Introduction
Prostate cancer is the second most common malignancy
in males worldwide, characterized by its high mortality and poor
prognosis (1). In spite of early
detection techniques and multidisciplinary therapeutic approaches,
the overall 5-year survival rate remains <30% in China (2). Anti-androgen treatment remains the
first-line therapy for patients with prostate cancer, among whom
the majority will eventually develop highly metastatic
androgen-independent prostate cancer (3). Prostate cancer is a complex and
heterogeneous disease characterized by various molecular signatures
in patients, which provides opportunities to explore targeted
therapies.
Glioma-associated oncogene 1 (Gli1) is highly
expressed in the prostate and serves various functions in prostate
development (4,5). Ectopic activation of the Hedgehog (HH)
signaling pathway has been demonstrated to be involved in the
initiation as well as the progression of prostate malignancy
(3,6).
The HH signaling pathway was first identified in Drosophila
as a central organizer for proper embryonic patterning and
development. Three HH ligands have been identified in vertebrate
organisms: Sonic Hedgehog (SHH), Indian Hedgehog and Desert
Hedgehog, which are all able to initiate signaling by binding and
inactivating the HH receptor Patched 1 (PTCH1) (5). In the canonical HH signaling pathway,
inactivation of PTCH1 releases the seven-pass transmembrane protein
Smoothened (SMO). SMO then transduces the signal to downstream
effectors (Gli proteins) via blocking the inhibitory partner,
suppressor of fused. Activated Gli proteins eventually translocate
into the nucleus and trigger the transcription of downstream target
genes (5,7). In addition to classical HH signal
transduction, the non-canonical HH pathway, in which Gli proteins
are regulated by phosphoinositide 3-kinase (PI3K)/V-Akt murine
thymoma viral oncogene (Akt), mitogen-activated protein kinase
(MAPK)/extracellular-signal-regulated kinase (ERK), nuclear factor
κB (NF-κB) and/or transforming growth factor β (TGFβ) pathways as
well as the key tumor suppressors tumor protein p53 (TP53) and
phosphatase and tensin homolog (PTEN) in a ligand-independent
manner, has been the focus of previous research (8–10).
Although the canonical pathway has been well-investigated, how Gli
proteins are regulated in a SMO-independent manner in prostate
cancer remains largely unknown.
The Akt/mammalian target of rapamycin (mTOR)/p70
ribosomal protein S6 kinase 1 (S6K1) signaling pathway is involved
in numerous aspects of molecular and cellular biology, such as mRNA
translation, ribosome biogenesis, cell proliferation, metabolism,
immunosuppression, development, aging and malignancies (8). As a serine/threonine protein kinase, the
activation of mTOR leads to the phosphorylation of S6K1 and
eukaryotic translation initiation factor 4E-binding protein 1
(4E-BP1) (11). S6K1 is also a
serine/threonine kinase and serves a role in target gene
translation following its phosphorylation by mTOR. More
importantly, tumor necrosis factor α (TNFα) is capable of
activating the mTOR/S6K1 signaling pathway to promote angiogenesis,
which mediates chronic inflammation-induced cancer, including
breast and prostate cancer (8,12).
In order to explore the potential therapeutic
targets for prostate cancer, an initial siRNA screen was performed
(data not shown) and it was identified that Gli1 and Gli2 are
critical in prostate cancer survival rates. Combined with other
published data, it was hypothesized that Gli1 and/or Gli2
contributes to prostate cancer cell proliferation. In the present
study, it was demonstrated that androgen-independent prostate
cancer cell lines are dependent on Gli1 expression for
proliferation and that its activation by mTOR/S6K1 is required for
this function.
Materials and methods
Cell culture
The human prostate cancer cell lines PC3
(CRL-1435™), DU145 (HTB-81™) and LNCaP (CRL-1740™) were purchased
from the American Type Culture Collection (Manassas, VA, USA). The
prostate cancer cell lines were maintained in RPMI-1640 medium
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with
10% fetal bovine serum (FBS; cat. no. 10082147 Thermo Fisher
Scientific, Waltham, MA, USA) and cultured at 37°C in a humidified
atmosphere containing 5% CO2. All cell lines were
confirmed to be Mycoplasma-free using an e-Myco kit (Boca
Scientific Inc., Boca Raton, FL, USA). The inhibitors used were
GANT61 (Tocris Bioscience, Bristol, UK), rapamycin (Sigma-Aldrich;
Merck KGaA) and BEZ235 (Selleck Chemicals, Houston, TX, USA).
Reverse transfection in prostate
cancer cells with small interfering RNA (siRNA) targeting Gli1 or
S6K1
SiRNA screening was performed as follows. The genome
wide siRNA library was purchased from Dharmacon (Lafayette, CO,
USA). The library contained a mixture of 4 individual siRNA oligos
for each gene. PC3 cells were cultured in RPMI-1640 medium
supplemented with 10% FBS at 37°C for three days to reach 60–70%
confluency. A total of 10 pmol of each siRNA pool (5 µl) was
reverse transfected to 95 µl serum-free media in empty 96-well
assay plates. Firstly, 5 µl siRNA was diluted in 25 µl OptiMEM
(cat. no. 31985-062; Thermo Fisher Scientific, Inc.) serum-free
medium and incubated for 5 min at room temperature. Then, 0.13 µl
RNAiMax (cat. no. 13778075; Thermo Fisher Scientific, Inc.) was
diluted in 10 µl OptiMEM medium. Secondly, siRNA was mixed with
Lipofectamine® RNAiMax (cat. no. 13778075; Thermo Fisher
Scientific, Inc.) transfection reagent, incubated for 15 min at
room temperature and transferred to 96-well plates. Thirdly, PC3
cells were harvested by incubation with trypsin for 2–4 min at 37°C
and centrifugation for 5 min at 100 × g and 4°C. A total of ~2,500
cells in 60 µl cell suspension were seeded in each well and
cultured for 96 h at 37°C. CellTiter-Glo Assay kit (Promega
Corporation, Madison, WI, USA) was used to measure the cell
viability. Cells were plated onto a 96-well plate at a density of
2,500 cells/well. After 96 h incubation at 37°C, CellTiter-Glo
reagent was added to the culture medium, and plates were agitated
at room temperature for 10 min. The luminescent signal was
determined using a GloMax absorbance plate reader at a wavelength
of 560 nm. siUBB and siTMEM114 were used as positive and negative
controls, respectively. Each screening was triplicated and repeated
three times. For Z scores, −3 was a cut-off value. The siRNAs
targeting human Gli1 or S6K1 were synthesized by Sigma-Aldrich;
Merck KGaA (siGli1: NM_005269, siS6K1: NM_003161). A scrambled
siRNA (SIC001, Sigma-Aldrich; Merck KGaA) was used as a negative
control. Transient knockdown of Gli1 or S6K1 with these siRNAs in
prostate cancer cells was carried out using Lipofectamine RNAiMax
(Thermo Fisher Scientific, Inc.) together with negative or positive
controls (GAPDH siRNA, NM_002046; Sigma; Merck KGaA), according to
the manufacturer's protocol. The cells were then cultured for 72 h
at 37°C in 5% CO2. Silencing efficiency was determined
using the reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blotting.
RT-qPCR
Total RNA was extracted from cultured cells using
the RNeasy Mini kit (Qiagen, Valencia, CA, USA). cDNA was generated
with oligo-dT primers from 0.5 µg RNA using an iScript cDNA
synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
according to the manufacturer's protocol. TaqMan probes (Thermo
Fisher Scientific, Inc.) of Gli1, Gli2, N-Myc, PTCH1 and CCND1
genes were used to quantitatively analyze mRNA transcript levels
with the 18S ribosomal RNA gene as an internal reference. PCR was
performed using the ABI 7300 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were 95°C for 3 min, 45 cycles at 95°C for 15 sec and
60°C for 45 sec. The primers were Gli1 (Hs00171790_m1), Gli2
(Hs01119974_m1), N-Myc (Hs00190768_m1), PTCH1 (Hs00181117_m1),
CCND1 (Hs00765553_m1), and reference gene GAPDH (Hs002786624_g1).
All primers were purchased from Thermo Fisher Scientific, Inc. The
results were analyzed using SDS Software v1.4.1 (Thermo Fisher
Scientific, Inc.). The comparative Cq method was used to
calculate relative mRNA expression levels (13).
Cell viability assays
Cell viability was examined using the CellTiter-Glo
Assay kit (Promega Corporation), according to the manufacturer's
protocol. Cells were plated onto a 96-well plate at a density of
2,000 cells/well. At 24, 48, 72 and 96 h, CellTiter-Glo reagent was
added to the culture medium, and plates were agitated at room
temperature for 10 min. The half-maximal inhibitory concentration
was the concentration of GANT61 required for 50% inhibition of the
cell viability in the curve. The luminescent signal was determined
using a GloMax absorbance plate reader at a wavelength of 560 nm.
Each individual experiment was performed at least three times
independently.
Liquid colony formation assays
To evaluate anchorage-dependent liquid colony
formation efficiency, 500 cells were suspended in 3 ml RPMI-1640
medium with 10% FBS and plated in a 6-well plate to grow for 2–3
weeks. The cells were fixed and stained with 1 ml 0.05% crystal
violet (Sigma-Aldrich; Merck KGaA) to count the colonies. Each
individual experiment was performed three times independently.
Western blotting
Treated cells were lysed in Protein Extraction
Reagent Type 4 (Sigma-Aldrich; Merck KGaA) with a PhosStop
phosphatase inhibitor and cOmplete protease inhibitor (Roche
Diagnostics, Indianapolis, IN, USA). The protein concentration of
cell lysates was measured using the Bradford reagent (Bio-Rad
Laboratories, Inc.). Equal amounts of total protein (30 µg) were
separated by SDS-PAGE on an 8–10% gel, and then transferred onto
nitrocellulose membranes (Bio-Rad Laboratories, Inc.). The
membranes were blocked in 5% milk followed by incubation with
primary antibodies in Tris-buffered saline with 0.1% Tween-20
solution at 4°C overnight. The following day, the membranes were
incubated with corresponding horseradish peroxidase-conjugated
secondary antibodies: Goat anti-mouse immunoglobulin (Ig)G (cat.
no. ab6789; Abcam, Cambridge, MA, USA; 1:3,000) and goat
anti-rabbit IgG antibodies (cat. no. ab97051; Abcam; 1:3,000) for 1
h at room temperature. Membranes were exposed to LucentBlue X-ray
film (Advansta, Menlo Park, CA, USA) at room temperature for
between 30 sec and 5 min. Antibodies used for western blotting were
as follows: Anti-Gli1 (cat. no. 2643), anti-poly(ADP-ribose)
polymerase (PARP)/cleaved PARP (cat. no. 9546) and
anti-phospho-S6K1 (Thr421/Ser424; cat. no. 9204; Cell Signaling
Technology, Inc., Danvers, MA, USA); anti-S6K1 (cat. no. ab14708;
Abcam); anti-Gli2 (cat. no. ABN506; EMD Millipore, Billerica, MA,
USA); and anti-β-actin (cat. no. sc-4778; Santa Cruz Biotechnology,
Dallas, TX, USA).
Statistical analysis
Student's t-test (two-tailed) was used to examine
the significance of in vitro cell viability and quantitative
real-time PCR data between different groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Overexpression of Gli1 and Gli2 in
androgen-independent prostate cancer cell lines
Kim et al (4)
demonstrated that the expression of certain HH signaling proteins
was significantly associated with poor prognosis, including larger
tumor size, increased level of prostate-specific antigen (PSA),
higher Gleason score and poorer invasiveness. In the present study,
it was examined whether Gli expression is associated with androgen
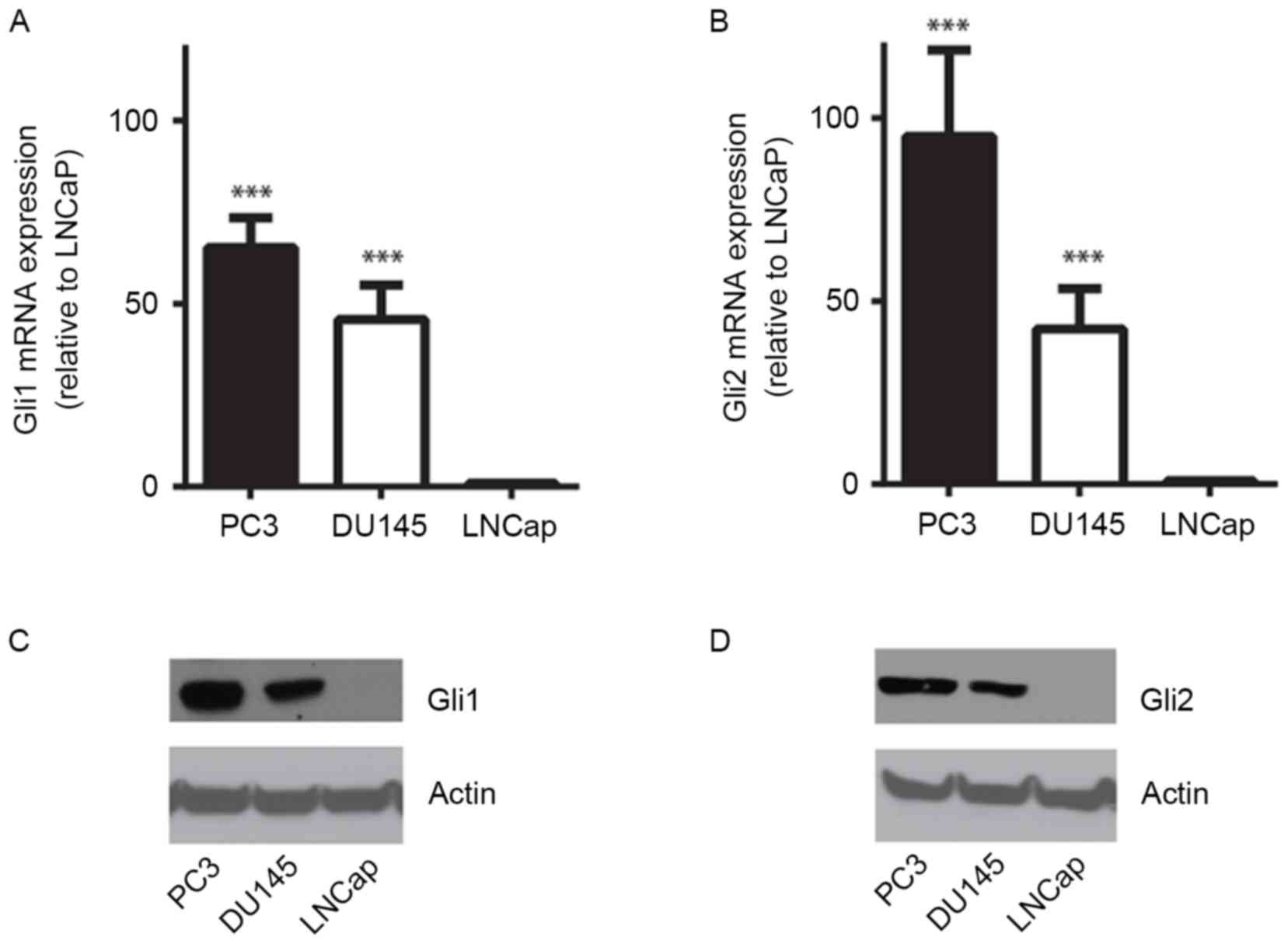
dependency in cultured prostate cancer cells. RT-qPCR revealed that
mRNA expression of Gli1 and Gli2 in the androgen-independent
prostate cancer cell lines PC3 and DU145 was significantly
increased compared with that in LNCaP cells, which is an
androgen-sensitive cell line (P<0.001; Fig. 1A and B). Immunoblotting of the cell
lysates using anti-Gli1 or -Gli2 antibody identified that Gli1 and
Gli2 were overexpressed at the protein level in PC3 and DU145 cells
compared with LNCaP cells, in which both Gli1 and Gli2 protein were
barely detected (Fig. 1C and D).
These results indicate that the HH signaling pathway may serve a
critical role in androgen-independent prostate cancer cells.
Gli1 depletion decreases prostate
cancer cell viability and liquid colony formation
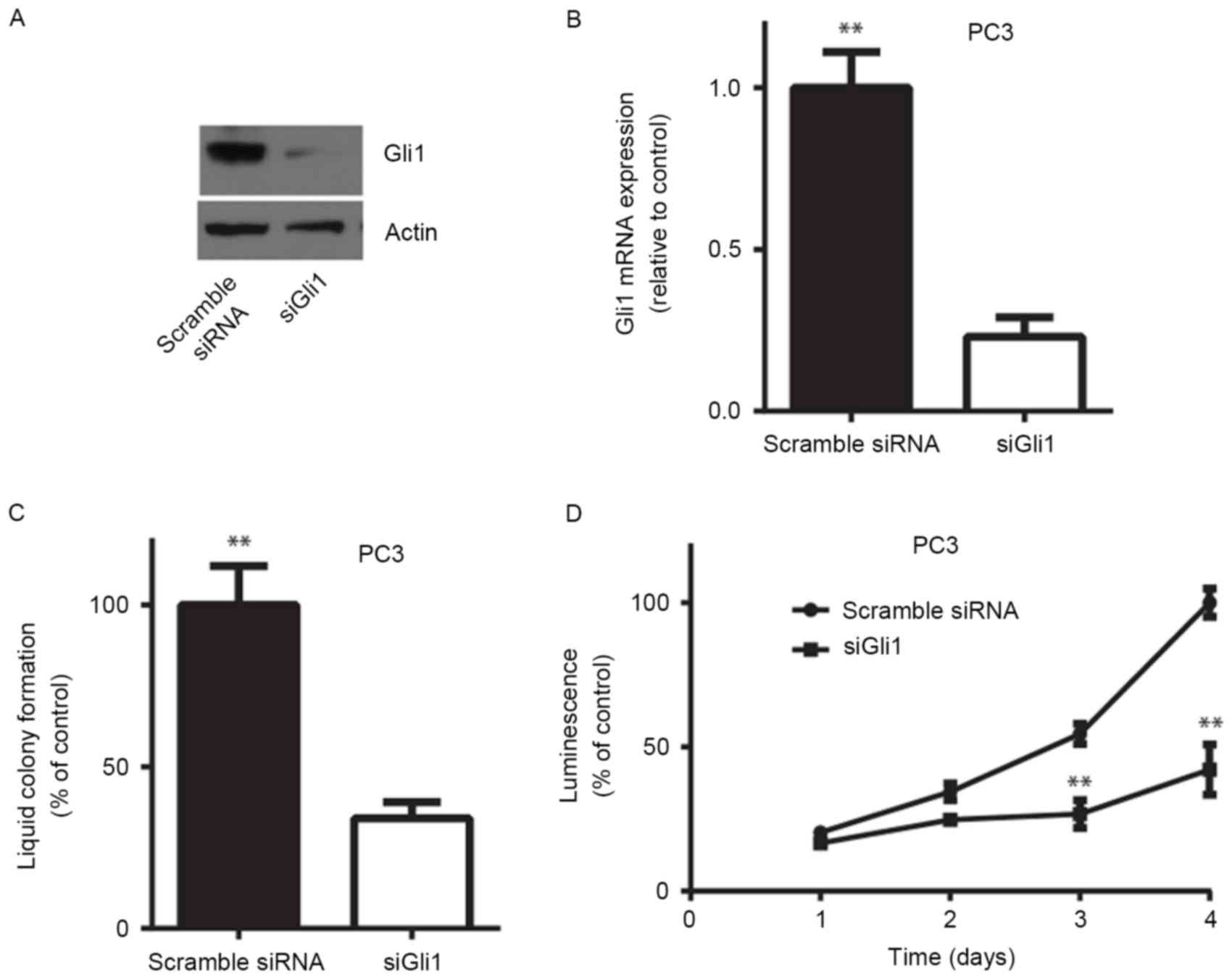
To investigate whether endogenous Gli1 serves a role
in prostate cancer cell proliferation, an siRNA targeting Gli1
(siGli1) was used to transiently knock down the expression of Gli1
in the PC3 cell line. RT-qPCR and western blotting demonstrated
that siGli1 significantly decreased Gli1 mRNA and protein
expression in PC3 cells (P<0.01; Fig.
2A and B). In vitro liquid colony formation assays
revealed that knocking down Gli1 resulted in a significant decrease
in anchorage-dependent colony formation efficiency in PC3 cells
(P<0.01; Fig. 2C). Similarly,
depletion of Gli1 expression led to a significant decrease in cell
viability determined by measuring the ATP level in PC3 cells
(P<0.01; Fig. 2D). These results
indicated that Gli1 is required for androgen-independent prostate
cancer cell survival in vitro.
Gli-specific small molecule inhibitor
GANT61 suppresses prostate cancer cell proliferation
Inhibiting Gli1 function may be a potential
therapeutic strategy for the treatment of prostate cancer. A number
of small-molecule inhibitors targeting Gli family proteins have
been developed (14,15). In the present study, it was examined
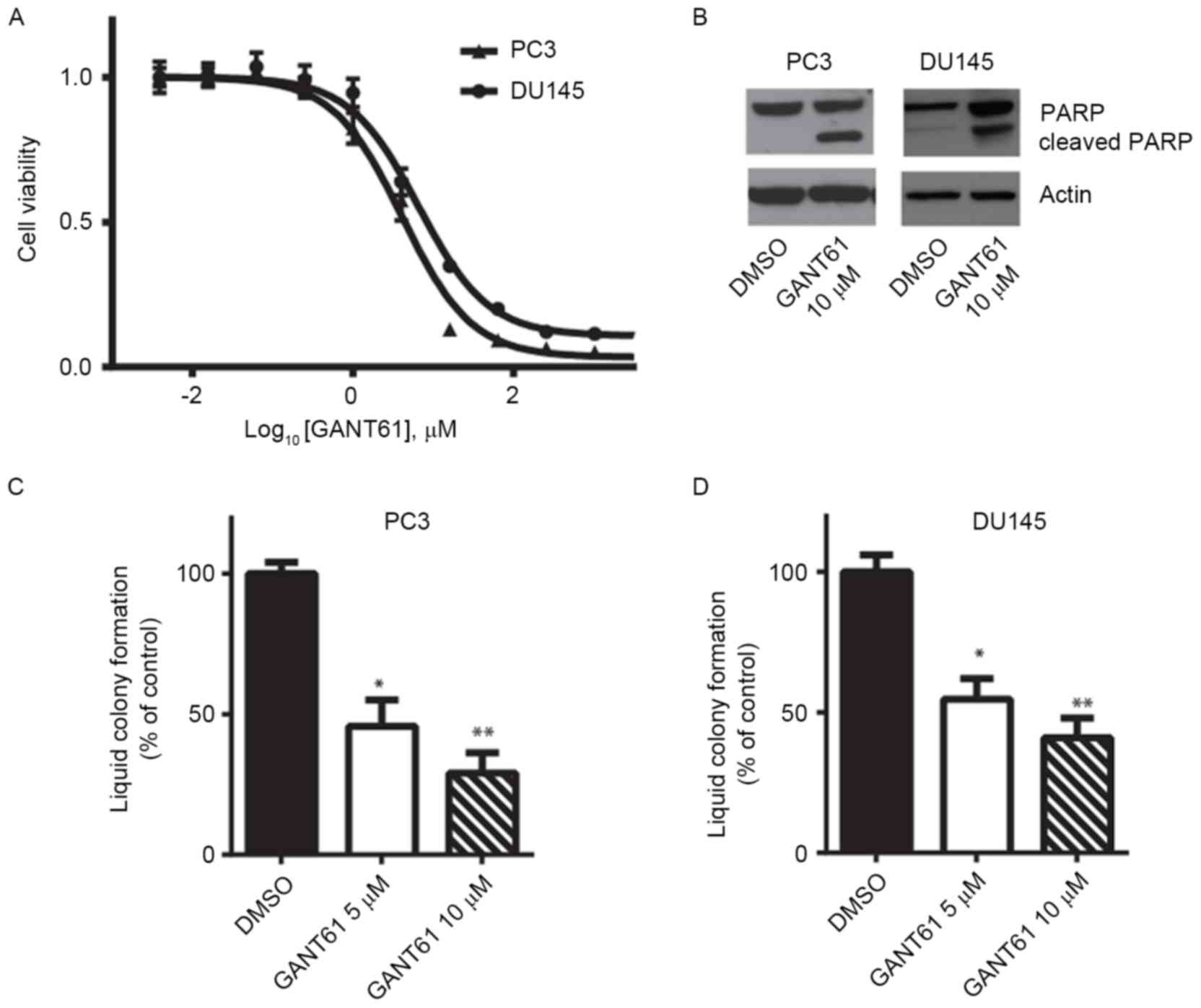
whether the Gli1/2-specific inhibitor GANT61 was able to suppress
prostate cancer cell viability. Cell viability assays revealed that
GANT61 inhibited both PC3 and DU145 cell viability in vitro.
The underlying molecular mechanisms by which GANT61 causes cell
death are not fully understood, although a previous study
demonstrated that GANT61 was able to inhibit the binding of Gli1/2
to the target gene promoter regions (16). PC3 cells exhibited slightly increased
sensitivity to GANT61 compared with DU145 cells, and their
half-maximal inhibitory concentration values were ~5 µM (Fig. 3A). Immunoblotting of the cell lysates
indicated that GANT61 treatment led to PARP cleavage, suggesting
that PC3 and DU145 cells underwent apoptosis (Fig. 3B). In addition, GANT61 treatment
significantly decreased the efficiency of PC3 and DU145 cells to
form liquid colonies (P<0.05 or P<0.01; Fig. 3C and D). These results indicate that
the Gli inhibitor GANT61 may be used as a potential targeted
therapy for androgen-independent prostate cancer.
mTOR/S6K1 signaling pathway is
involved in the regulation of Gli1 expression in prostate cancer
cells
Wang et al (8)
reported that the activated TNFα/mTOR/S6K1 signaling pathway
promotes Gli1 transcriptional activity and oncogenic function in
esophageal adenocarcinoma. To study the upstream pathway further,
rather than the canonical HH pathway, involved in the regulation of
Gli1 expression, it was investigated whether mTOR/S6K1 regulates
Gli1 and its downstream target gene expression in prostate cancer
cells. The documented Gli1 target genes include Gli1 and
PTCH1, of which the corresponding proteins are important
regulators of the canonical HH pathway itself. Other validated
target genes include the cell cycle regulator cyclin D1
(CCND1), epithelial-mesenchymal transition regulator
SNAIL, and self-renewal-associated molecules NANOG
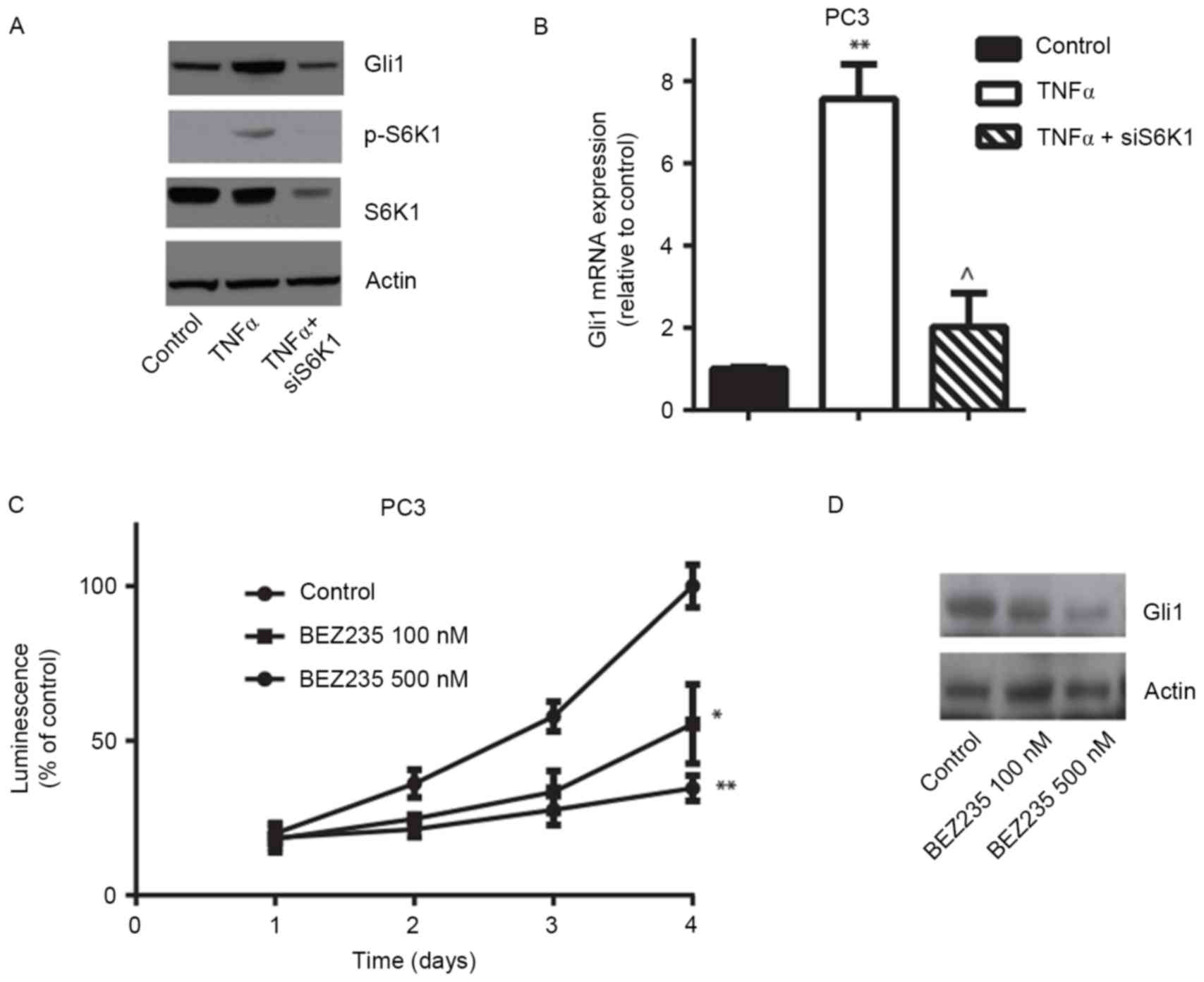
and OCT4. PC3 cells were treated with 5 ng/ml TNFα for 24 h
and it was identified that the PTCH1, CCND1,
N-MYC and Gli1 mRNA expression levels significantly
increased, whereas the presence of rapamycin, an mTOR inhibitor,
significantly decreased gene expression (Fig. 4A-D). Western blotting confirmed that
TNFα stimulation induced the activation of S6K1 and increased Gli1
protein expression, and that rapamycin inhibited TNFα-induced S6K1
phosphorylation and decreased Gli1 expression in PC3 cells
(Fig. 4E). Similarly, depletion of
S6K1 expression using siRNA, which was confirmed using RT-qPCR,
markedly decreased TNFα-induced Gli1 mRNA and protein expression
(Fig. 5A and B). Consistent with
genetic manipulation, pharmacological inhibition of mTOR/S6K1
pathway using the PI3K/mTOR dual inhibitor BEZ235 markedly
decreased PC3 cell viability and Gli1 protein expression (Fig. 5C and D). These results suggest that
the TNFα/mTOR/S6K1 signaling pathway contributes to the regulation
of Gli1 gene expression and its transcriptional activity in
prostate cancer cells.
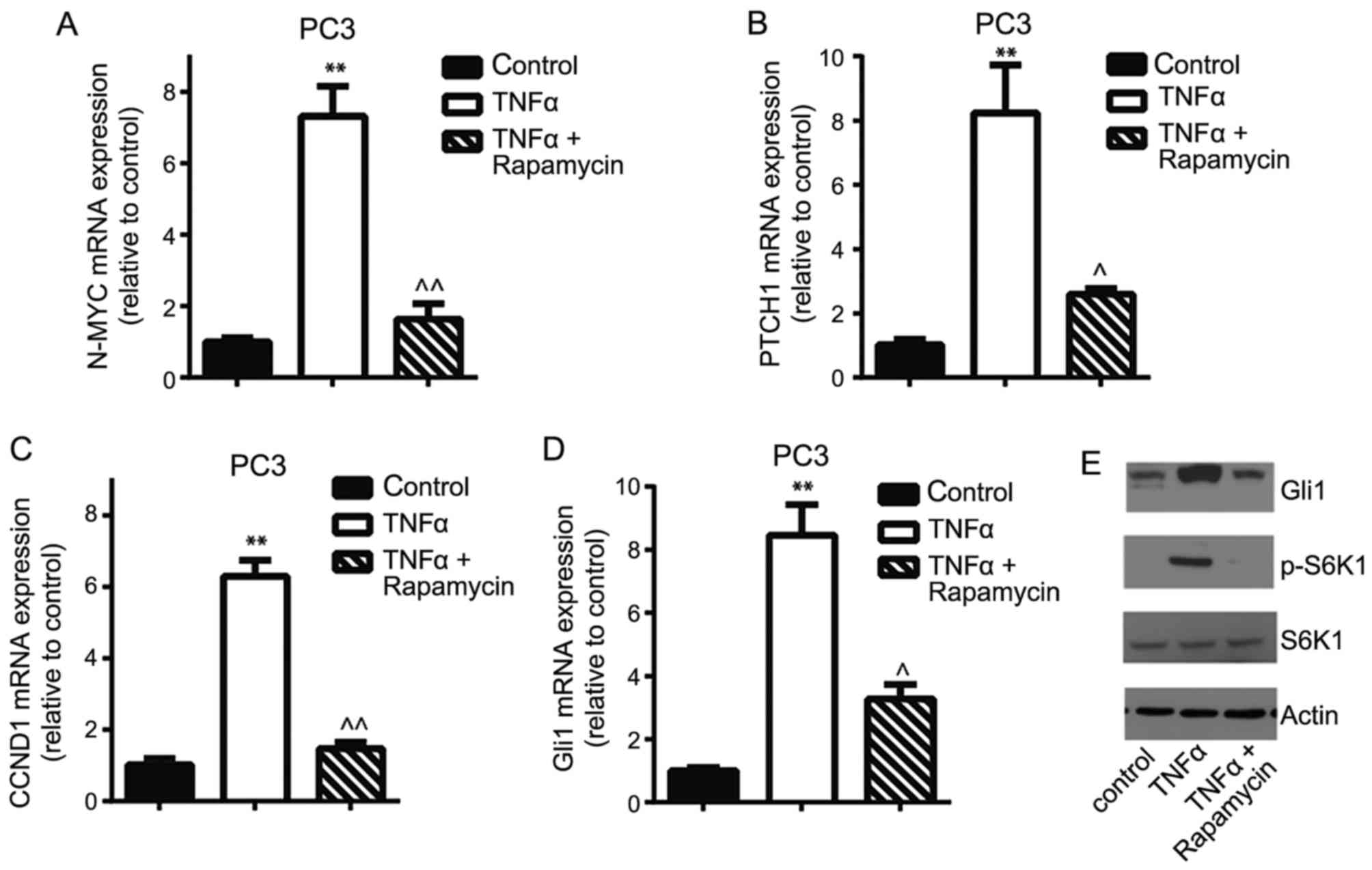 | Figure 4.mTOR/S6K1 signaling pathway is
involved in the regulation of Gli1 expression in PC3 cells. The
reverse transcription-quantitative polymerase chain reaction
demonstrated that 5 ng/ml TNFα treatment for 24 h stimulated the
Gli1 downstream target genes and the Gli1 gene (A) N-MYC,
(B) CCND1, (C) PTCH1 and (D) Gli1 mRNA
expression compared with controls, whereas the mTOR inhibitor
rapamycin decreased TNFα-induced mRNA expression. (E) Western
blotting confirmed TNFα-triggered phosphorylation of S6K1 and
increased Gli1 expression. However, rapamycin inhibited
TNFα-induced S6K1 activation and decreased Gli1 expression in PC3
cells. **P<0.01 vs. control; ^P<0.05,
^^P<0.01 compared with TNFα treatment. mTOR,
mammalian target of rapamycin; S6K1, p70 ribosomal S6 kinase 1;
Gli, glioma-associated oncogene; PTCH1, Patched 1; CCND1, cyclin
D1; TNFα, tumor necrosis factor α; p-, phosphorylated. |
Discussion
The results of the present study identified that
Gli1 is overexpressed at the protein level in androgen-independent
prostate cancer cell lines compared with androgen-dependent
prostate cancer cells. Silencing of Gli1 inhibited prostate cancer
cell proliferation and liquid colony formation in vitro. The
Gli1/2-specific inhibitor GANT61 markedly decreased prostate
cancer.cell viability by inducing cell apoptosis. Pharmacological
and genetic manipulation of the TNFα/mTOR/S6K1 signaling pathway
altered Gli1 expression and cancer cell survival. These results
suggest that Gli1 is a suitable target for drug development for
androgen-independent prostate cancer.
Previous studies have indicated that the HH pathway
contributes to the initiation as well as the progression of
prostate cancer (17–19). Previous studies have assessed the
protein expression of the HH components in tissue microarrays and
identified that the expression of SHH, SMO and PTCH1 in the tumor
was upregulated compared with adjacent normal tissue. However,
stromal PTCH1, SMO and Gli1 expression were downregulated in the
tumor compared with normal tissue (20,21).
Canonical HH pathway activation appears to be more marked in
late-stage prostate cancer (22,23). In
addition, results of the present study and previous studies
observed a potential association between HH signaling component
expression and androgen-independent prostate cancer cells (1,15).
Long-term androgen deprivation may induce an upregulation of HH
signaling in human specimens and in cell lines. Inhibition of HH
signaling led to a decrease in androgen receptor activation, partly
because of the interaction between Gli1/2 and the androgen receptor
(24). The present study provides
experimental evidence for the application of Gli1/2 inhibitors in
the treatment of androgen-insensitive prostate cancer.
In the present study, the main focus was on Gli1
activity and function in androgen-independent prostate cancer lines
and it was identified that the Gli1/2-specific small-molecule
inhibitor GANT61 significantly impaired prostate cancer cell
proliferation. Further studies are required to elucidate the
function of Gli1 and pharmacological effect of GANT61 in
vivo. Gli2 has been demonstrated to be involved in the
malignant transformation of prostate cancer cells. Thiyagarajan
et al (25) reported that
knockdown of Gli2 in prostate cancer cells suppressed tumor growth
in vitro and in vivo. The mechanisms by which the HH
signaling pathway serves a role in the initiation and progression
of prostate cancer include the anti-apoptotic effect and the
inhibition of invasiveness and metastasis. Karlou et al
(26) also demonstrated that the SMO
inhibitor GDC-0449 exhibited the ability to inhibit prostate cancer
xenograft tumor growth. Despite the promising results of HH
inhibition in prostate cancer cell lines and mouse models, this has
not been fully translated into the clinic.
Previously, aberrant HH signaling in prostate tumors
was reported to be ligand-dependent, although it is controversial
whether this is in a paracrine or autocrine manner (3). In fact, the HH pathway is part of a
complex signaling network that remains incompletely understood
(9). The PI3K/Akt and Ras/MAPK/ERK
kinase (MEK)/ERK signaling pathways have been demonstrated to
activate Gli1 in a SMO-independent manner (10,11). In
prostate cancer, alterations in the PI3K/Akt signaling pathway are
common in primary and metastatic lesions (27). The Ras/MEK/ERK pathway is also
constitutively activated in a number of prostate tumor tissues and
appears to exhibit an association with advanced and
androgen-independent prostate cancer (27). Furthermore, the tumor suppressor PTEN,
which is a negative regulator of the signaling pathway, is lost in
<80% of prostate cancers, leading to constitutive activation of
Akt pathway (22); however, the
underlying molecular mechanism is not fully understood. In the
present study, it was identified that the TNFα/mTOR/S6K1 signaling
pathway was partly responsible for the aberrant overexpression of
Gli protein. Inhibiting mTOR/S6K1 activity markedly blocked Gli1
function in androgen-independent prostate cancer cells. The next
step is to determine the in vivo effect of inhibiting the
mTOR/S6K1 signaling pathway on prostate xenograft tumor growth.
Additionally, Narita et al (28) have demonstrated that inhibition of HH
signaling may increase the chemosensitivity of prostate cancer
cells.
The results of the present study indicate that Gli1
transcriptional activity is critical in androgen-independent
prostate cancer cell proliferation. The PI3K/mTOR/S6K1 signaling
pathway is implicated in the regulation of Gli1 expression and
functions in a SMO-independent manner. Blocking the non-canonical
HH pathway or directly inhibiting Gli1 transcriptional activity may
open a new avenue for targeted therapies. Further study using mouse
models may lead to a better understanding of the role of Gli1 in
prostate cancer growth and eventually to inhibitors that may be
used as tools for research or treatment.
Acknowledgements
This research was supported by grants from the
National Natural Science Foundation of China (grant no. 81201767)
and the Joint Special Funds for the Department of Science and
Technology of Yunnan Province-Kunming Medical University (grant
nos. 2011FB202, 2013FZ277 and 2017FE467-191).
References
|
1
|
Gonnissen A, Isebaert S and Haustermans K:
Hedgehog signaling in prostate cancer and its therapeutic
implication. Int J Mol Sci. 14:13979–14007. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo R, Cai L, Fan Y, Jin J, Zhou L and
Zhang K: Magnetic resonance imaging on disease reclassification
among active surveillance candidates with low-risk prostate cancer:
A diagnostic meta-analysis. Prostate Cancer Prostatic Dis.
18:221–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen M, Carkner R and Buttyan R: The
hedgehog/Gli signaling paradigm in prostate cancer. Exp Rev
Endocrinol Metab. 6:453–467. 2011. View Article : Google Scholar
|
|
4
|
Kim TJ, Lee JY, Hwang TK, Kang CS and Choi
YJ: Hedgehog signaling protein expression and its association with
prognostic parameters in prostate cancer: A retrospective study
from the view point of new 2010 anatomic stage/prognostic groups. J
Sur Oncol. 104:472–479. 2011. View Article : Google Scholar
|
|
5
|
Jiang J and Hui CC: Hedgehog signaling in
development and cancer. Dev Cell. 15:801–812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Onishi H and Katano M: Hedgehog signaling
pathway as a therapeutic target in various types of cancer. Cancer
Sci. 102:1756–1760. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rubin LL and de Sauvage FJ: Targeting the
Hedgehog pathway in cancer. Nat Rev Drug Discov. 5:1026–1033. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Ding Q, Yen CJ, Xia W, Izzo JG,
Lang JY, Li CW, Hsu JL, Miller SA, Wang X, et al: The crosstalk of
mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 21:374–387. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lauth M and Toftgård R: Non-canonical
activation of GLI transcription factors: Implications for targeted
anti-cancer therapy. Cell. 6:2458–2463. 2007.
|
|
10
|
Riobo NA, Haines GM and Emerson CP Jr:
Protein kinase C-delta and mitogen-activated protein/extracellular
signal-regulated kinase-1 control GLI activation in hedgehog
signaling. Cancer Res. 66:839–845. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Riobo NA, Lu K, Ai X, Haines GM and
Emerson CP Jr: Phosphoinositide 3-kinase and Akt are essential for
Sonic Hedgehog signaling. Proc Natl Acad Sci USA. 103:pp.
4505–4510. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang L, Xie G, Fan Q and Xie J: Activation
of the hedgehog-signaling pathway in human cancer and the clinical
implications. Oncogene. 29:469–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vandesompele J, De Preter K, Pattyn F,
Poppe B, Van Roy N, De Paepe A and Speleman F: Accurate
normalization of real-time quantitative RT-PCR data by geometric
averaging of multiple internal control genes. Genome Biol.
3:RESEARCH00342002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ebrahimi A, Larijani L, Moradi A and
Ebrahimi MR: Hedgehog signalling pathway: Carcinogenesis and
targeted therapy. Iran J Cancer Prev. 6:36–43. 2013.PubMed/NCBI
|
|
15
|
Chen G, Goto Y, Sakamoto R, Tanaka K,
Matsubara E, Nakamura M, Zheng H, Lu J, Takayanagi R and Nomura M:
GLI1, a crucial mediator of sonic hedgehog signaling in prostate
cancer, functions as a negative modulator for androgen receptor.
Biochem Biophys Res Commun. 404:809–815. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lauth M, Bergström A, Shimokawa T and
Toftgård R: Inhibition of GLI-mediated transcription and tumor cell
growth by small-molecule antagonists. Proc Natl Acad Sci USA.
104:pp. 8455–8460. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thayer SP, di Magliano MP, Heiser PW,
Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del
Castillo C, Yajnik V, et al: Hedgehog is an early and late mediator
of pancreatic cancer tumorigenesis. Nature. 425:851–856. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pasca di Magliano M and Hebrok M: Hedgehog
signalling in cancer formation and maintenance. Nat Rev Cancer.
3:903–911. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Podlasek CA, Barnett DH, Clemens JQ, Bak
PM and Bushman W: Prostate development requires Sonic hedgehog
expressed by the urogenital sinus epithelium. Dev Biol. 209:28–39.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Datta S and Datta MW: Sonic Hedgehog
signaling in advanced prostate cancer. Cell Mol Life Sci.
63:435–448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sheng T, Li C, Zhang X, Chi S, He N, Chen
K, McCormick F, Gatalica Z and Xie J: Activation of the hedgehog
pathway in advanced prostate cancer. Mol Cancer. 3:292004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karhadkar SS, Bova GS, Abdallah N, Dhara
S, Gardner D, Maitra A, Isaacs JT, Berman DM and Beachy PA:
Hedgehog signalling in prostate regeneration, neoplasia and
metastasis. Nature. 431:707–712. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan L, Pepicelli CV, Dibble CC, Catbagan
W, Zarycki JL, Laciak R, Gipp J, Shaw A, Lamm ML, Munoz A, et al:
Hedgehog signaling promotes prostate xenograft tumor growth.
Endocrinology. 145:3961–3970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Efstathiou E, Karlou M, Wen S, Hoang A,
Pettaway CA, Pisters LL, Maity S, Troncoso P and Logothetis CJ:
Integrated Hedgehog signaling is induced following castration in
human and murine prostate cancers. Prostate. 73:153–161. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thiyagarajan S, Bhatia N, Reagan-Shaw S,
Cozma D, Thomas-Tikhonenko A, Ahmad N and Spiegelman VS: Role of
GLI2 transcription factor in growth and tumorigenicity of prostate
cells. Cancer Res. 67:10642–10646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karlou M, Lu JF, Wu G, Maity S, Tzelepi V,
Navone NM, Hoang A, Logothetis CJ and Efstathiou E: Hedgehog
signaling inhibition by the small molecule smoothened inhibitor
GDC-0449 in the bone forming prostate cancer xenograft MDA PCa
118b. Prostate. 72:1638–1647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Taylor BS, Schultz N, Hieronymus H,
Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva
B, et al: Integrative genomic profiling of human prostate cancer.
Cancer Cell. 18:11–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Narita S, So A, Ettinger S, Hayashi N,
Muramaki M, Fazli L, Kim Y and Gleave ME: GLI2 knockdown using an
antisense oligonucleotide induces apoptosis and chemosensitizes
cells to paclitaxel in androgen-independent prostate cancer. Clin
Cancer Res. 14:5769–5777. 2008. View Article : Google Scholar : PubMed/NCBI
|