G and Reali E: T cell
responses in psoriasis and psoriatic arthritis. Autoimmun Rev.
14:286–292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madankumar R, Teperman LW and Stein JA:
Use of etanercept for psoriasis in a liver transplant recipient.
JAAD Case Rep. 1:S36–S37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Diani M, Altomare G and Reali E: T Helper
Cell Subsets in Clinical Manifestations of Psoriasis. J Immunol
Res. 2016:76920242016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lowes MA, Russell CB, Martin DA, Towne JE
and Krueger JG: The IL-23/T17 pathogenic axis in psoriasis is
amplified by keratinocyte responses. Trends Immunol. 34:174–181.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kolios AG, Yawalkar N, Anliker M, Boehncke
WH, Borradori L, Conrad C, Gilliet M, Häusermann P, Itin P,
Laffitte E, et al: Swiss S1 guidelines on the systemic treatment of
psoriasis vulgaris. Dermatology. 232:385–406. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quesada JR and Gutterman JU: Psoriasis and
alpha-interferon. Lancet. 1:1466–1468. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baker BS, Griffiths CE, Lambert S, Powles
AV, Leonard JN, Valdimarsson H and Fry L: The effects of
cyclosporine A on T lymphocyte and dendritic cell subpopulations in
psoriasis. Transplant Proc. 20 3 Suppl 4:S72–S77. 1988.
|
|
11
|
Balato A, Schiattarella M, Di Caprio R,
Lembo S, Mattii M, Balato N and Ayala F: Effects of adalimumab
therapy in adult subjects with moderate-to-severe psoriasis on Th17
pathway. J Eur Acad Dermatol Venereol. 28:1016–1124. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foroncewicz B, Mucha K, Lerut J, Majewski
S, Krawczyk M and Pączek L: Cyclosporine is superior to tacrolimus
in liver transplant recipients with recurrent psoriasis. Ann
Transplant. 19:427–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang ZS, Lin NN, Li L and Li Y: The effect
of TNF inhibitors on cardiovascular events in psoriasis and
psoriatic arthritis: An updated meta-analysis. Clin Rev Allergy
Immunol. 51:240–247.. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krueger GG and Duvic M: Epidemiology of
psoriasis: Clinical issues. J Invest Dermatol. 102:14S–18S. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asadullah K, Prösch S, Audring H,
Büttnerova I, Volk HD, Sterry W and Döcke WD: A high prevalence of
cytomegalovirus antigenaemia in patients with moderate to severe
chronic plaque psoriasis: An association with systemic tumour
necrosis factor alpha overexpression. Br J Dermatol. 141:94–102.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mehraein Y, Lennerz C, Ehlhardt S, Zang KD
and Madry H: Replicative multivirus infection with cytomegalovirus,
herpes simplex virus 1, and parvovirus B19, and latent Epstein-Barr
virus infection in the synovial tissue of a psoriatic arthritis
patient. J Clin Virol. 31:25–31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Quintin E, Scoazec JY, Marotte H and
Miossec P: Rare incidence of methotrexate-specific lesions in liver
biopsy of patients with arthritis and elevated liver enzymes.
Arthritis Res Ther. 12:R1432010. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Howel D, Fischbacher CM, Bhopal RS, Gray
J, Metcalf JV and James OF: An exploratory population based
case-control study of primary biliary cirrhosis. Hepatology.
31:1055–1060. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prince MI, Ducker SI and James OF:
Case-control studies of risk factors for primary biliary cirrhosis
in two United Kingdom populations. Gut. 59:508–512. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reich K, Burden AD, Eaton JN and Hawkins
NS: Efficacy of biologics in the treatment of moderate to severe
psoriasis: A network meta-analysis of randomized controlled trials.
Br J Dermatol. 166:179–188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Collazo MH, González JR and Torres EA:
Etanercept therapy for psoriasis in a patient with concomitant
hepatitis C and liver transplant. P R Health Sci J. 27:346–347.
2008.PubMed/NCBI
|
|
22
|
Hoover WD: Etanercept therapy for severe
plaque psoriasis in a patient who underwent a liver transplant.
Cutis. 80:211–214. 2007.PubMed/NCBI
|
|
23
|
Steglich RB, Meneghello LP, Carvalho AV,
Cheinquer H, Muller FM and Reginatto F: The use of ustekinumab in a
patient with severe psoriasis and positive HBV serology. An Bras
Dermatol. 89:652–654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho YT, Chen CH, Chiu HY and Tsai TF: Use
of anti-tumor necrosis factor-α therapy in hepatitis B virus
carriers with psoriasis or psoriatic arthritis: a case series in
Taiwan. Dermatol. 39:269–273. 2012. View Article : Google Scholar
|
|
25
|
Cassano N, Mastrandrea V, Principi M,
Loconsole F, De Tullio N, Di Leo A and Vena GA: Anti-tumor necrosis
factor treatment in occult hepatitis B virus infection: a
retrospective analysis of 62 patients with psoriatic disease. J
Biol Regul Homeost Agents. 25:285–289. 2011.PubMed/NCBI
|
|
26
|
Balato A, Caprio RD, Lembo S, Mattii M,
Megna M, Schiattarella M, Tarantino G, Balato N, Ayala F and
Monfrecola G: Mammalian Target of Rapamycin in Inflammatory Skin
Conditions. European Journal of Inflammation. 12:341–350. 2014.
View Article : Google Scholar
|
|
27
|
Balato A, Lembo S, Ayala F, Balato N,
Caiazzo G, Raimondo A, Di Caprio R and Monfrecola G: Mechanistic
target of rapamycin complex 1 is involved in psoriasis and
regulated by anti-TNF-α treatment. Exp Dermatol. 26(4): 325–327.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geissler EK, Schnitzbauer AA, Zülke C,
Lamby PE, Proneth A, Duvoux C, Burra P, Jauch KW, Rentsch M and
Ganten TM: Sirolimus use in liver transplant recipients with
hepatocellular carcinoma: A randomized, multicenter, open-label
phase 3 trial. Transplantation. 100:116–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lythgoe M and Abraham S: Tacrolimus: an
effective treatment in refractory psoriatic arthritis following
biologic failure. Clin Exp Rheumatol. 34:S12–S13. 2016.PubMed/NCBI
|
|
30
|
Wei KC and Lai PC: Combination of
everolimus and tacrolimus: a potentially effective regimen for
recalcitrant psoriasis. Dermatol Ther. 28:25–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tirado-Sánchez A and Ponce-Olivera RM:
Preliminary study of the efficacy and tolerability of combination
therapy with calcipotriene ointment 0.005% and tacrolimus ointment
0.1% in the treatment of stable plaque psoriasis. Cutis.
90:140–144. 2012.PubMed/NCBI
|
|
32
|
Laino L and Di Carlo A: Palmoplantar
pustular psoriasis: clinical and video thermographic evaluation
before and after topical tacrolimus treatment. Arch Dermatol.
147:7602011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Foroncewicz B, Mucha K, Paczek L,
Oldakowska-Jedynak U, Górnicka B, Zieniewicz K, Nyckowski P and
Krawczyk M: Anti-CD25 and tacrolimus therapy may not prevent early
primary biliary cirrhosis recurrence after liver transplantation:
two case reports. Transplant Proc. 35:pp. 2310–2312. 2003,
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gilbert SC, Klintmalm G, Menter A and
Silverman A: Methotrexate-induced cirrhosis requiring liver
transplantation in three patients with psoriasis. a word of caution
in light of the expanding use of this ‘steroid-sparing’ agent. Arch
Intern Med. 150:889–891. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abu-Elmagd K, Van Thiel D, Jegasothy BV,
Ackerman CD, Todo S, Fung JJ, Thomson AW and Starzl TE: FK506: a
new therapeutic agent for severe recalcitrant psoriasis. Transplant
Proc. 23:3322–3324. 1991.PubMed/NCBI
|