Introduction
Colorectal cancer (CRC) is one of the most common
gastrointestinal malignancies worldwide (1,2). In
previous years, with the improvement of living conditions,
increases in lifespan and alterations to environmental pollution
factors, the morbidity and mortality of colorectal cancer has
increased significantly, alongside a decrease in the age of disease
onset (3–5). The pathogenesis of colorectal cancer is
complex, and therefore diagnosis is often difficult in the earlier
stages of disease (6). At present,
surgery, chemotherapy and radiotherapy are the most effective
treatments, and the primary methods used to treat colorectal cancer
(7). However, the success rate of
this therapy is not sufficient, and the rate of CRC mortality is
increasing year by year (8,9), which indicates that innovative
strategies are required to control this deadly disease.
A number of previous studies have reported the
potential anti-tumor activities of various herbal medicines in
various experimental models of cancer (10–12).
Clinical reports have also demonstrated the beneficial effects of
herbal medicines in patients with tumors (13,14).
Therefore, it is necessary to identify novel drugs for the
prevention and treatment of tumors.
Oxysophoridine (OSR) is a major active alkaloid
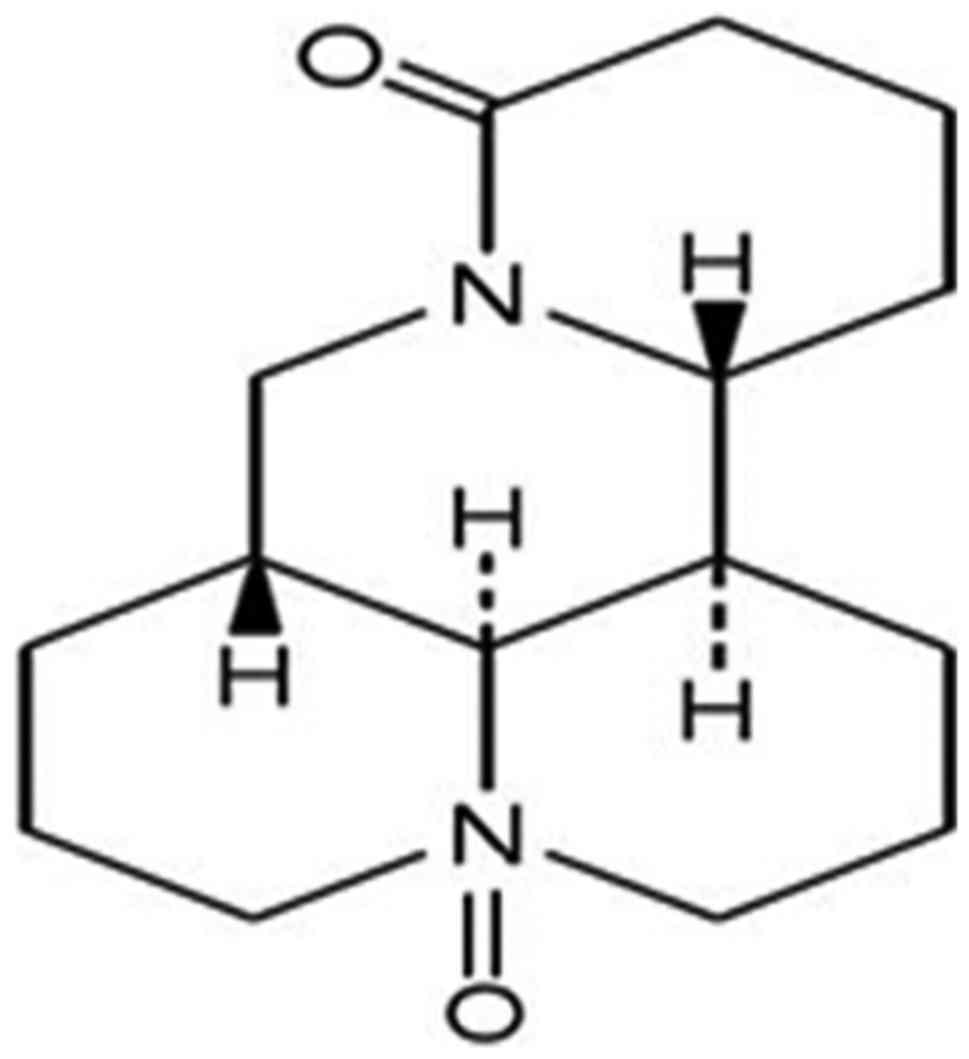
extracted from Sophoraalopecuroides L. (15). The chemical structure of OSR comprises
two piperidine rings (Fig. 1), and it
belongs to the family of quinolizidine alkaloids. OSR has been
identified to possess several pharmacological activities such as
anti-oxidative and anti-inflammatory effects, suppression of the
growth of hepatocellular carcinoma and analgesic and central
inhibitory effects (16–20). However, the anti-tumor potential of
OSR in CRC has not been characterized. Therefore, the present study
aimed to investigate the anti-tumor effects of OSR in CRC and to
investigate the induction of the apoptotic effects of OSR in the
CRC HCT116 cell line and mouse CT26 tumor model. The findings of
the present study suggest that OSR mediates its anti-colorectal
cancer activity via the regulation of the B-cell lymphoma 2
(Bcl-2)/Bcl-2-associated X protein (Bax)/caspase-3 signaling
pathway to induce apoptosis in vitro and in vivo.
Materials and methods
Cell culture and experimental
reagents
Colorectal cancer HCT116 and CT26 cell lines were
purchased from Nanjing Keygen Biotech Co., Ltd. (Nanjing, China).
Cells were maintained in Dulbecco's modified Eagle's medium
(DMEM)/high glucose medium (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 12% heat-inactivated
fetal bovine serum (Minhai Biological Engineering Co., Ltd.,
Lanzhou, China), and 1% penicillin-streptomycin (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), and cultured at 37°C in a
humidified atmosphere of 5% CO2. When the concentration
of cells reached 1×105 cells/ml for subculture the
treatments were performed and all experiments were performed with
cells in the logarithmic phase of growth. OSR was extracted from
Sophoraalopecuroides L. (purity ≥98%; Chengdu Herbpurify
Co., Ltd., Chengdu, China) and dissolved in normal saline (NS) at
an initial concentration of 200 mg/ml and stored at −20°C.
Fluorouracil (5-Fu) was obtained from Shanghai Xudong Haipu
Pharmaceutical Co., Ltd., Shanghai, China. Primary antibodies
against caspase-3, cytochrome c, Bcl-2, Bax and poly (adenosine
5′-diphosphate-ribose) polymerase 1 (PARP-1) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Trypsin and MTT
were obtained from Sigma-Aldrich; Merck KGaA.
Assessment of cell growth inhibition
ratio
To assess cell growth inhibition ratio, HCT116 cells
(5×103) were seeded into 96-well plates (Guangzhou Jet
Bio-Filtration, Co., Ltd., Guangzhou, China). Following overnight
incubation at 37°C, different concentrations of OSR (3, 6, 12, 24,
48, 96 and 192 mg/l) were used to treat the cells, and the cells
were incubated at 37°C for 48 h (6 wells for each concentration).
Briefly, 20 µl MTT solutions (5 mg/ml) was added to each well and
incubated for 4 h at 37°C, then 150 µl dimethyl sulfoxide was added
to each well to dissolve MTT formazan, and the plate was shaken at
room temperature for 15 min. The absorbance was measured at 490 nm
with a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA).
The half maximal inhibitory concentration (IC50) was
calculated using SPSS version 17.0 (SPSS, Inc., Chicago, IL,
USA).
Growth curve assay
To evaluate the cell growth curve, HCT116 cells
(1×103 cells/well) were inoculated into 96-well plates.
After 24 h, the cells were treated with OSR at different
concentrations (100, 50, and 25 mg/l) for 1 to 7 days. The control
group was incubated in a drug-free medium, and the positive group
was exposed to 5-Fu (20 mg/l) (21).
The number of surviving cells from each group was counted daily
using Trypan Blue staining assay, according to the directions
below. A total of 10 µl 0.4% Trypan Blue solution was added to 90
µl of cell-suspension with sufficient mixing, and 10 µl solution
was added on to the cell-count boards. Cells were then counted to
assess the number of viable cells, under a light microscope
(Olympus CX31) at ×10 magnification.
Colony formation assay
For the colony formation assay, 200 cells were
seeded into 6-well plates and exposed to various concentrations of
OSR (100, 50 and 25 mg/l) for 14 days at 37°C in 5% CO2
atmosphere with a relative humidity of 95%. The colonies were fixed
in pure methanol solution at room temperature for 15 min and then
stained with Giemsa (3%) at room temperature for 30 min and washed
3 times with PBS, 5 min/time. Images of the colonies were captured
and counted under a light microscope, and the colony formation
inhibitory rate was calculated as follows: (1-mean number of
colonies in the OSR group/mean number of colonies in the negative
control group) ×100.
Hoechst 33258 staining assay
The HCT116 cells were seeded at a density of
5×103 cells/ml into a 24-well plate. After 24 h, the
cells were treated with OSR at various concentrations (100, 50, and
25 mg/l) for 48 h. The cells were washed with PBS three times,
fixed with 4% neutral paraformaldehyde at room temperature for 30
min, washed with PBS three times and stained with 2 mg/l Hoechst
for 30 min at room temperature. The chromatin structure of the
cells was observed by fluorescence microscopy at ×200
magnification, and the apoptosis rate was calculated as follows:
Apoptosis rate (%) = apoptosis cell number/(normal cell number +
apoptosis cell number) ×100.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from treated cells or tissues
with TRIzol® reagent (Bio Basic, Inc., Markham, ON,
Canada), according to the manufacturer's protocol. Total RNA was
reverse transcribed with the RevertAid™ First Strand cDNA Synthesis
kit (Fermentas; Thermo Fisher Scientific, Inc.) according to
manufacturer's protocol. A total of 2 µl RNA samples were diluted
by DEPC water, and the absorbance value (A) of RNA solution was
detected by UV spectrophotometer at 260 and 280 nm respectively.
The purity of RNA samples was determined according to the ratio of
A260 nm/A280 nm. A ratio between 1.8–2.0, indicates that the purity
of the RNA samples are high, whereas <1.8 demonstrates that the
samples are contaminated by protein, and >2.0 demonstrated that
the samples have RNA degradation. Primer sequences of all the genes
are listed in Table I. A total
reaction volume of 25 µl was used, composed of 2 µl cDNA, and 12.5
µl Taq Master mix (Beyotime Institute of Biotechnology, Haimen,
China) and 8.5 µl nuclease-free water. The reaction conditions were
as follows: 30 sec at 95°C for denaturation (1 cycle), annealing at
58°C for 30 sec and extension at 72°C for 30 sec, for a total of 35
cycles. The PCR products (10 µl) were visualized using
electrophoresis on 2.0% agarose gel. The expression levels of the
target mRNAs were normalized to the reference gene β-actin
(22).
 | Table I.Primer sequences and product
length. |
Table I.
Primer sequences and product
length.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | Product length
(bp) |
|---|
|
Caspase-3 |
TGGGTGCTATTGTGAGGCGG |
GCACACCCACCGAAAACCAG | 168 |
| Cytochrome
c |
CGTTGTGCCAGCGACTAAAAA |
GATTTGGCCCAGTCTTGTGC | 129 |
| Bcl-2 |
TGAACTGGGGGAGGATTGTG |
AAATCAAACAGAGGCCGCAT | 211 |
| Bax |
CCCAGAGGCGGGGTTTCA |
GGAAAAAGACCTCTCGGGGG | 207 |
| PARP-1 |
GAAGCCACAGCTAGGCATGA |
CGCCACTTCATCCACTCCAT | 220 |
| β-actin |
GGCACCCAGCACAATGAAGA |
CATCTGCTGGAAGGTGGACA | 106 |
Western blot analysis
The cells were collected and lysed with lysis buffer
(150 mM NaCl, 1.0 mM EDTA, 1% NP-40, 50 Mm Tris-HCl, pH 7.4, 1 mM
phenylmethylsulfonyl fluoride, 1 µg/ml Leupeptin, 1 µg/ml aprotinin
and 1 g/ml pepstatin) by incubating for 30 min at 4°C. The lysates
were centrifuged at 13,500 × g for 15 min at 4°C, and the protein
concentrations were determined using the Bio-Rad Protein Assay kit
(Bio-Rad Laboratories, Inc.). Equal amounts of total proteins (50
µg) were mixed with loading buffer, at 95°C for 5 min, and
separated on 12% SDS-PAGE gels, and then blotted onto
polyvinylidene fluoride membranes. The membranes were blocked with
5% non-fat milk at room temperature for 1 h, and incubated with
specific primary antibodies at room temperature for 2 h. The
primary antibodies used in this study were as follows: PARP-1
antibody (1:400; sc-8007), cytochrome c antibody (1:400;
sc-514435), Bcl-2 antibody (1:400; sc-509), Bax antibody (1:400;
sc-4239), caspase-3 antibody (1:400; sc-136219; all from Santa Cruz
Biotechnology, Inc.). All of these antibodies were rabbit
monoclonal antibodies. After washing with TBST (TBS containing
0.05% Tween-20, pH 7.6) three times, the membranes were incubated
with goat anti-rabbit immunoglobulin G conjugated to horseradish
peroxidase (1:1,000; cat. no. Ba1055; Boster Biological Technology,
Pleasanton, CA, USA) for 1 h at room temperature. Following washing
with TBST three times, the proteins signal was detected using an
electrochemiluminescence kit (Beyotime Institute of Biotechnology).
Densitometric analyses of resultant western blots were performed
with ImageJ software (ImageJ version 1.47 public domain software;
National Institutes of Health, Bethesda, MD, USA).
Anti-tumor activities in vivo
Five-week old ICR male mice (weight, 18–22 g) were
obtained from the Experimental Animal Center of Sichuan University
(Chengdu, China). The mice were housed in an air-conditioned room,
which was maintained at 23±2°C, and the relatively humidity of the
house was kept at 55±5% CO2 with a 12:12 h light/dark
cycle, with free access to food and water. The CT26 cells were
homogenized with NS at a ratio of (cell solution, NS=1:4; cell
concentration, ~1×106/ml). The mice were injected with
the cell suspension (0.2 ml/mouse) subcutaneously at the right
front axilla. Once transplanted, the tumor (~50 mg) was palpable.
The mice were randomly divided into different treatment groups,
with 10 mice in each group. The mice in the three OSR groups were
administered daily with OSR at 300, 150 and 75 mg/kg of body weight
via intraperitoneal injection (i.p). The mice in the positive
control group were injected with (30 mg/kg) 5-Fu once a day, and
the mice in the negative control group were administered daily with
an equal volume of NS. From the third day, tumor volume (V) was
measured using calipers on alternate days and calculated using the
standard formula: V (mm3) = AB2/2, where A is
the longest superficial diameter, and B is the smallest superficial
diameter. After 13 days, all mice were sacrificed by dislocation of
cervical vertebra, and the tumor tissues were removed and weighed.
Then, the tumor tissues were used to detect the expression levels
of caspase-3, Bax, Bcl-2, cytochrome c and PARP-1 by RT-PCR and
western blotting. The animal experiments were approved by the
Animal Ethics Committee of the Luohe Medical College (Luohe,
China).
Statistical analysis
The data are presented as the mean ± standard
deviation. Data were analyzed by one-way analysis of variance
followed by Duncan's post-hoc test using the SPSS software package
version 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
OSR inhibits the proliferation of
human colorectal HCT116 cancer cells
To detect the growth inhibition effects of OSR in
HCT116 cells, cell growth inhibition rate, growth curve and
colony-forming assays were performed. The HCT116 cells were treated
with serial concentrations of OSR (3, 6, 12, 24, 48, 96 and 192
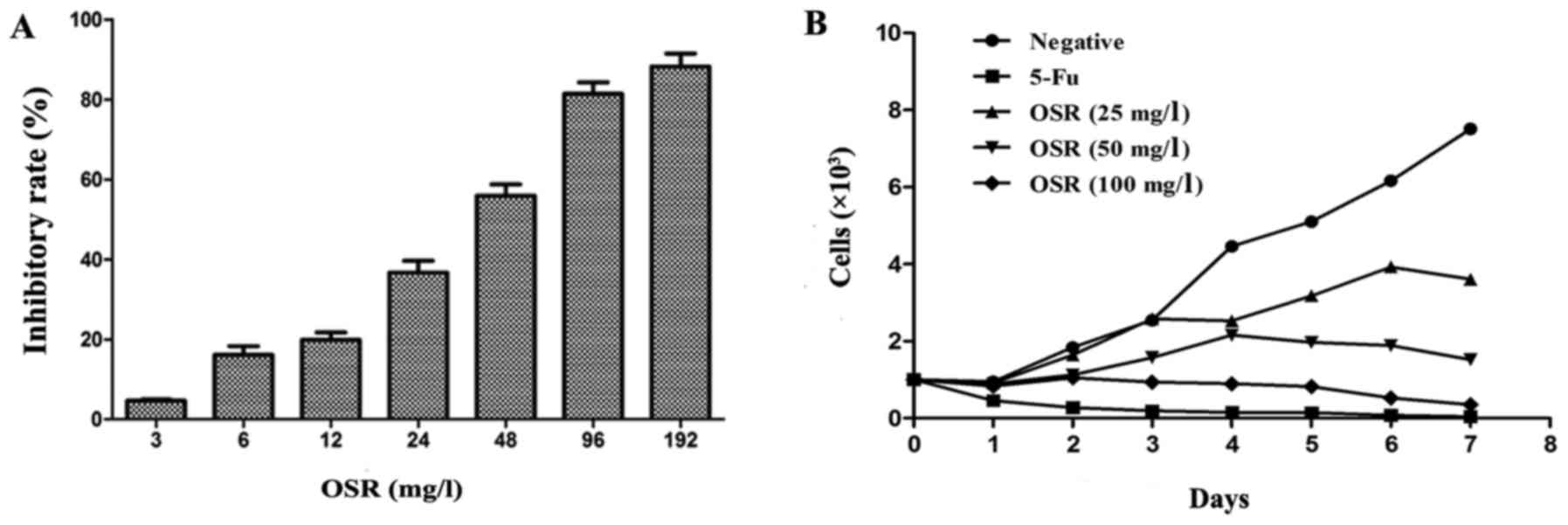
mg/l) for 48 h. The results demonstrate that the cell growth
inhibition rate significantly increased in a dose-dependent manner
(Fig. 2A), and that the
IC50 value was 59.28 mg/l. The cell growth curve assay
indicated that OSR may inhibit HCT116 cell growth in a time and
dose-dependent manner (Fig. 2B).
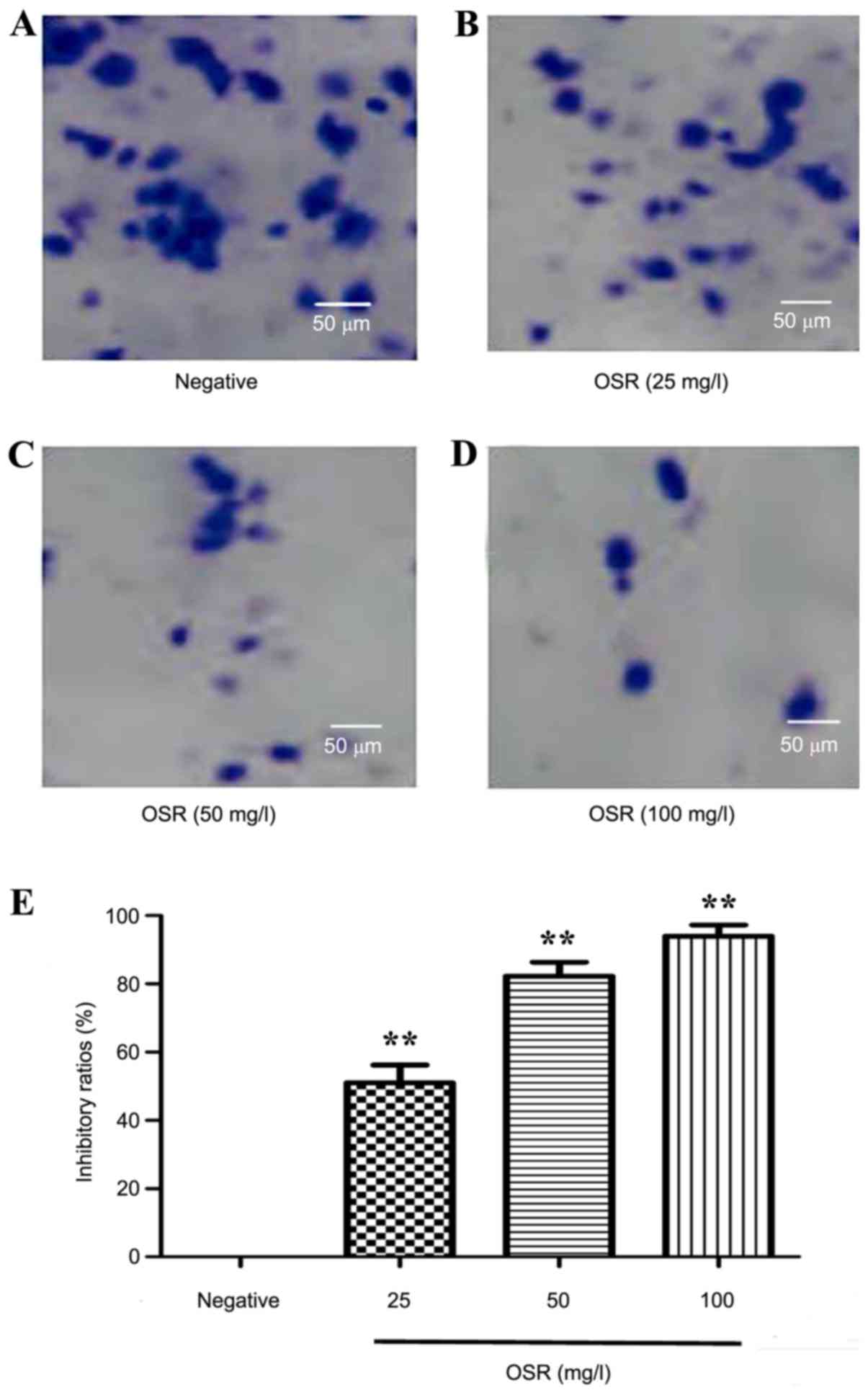
Compared with the negative group, the colony formation assay
demonstrated that OSR may inhibit the proliferation of HCT116 cells
markedly. The colony formation inhibition rate significantly
increased from 51.1% for 25 mg/l OSR to 93.9% for 100 mg/l OSR
(P<0.01; Fig. 3).
OSR promotes apoptosis of HCT116
cells
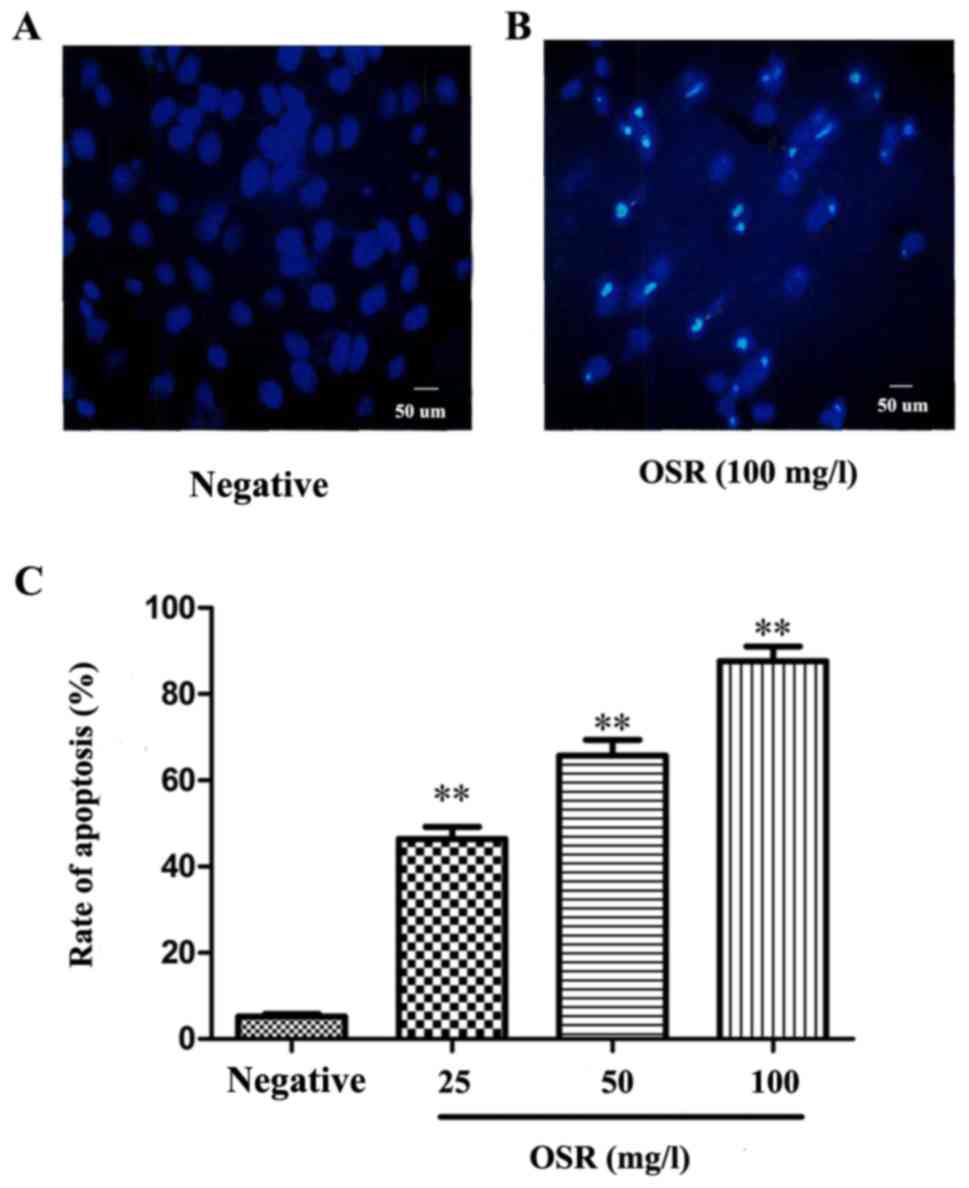
The changes in the levels of apoptosis in
OSR-treated HCT116 cells were detected via Hoechst 33258 nucleus
staining. Compared with the negative group, the cells exhibited a
marked increase in apoptosis following treatment with OSR for 48 h,
exhibiting nuclear condensation, DNA fragmentation and the
formation of apoptotic bodies. The cell apoptosis rate of the
negative control group was 5.21%. Treatment with 25, 50 and 100
mg/l OSR significantly upregulated the rate of apoptosis from
46.37% (25 mg/1) to 87.62% (100 mg/l; P<0.01; Fig. 4).
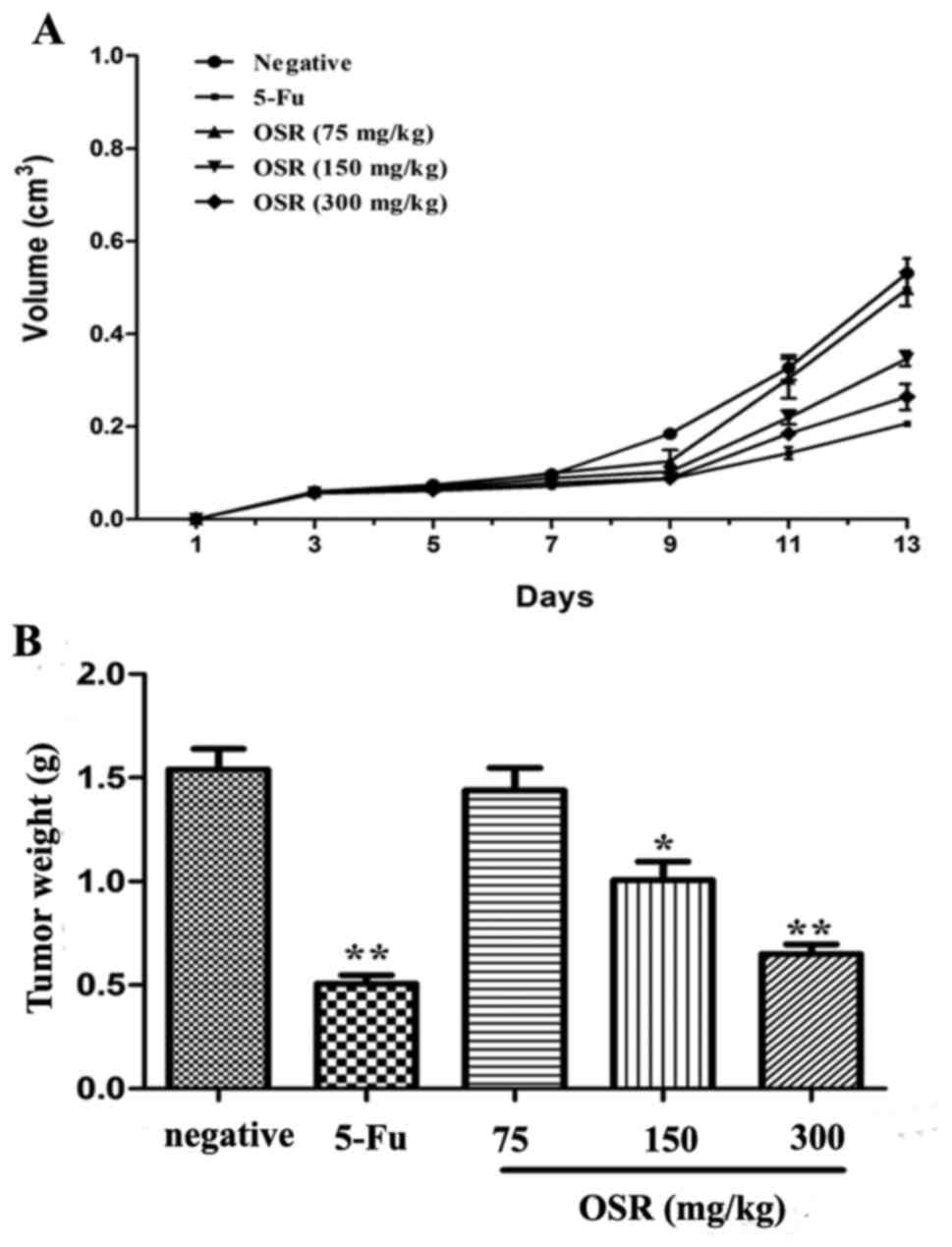
OSR inhibits CRC growth in vivo
To determine whether OSR is able to inhibit tumor
growth in vivo, CT26-xenografts were established in ICR
mice. It was identified that the tumor volume of the negative
control group was higher compared with the tumor volume of the
OSR-treated (150 and 300 mg/kg) mice from day 9 to 13. The growth
curve assay indicated that OSR was able to inhibit tumor tissue
growth significantly (P<0.05, P<0.01; Fig. 5).
Effects of OSR treatmenton caspase-3,
Bax, Bcl-2, cytochrome c and PARP-1expression in HCT116 cells
The levels of caspase-3, Bax, Bcl-2, cytochrome c
and PARP-1expression in HCT116 cells were analyzed by RT-qPCR and
western blotting. The HCT116 cells were treated with different
concentrations of OSR (25, 50 and 100 mg/l). Compared with the
control group, treatment with 50 and 100 mg/l OSR downregulated the
expression of Bcl-2 and PARP-1, whereas the levels of caspase-3,
Bax and cytochrome c were upregulated (Fig. 6).
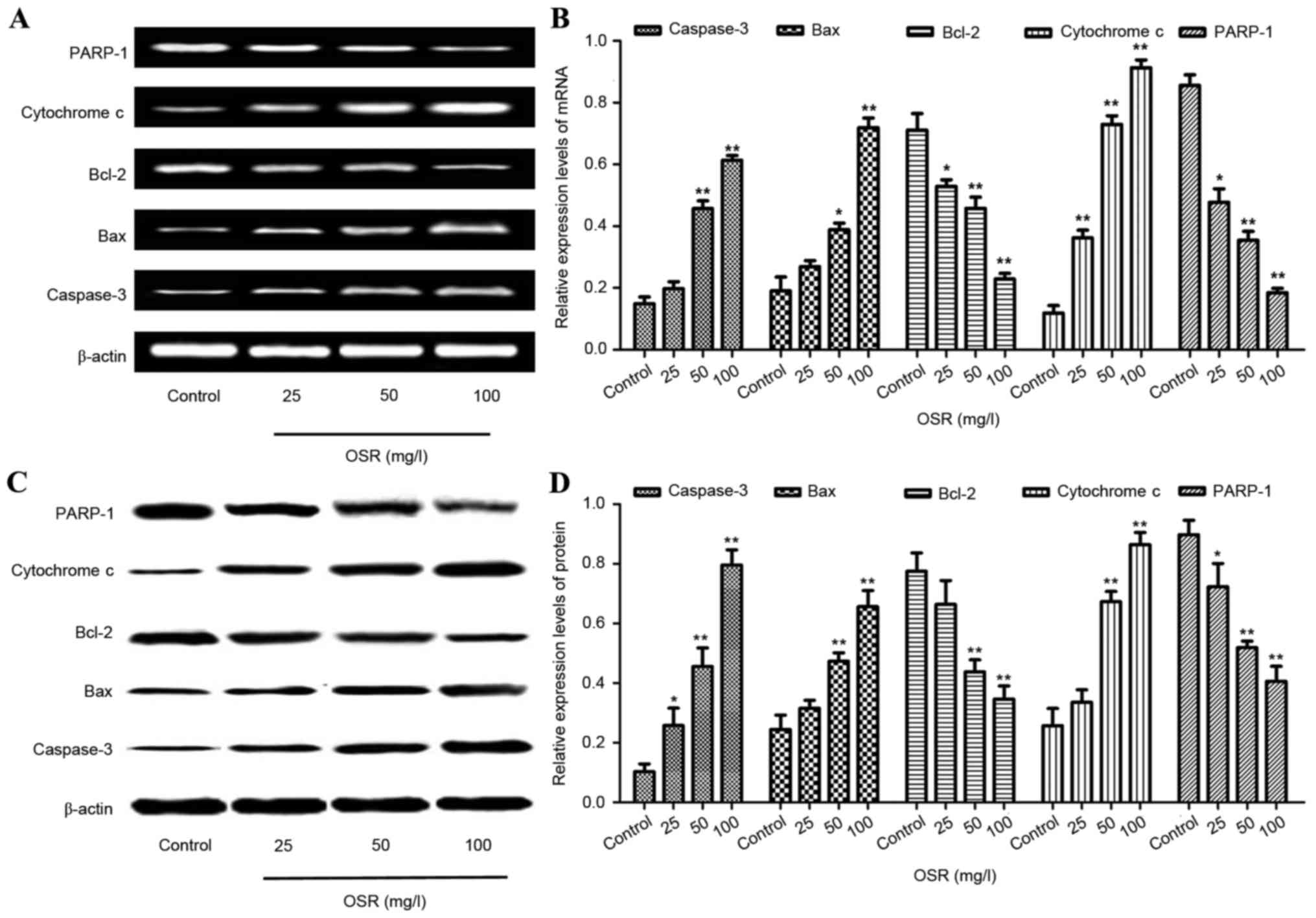 | Figure 6.OSR upregulates the levels of
caspase-3, Bax and cytochrome c expression, and downregulates the
levels of Bcl-2 and PARP-1 in the HCT116 cells. (A and B) The
levels of caspase-3, Bax, cytochrome c, Bcl-2 and PARP-1 mRNA
expression in HCT116 cells. (C and D) The levels of caspase-3, Bax,
cytochrome c, Bcl-2 and PARP-1 protein in HCT116 cells. *P<0.05,
**P<0.01 vs. the control group. OSR, oxysophoridine; Bcl-2,
B-cell lymphoma 2; Bax, Bcl-2 associated X protein; PARP-1, poly
(adenosine 5′-diphosphate-ribose) polymerase 1. |
Effects of OSR treatment on caspase-3,
Bax, Bcl-2, cytochrome c and PARP-1expression in transplanted
CT26CRC tissues
The caspase-3, Bax, Bcl-2, cytochrome c and PARP-1
expression levels in the transplanted CT26CRC tissues were also
detected by RT-PCR and western blot analysis. Compared with the
negative control group, the levels of Bcl-2 and PAPR-1 were
significantly decreased in cells treated with 150 and 300 mg/kg
OSR, and the levels of caspase-3, Bax and cytochrome c were
increased in the OSR groups (150 and 300 mg/kg; Fig. 7).
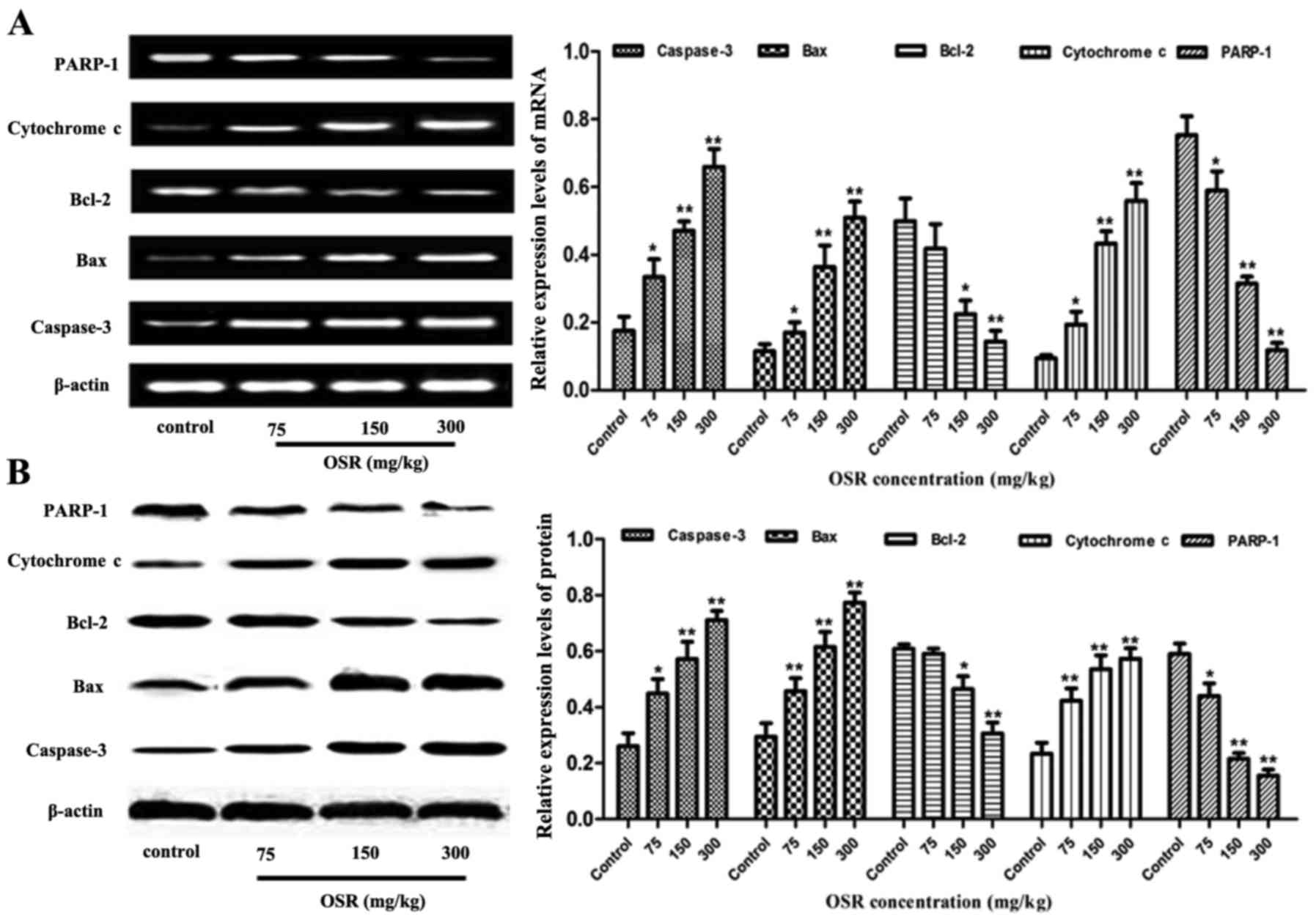 | Figure 7.OSR upregulates the levels of
caspase-3, Bax and cytochrome c expression, and downregulates the
levels of Bcl-2 and PARP-1 expression in the transplanted mouse
CT26 colorectal cancer tissues. (A and B) The levels of caspase-3,
Bax, cytochrome c, Bcl-2 and PARP-1 mRNA expression in the
transplanted mouse CT26 colorectal cancer tissues. (C and D) The
levels of caspase-3, Bax, cytochrome c, Bcl-2 and PARP-1 protein in
transplanted mouse CT26 colorectal cancer tissues.*P<0.05,
**P<0.01 vs. the control group. OSR, oxysophoridine; Bcl-2,
B-cell lymphoma 2; Bax, Bcl-2 associated X protein; PARP-1, poly
(adenosine 5′-diphosphate-ribose) polymerase 1. |
Discussion
Colorectal cancer is one of the most common
digestive tract malignancies worldwide, with a poor prognosis
(23). Apoptosis or programmed cell
death (PCD) is a mechanism of nucleated cell death, which is
regulated by physiological processes, including alterations to the
intracellular and extracellular environments or cell death
signaling activation. Cell membrane shrinkage, nucleus
pyknosisorkaryolysis, DNA fragmentation and formation of apoptotic
bodies are characteristics of apoptotic cells. A number of previous
studies indicated that cellular apoptosis was associated with the
occurrence, development, therapy and prognosis of numerous types of
human tumor (24–26). To the best of our knowledge, apoptin
plays a paramount role in apoptosis, by upregulating pro-apoptotic
proteins (caspase-3, cytochrome c, and Bax) and downregulating
anti-apoptotic proteins (Bcl-2 and PARP-1) (27–34).
Previous studies have indicated that increased Bax expression may
induce apoptosis and that increased Bcl-2 expression may inhibit
apoptosis (35). Caspase-3, belongs
to the family of cysteine proteases, and is a crucial mediator of
apoptosis. Caspases are divided into three broad categories:
Apoptotic initiators (caspase-2, −8, −9 and −10), apoptotic
inhibitors (caspase-3, −6 and −7) and inflammatory mediators
(caspase-1, −4, −5 and −11) (36,37). To
date, multiple previous studies have demonstrated that caspase-3 is
a major effector in the process of apoptosis, and that its
activation marks the irreversible stage of apoptosis (38). The release of cytochrome c in the
mitochondria is one of the inchoate diagnostic characteristics in
nucleated cell apoptosis (39). Bcl-2
inhibits cell apoptosis by reducing the release of mitochondrial
cytochrome c in order to inhibit the activation of caspase-3. Bax
protein, as a crucial component of mitochondrial membrane ion
channels, induces the transfer of cytochrome c across mitochondrial
membranes and the formation of apoptotic bodies; activates
caspase-9 andcaspase-3, which consequently leads to apoptosis
(40). PARP-1 is a type of
post-translational modification protein enzyme, which exists in the
cell nucleus and cytoplasm and is involved in DNA damage repair,
gene transcription regulation, telomerase activity regulation and
protein degradation. PAPR-1 is cleaved into two fragments, p89 and
p24, by caspase-3, which causes a loss of PARP-1 function and leads
to apoptosis (41–44).
In the present study, the inhibitory effects of OSR
on the proliferation of the human CRC HCT116 cells were detected.
Cell growth inhibition, growth curve and colony-forming assays
indicated that treatment with OSR was able to inhibit the growth of
HCT116 cells in a time and dose-dependent manner. Hoechst 33258
staining demonstrated that OSR was able to induce apoptosis
inHCT116 cells. An antitumor effect of OSR on CRC in a mouse model
of transplanted CT26 CRC was also observed. Furthermore, it was
observed that OSR was able to significantly inhibit the growth of
tumors at doses of 150 and 300 mg/kg/day. Concomitantly, in order
to additionally investigate the mechanism of OSR-induced apoptosis,
the expression levels of apoptotic-associated proteins were
detected in vivo and in vitro. The results
demonstrated that treatment with OSR was able to suppress Bcl-2 and
PARP-1 expression, and augment caspase-3, Bax and cytochrome c
expression.
Therefore, the present study concluded that OSR is
able to mediate anti-tumor activity in CRC cells in vivo and
in vitro, via the induction of apoptosis, and its mechanism
may be associated with the Bcl-2/Bax/caspase-3 signaling
pathway.
Acknowledgements
The present study was supported by the Dr Startup
Funds of Luohe Medical College, China (grant no. 2014-DF-003), the
Science Foundation of Health and Family Planning Commission of
Jiangxi Province, China (grant no. 20165176), the Foundation of
Educational Commission of Jiangxi Province, China (grant no.
GJJ150129) and the Youth Natural Science Foundation of Jiangxi
Province, China (grant no. 20161BAB215226).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Young GP, Senore C, Mandel JS, Allison JE,
Atkin WS, Benamouzig R, Bossuyt PM, Silva MD, Guittet L, Halloran
SP, et al: Recommendations for a step-wise comparative approach to
the evaluation of new screening tests for colorectal cancer.
Cancer. 122:826–839. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aykan NF: Red meat and colorectal cancer.
Oncol Rev. 9:2882015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martinez-Useros J and Garcia-Foncillas J:
Obesity and colorectal cancer: Molecular features of adipose
tissue. J Transl Med. 14:212016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cetinkaya E, Dogrul AB and Tirnaksiz MB:
Role of self-expandable stents in management of colorectal cancers.
World J Gastrointest Oncol. 8:113–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palma S, Zwenger AO, Croce MV, Abba MC and
Lacunza E: From molecular biology to clinical trials: Toward
personalized colorectal cancer therapy. Clin Colorectal Cancer.
15:104–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buckley H, Wilson C and Ajithkumar T:
High-dose-rate brachytherapy in the management of operable rectal
cancer: A systematic review. Int J Radiat Oncol Biol Phys.
99:111–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hammond WA, Swaika A and Mody K:
Pharmacologic resistance in colorectal cancer: A review. Ther Adv
Med Oncol. 8:57–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chibaudel B, Tournigand C, Bonnetain F,
Richa H, Benetkiewicz M, André T and de Gramont A: Therapeutic
strategy in unresectable metastatic colorectal cancer: An updated
review. Ther Adv Med Oncol. 7:153–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu X, Li Y, Li X and Aisa HA: Luteolin
induces apoptosis in vitro through suppressing the MAPK and PI3K
signaling pathways in gastric cancer. Oncol Lett. 14:1993–2000.
2017.PubMed/NCBI
|
|
11
|
Li B, Chen D, Li W and Xiao D:
20(S)-Protopanaxadiol saponins inhibit SKOV3 cell migration. Oncol
Lett. 11:1693–1698. 2016.PubMed/NCBI
|
|
12
|
Chang A, Cai Z, Wang Z and Sun S:
Extraction and isolation of alkaloids of Sophora alopecuroides and
their anti-tumor effects in H22 tumor-bearing mice. Afr J Tradit
Complement Altern Med. 11:245–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang CZ, Zhang Z, Anderson S and Yuan CS:
Natural products and chemotherapeutic agents on cancer: Prevention
vs. treatment. Am J Chin Med. 42:1555–1558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park GH, Park JH, Song HM, Eo HJ, Kim MK,
Lee JW, Lee MH, Cho KH, Lee JR, Cho HJ and Jeong JB: Anti-cancer
activity of Ginger (Zingiber officinale) leaf through the
expression of activating transcription factor 3 in human colorectal
cancer cells. BMC Complement Altern Med. 14:4082014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng C, Liu C, Liu Y and Wu F:
Oxysophoridine attenuates the injury caused by acute myocardial
infarction in rats through anti-oxidative, anti-inflammatory and
anti-apoptotic pathways. Mol Med Rep. 11:527–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang YS, Li YX, Zhao P, Wang HB, Zhou R,
Hao YJ, Wang J, Wang SJ, Du J, Ma L, et al: Anti-inflammation
effects of oxysophoridine on cerebral ischemia-reperfusion injury
in mice. Inflammation. 38:2259–2268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang G, Gao J, Jia Y, Yan L, Yu J and
Jiang Y: Oxysophoridine through intrathecal injection induces
antinociception and increases the expression of the GABAAa1
receptor in the spinal cord of mice. Planta Med. 78:874–880. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao XQ, Zhang YH, Long W and Liu PX:
Oxysophoridine suppresses the growth of hepatocellular carcinoma in
mice: In vivo and cDNA microarray studies. Chin J Integr Med.
18:209–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu J, Li Y, Zhao C, Gong X, Liu J, Wang F
and Jiang Y: Effect of oxysophoridine on electric activities and
its power spectrum of reticular formation in rats. Zhongguo Zhong
Yao Za Zhi. 35:1170–1172. 2010.(In Chinese). PubMed/NCBI
|
|
20
|
Zhang HM and Li HQ: Anti-arrhythmic
effects of sophoridine and oxysophoridine. Zhongguo Yao Li Xue Bao.
20:517–520. 1999.PubMed/NCBI
|
|
21
|
Zhang JT, Zhou WL, He C, Liu T, Li CY and
Wang L: 5-Fluorouracil induces apoptosis of colorectal cancer
cells. Genet Mol Res. 15:150173262016.PubMed/NCBI
|
|
22
|
Little EC, Kubic JD, Salgia R, Grippo PJ
and Lang D: Canonical and alternative transcript expression of PAX6
and CXCR4 in pancreatic cancer. Oncol Lett. 13:4027–4034.
2017.PubMed/NCBI
|
|
23
|
Aakif M, Balfe P, Elfaedy O, Awan FN,
Pretorius F, Silvio L, Castinera C and Mustafa H: Study on
colorectal cancer presentation, treatment and follow-up. Int J
Colorectal Dis. 31:1361–1363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bold RJ, Termuhlen PM and McConkey DJ:
Apoptosis, cancer and cancer therapy. Surg Oncol. 6:133–142. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scatena R: Mitochondria and cancer: A
growing role in apoptosis, cancer cell metabolism and
dedifferentiation. Adv Exp Med Biol. 942:287–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elkholi R, Renault TT, Serasinghe MN and
Chipuk JE: Putting the pieces together: How is the mitochondrial
pathway of apoptosis regulated in cancer and chemotherapy? Cancer
Metab. 2:162014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang D, Okamura H, Teramachi J and Haneji
T: Histone demethylase Jmjd3 regulates osteoblast apoptosis through
targeting anti-apoptotic protein Bcl-2 and pro-apoptotic protein
Bim. Biochim Biophys Acta. 1863:650–659. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luna-Vargas MP and Chipuk JE: The deadly
landscape of pro-apoptotic BCL-2 proteins in the outer
mitochondrial membrane. FEBS J. 283:2676–2689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Um HD: Bcl-2 family proteins as regulators
of cancer cell invasion and metastasis: A review focusing on
mitochondrial respiration and reactive oxygen species. Oncotarget.
7:5193–5203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lalier L, Cartron PF, Juin P, Nedelkina S,
Manon S, Bechinger B and Vallette FM: Bax activation and
mitochondrial insertion during apoptosis. Apoptosis. 12:887–896.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horwacik I and Rokita H: Targeting of
tumor-associated gangliosides with antibodies affects signaling
pathways and leads to cell death including apoptosis. Apoptosis.
20:679–688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ishii Y, Nhiayi MK, Tse E, Cheng J,
Massimino M, Durden DL, Vigneri P and Wang JY: Knockout serum
replacement promotes cell survival by preventing BIM from inducing
mitochondrial cytochrome C release. PLoS One. 10:e01405852015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Węsierska-Gądek J, Mauritz M, Mitulovic G
and Cupo M: Differential potential of pharmacological PARP
inhibitors for inhibiting cell proliferation and inducing apoptosis
in human breast cancer cells. J Cell Biochem. 116:2824–2839. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dias MM, Noratto G, Martino HS, et al:
Pro-apoptotic activities of polyphenolics from açai (Euterpe
oleracea Martius) in human SW-480 colon cancer cells. Nutr Cancer.
66:1394–1405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng JH, Follis Viacava A, Kriwacki RW
and Moldoveanu T: Discoveries and controversies in BCL-2
protein-mediated apoptosis. FEBS J. 283:2690–2700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Julien O and Wells JA: Caspases and their
substrates. Cell Death Differ. 24:1380–1389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yuan J, Najafov A and Py BF: Roles of
caspases in necrotic cell death. Cell. 167:1693–1704. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fiandalo MV and Kyprianou N: Caspase
control: Protagonists of cancer cell apoptosis. Exp Oncol.
34:165–175. 2012.PubMed/NCBI
|
|
39
|
Caroppi P, Sinibaldi F, Fiorucci L and
Santucci R: Apoptosis and human diseases: Mitochondrion damage and
lethal role of released cytochrome C as proapoptotic protein. Curr
Med Chem. 16:4058–4065. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao Y, Jing Z, Lv J, Zhang Z, Lin J, Cao
X, Zhao Z, Liu P and Mao W: Berberine activates
caspase-9/cytochrome c-mediated apoptosis to suppress
triple-negative breast cancer cells in vitro and in vivo. Biomed
Pharmacother. 95:18–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koh DW, Dawson TM and Dawson VL: Mediation
of cell death by poly(ADP-ribose) polymerase-1. Pharmacol Res.
52:5–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu SW, Wang H, Poitras MF, Coombs C,
Bowers WJ, Federoff HJ, Poirier GG, Dawson TM and Dawson VL:
Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by
apoptosis-inducing factor. Science. 297:259–263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schon EA and Manfredi G: Neuronal
degeneration and mitochondrial dysfunction. J Clin Invest.
111:303–312. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Voutsadakis IA: Apoptosis and the
pathogenesis of lymphoma. Acta Oncol. 39:151–156. 2000. View Article : Google Scholar : PubMed/NCBI
|