Introduction
Hepatoid adenocarcinoma (HAC) represents
approximately 0.2 to 0.8% of diagnosed gastric cancer (1). Ishikura et al defined HAC as
extrahepatic tumor with hepatocyte differentiation and potentially
increased α-fetoprotein (AFP) secretion (2). HAC diagnosis is mainly histological and
is characterized by large and polygonal hepatocyte-like cells with
intense eosinophil cytoplasm and big central nucleus. HAC is mainly
located in oesophagus and stomach, however other sites were also
described (3). According to a Chinese
analysis, which includes a total of 180 gastric HACs from 62
different cases reports, the 3-year survival rate was only of 7.36%
and the median survival time of 10 months (1). Another analysis including 31 gastric HAC
patients described that: i) 87% of them exhibited an increased
secretion of AFP serum level; ii) 55% had a locoregional
synchronous node extension; and iii) 25% had synchronous
metastatic, especially hepatic. An overexpression of AFP was
detected by immunohistochemistry (IHC) in 90.3% of cases (4). Conversely, Nagai et al showed
that in 46% of HAC patients, any increase of AFP secretion was
observed. Therefore, they proposed a novel description of HAC as an
extrahepatic tumor with typical hepatocyte cells with or without
AFP secretion (5). Importantly, HAC
must not be confounded with classical gastric adenocarcinoma, which
can be associated with AFP overexpression, and having a better
prognosis. Indeed, gastric HAC prognosis is poorer than classical
histological types ones (4,6). Currently, the treatment of metastatic
HAC (mHAC) remains not elucidated. Herein, we report two
case-reports of mHAC patients. Moreover, through a pertinent review
of the literature, we aim to analyse the different current
therapeutic approaches for mHAC.
Materials and methods
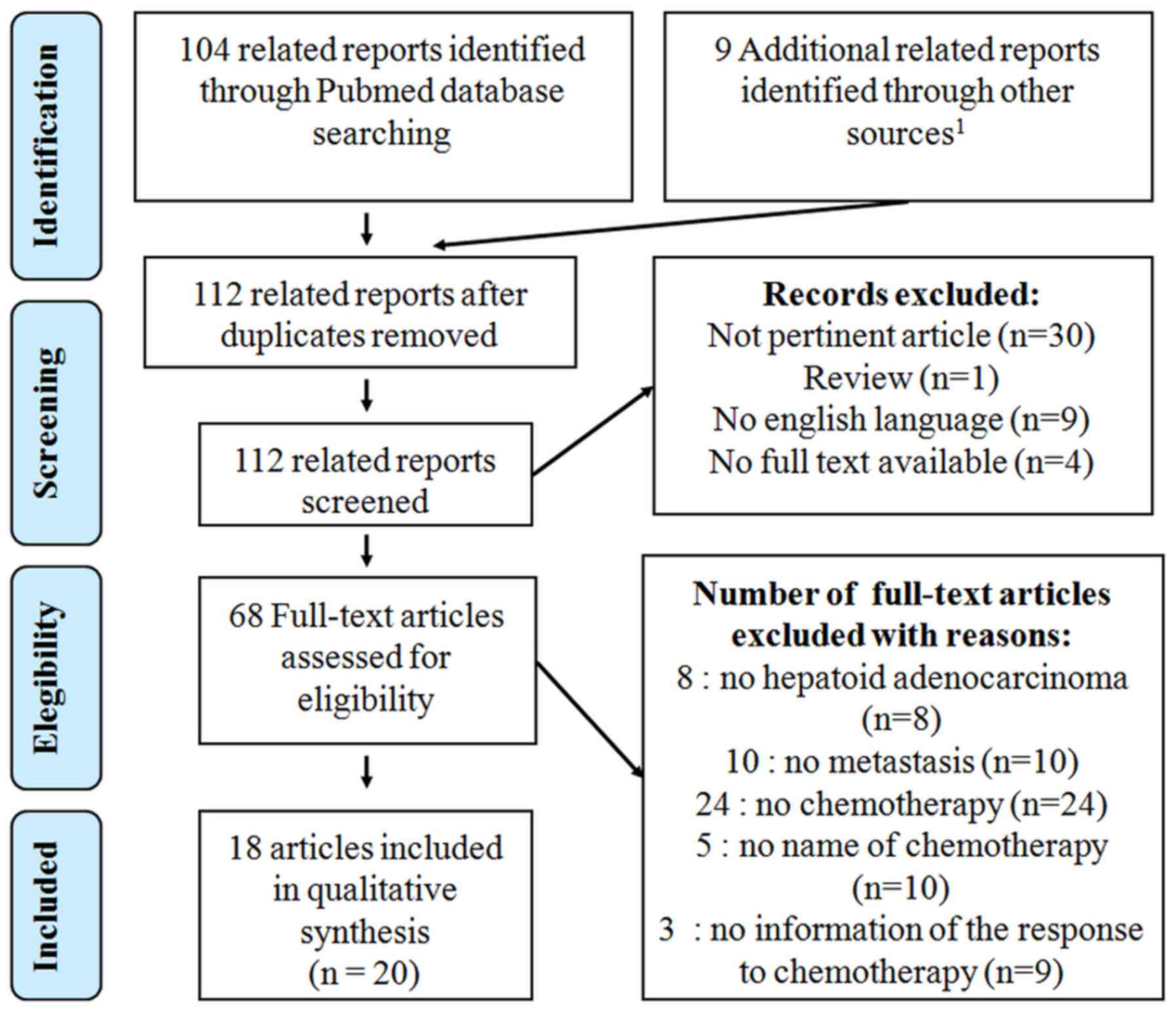
We have performed a PRISMA-compliant systematic
review of literature concerning the use of chemotherapy in mHAC
(7). Inclusion criteria for articles
published from January 2001 to October 2016 were: i) mHAC; ii)
treated by chemotherapy; and iii) with an evaluation of the
response. The following term was used in the Pubmed and Science
Direct database exploration: ‘metastatic hepatoid carcinoma’, or
‘hepatoid’ with mesh term ‘drug therapy’. We also performed an
investigation in the Grey literature database and in all case
reports published in open case reports journals, which are not
indexed in Pubmed databases (Journal of Medical Case Report, BMJ
Case Report, American Journal of Case Report, International Journal
of Case Reports, Clinical Case Reports) with the following term,
‘hepatoid’. The flow chart respect PRISMA criteria, but no
registration was carried out prospectively, explaining the lack of
PROSPERO number (7). We identified
104 related reports in Pubmed Database and 9 in open case reports
journals (Fig. 1). Though, no related
article was found in Grey Literature. Thirty articles were not
pertinent and 13 were not available in English full text.
Sixty-eight full text articles were assessed for eligibility. Among
them, 8 were not related to HAC, 10 exhibited no metastasis
features and 24 were not associated with chemotherapy. The nature
of chemotherapy was not described in 10 case reports from 5
articles. The response to chemotherapy was not related in 9 case
reports from 3 articles. Finally, only 18 articles concerning 20
patients were included. The best overall response (BOR),
corresponding to the addition of best metastatic response (BMR) and
the best primitive lesion response (BPR) to chemotherapy was
reported and assessed according to Response Evaluation Criteria In
Solid Tumors (RECIST version 1.1). The decrease of serum AFP levels
following the treatment, named ‘biological response’ (BioR), was
also assessed.
First case report
Mr. B., a 64-year-old French man, suffering from
gastric reflux with hiatal hernia treated by proton inhibitor, was
admitted to the gastroenterology department in February 2006 for
epigastric and right hypochondrium pains associated with an
anorexia and lose of weight. During the physical examination, the
patient had a rapidly worsening general state with i) a Performans
Status estimated to 4; ii) an icterus; iii) a paraneoplasic fever;
and iv) a painful massive hepatomegaly. The laboratory
investigation showed a biological chronic inflammation with LDH 25
times upper limit of normal (ULN), ASAT 2 ULN, and icteric
cholestasis with total bilirubin at 120 µmol/l. Carcinoembryonic
antigen (CEA) and CA 19-9 were normal, but serum AFP was elevated
to 2,600 ng/ml. An oesogastroduodenoscopy discovered an ulcerated
cardial tumoral lesion. Ultrasound scanning and computed tomography
(CT) scans found three lesions in the right liver. A
histopathologic examination of the cardia biopsy showed an ulcered
HAC of the cardia, with tubulopapillary contingents. It was
composed of large cells, with large nucleus, eosinophilic
cytoplasm, and few hyaline balls. The periodic acid-Schiff (PAS)
coloration, as well as the IHC of Hep Par antibodies, was negative.
The histological examination of the hepatic biopsy showed the same
HAC with the same cellular features, but with more necrosis
aspects. The IHC study revealed CK19 positive (+), CK7 negative
(−), CD10-, Chromogranine-, and Anti-synaptophysine negative
cells.
A systemic chemotherapy was delivered associating
cisplatin 25 mg/m2 with etoposide 100 mg/m2
each day, day 1 to day 3, every 3 weeks. After one cycle, an
impressive clinical improvement was notified with a decrease of
abdominal pains and an extinction of hepatomegaly, with a straight
decrease of icteric cholestasis although the AFP level was enhanced
to 6,900 ng/ml. After 3 cycles, CT scans showed a good partial
response on the liver metastasis and on the cardia lesion,
confirmed after 6 cycles with a reduction of more than 80% of the
size of liver metastasis. Moreover, the serum AFP levels were
normalized. The Positron Emission Tomography (PET), realised two
months after the end of chemotherapy, showed a complete metabolic
gastric response and the persistence of two lesions in the segment
VIII. The oesogastroduodenoscopy revealed an inflammatory cardia
with complete histopathologic responses. After a one-year
follow-up, a radical surgery associating gastrectomy and right
hepatotectomy was decided by pluridisciplinary committee, and
carried out. The patient was still alive, in complete remission at
the last CT scan, more than 9 years after his diagnosis (Tables I and II; case 1).
 | Table I.Characteristics of metastatic HAC
cases reported in literature. |
Table I.
Characteristics of metastatic HAC
cases reported in literature.
| Case | Year | First author | Nationality | Sex | Age | Primitive site | Metastasis
site(s) | Synchrone
metastasis | IHC AFP | Initial
AFPa | CEAa | Initial surgery | (Refs.) |
|---|
| 3 | 2002 | Shimada | Japanese | F | 71 | Stomach | Liver | Yes | + | 5190 | NA | No | (9) |
| 4 | 2002 | Shimada | Japanese | M | 63 | Stomach | Liver | Yes | + | 156 | NA | No | (9) |
| 5 | 2005 | Chiba | Japanese | M | 47 | Stomach | Liver | No | – | 606.8 | ULN | Yes | (15) |
| 6 | 2007 | Takayema | Japanese | M | 64 | Stomach | Liver | Yes | + | 1497.8 | 72.7 | No | (10) |
| 7 | 2009 | Takahashi | Japanese | M | 51 | Stomach | Liver | Yes | + | 91 | NA | No | (11) |
| 8 | 2009 | Lin | Chinese | F | 56 | Stomach | Liver | Yes | + | 9457 | ULN | No | (16) |
| 9 | 2009 | Gálvez-Muñoz | European | M | 75 | Stomach | Liver/nodes | Yes | + | 4500 | 460 | Yes | (17) |
| 10 | 2011 | Mokrim | Moroccan | M | 52 | Lung | Lung/nodes | Yes | + | 5000 | NA | No | (27) |
| 11 | 2012 | Cappetta | European | F | 75 | Colon |
Peritoneal/nodes | No | + | ULN | ULN | Yes | (18) |
| 12 | 2013 | Ye | Chinese | M | 54 | Stomach | Lung | No | + | 99 | ULN | Yes | (19) |
| 13 | 2013 | Ye | Chinese | F | 61 | Stomach | Spleen | Yes | + | >50000 | ULN | No | (19) |
| 14 | 2013 | Ahn | Korean | M | 68 | Stomach | Liver | No | + | NA | NA | Yes | (20) |
| 15 | 2013 | Majumder | American | M | 60 | Pancreas | Liver | Yes | – | ULN | ULN | No | (21) |
| 16 | 2014 | Nagai | Japanese | M | 62 | Stomach | Liver | Yes | NA | NA | NA | Yes | (22) |
| 17 | 2015 | Chen | American | M | 36 | Colon |
Peritoneal/nodes | No | + | 4896 | ULN | Yes | (23) |
| 18 | 2015 | Hu | Chinese | M | 28 | Mediastinum | Liver/lung | Yes | + | 155000 | NA | Yes | (24) |
| 1 | 2017 | Simmetb | European | M | 64 | Stomach | Liver | Yes | – | 2600 | ULN | No |
|
| 2 | 2017 | Simmetb | European | F | 60 | Stomach | Liver | Yes | – | 76000 | 176 | No |
|
 | Table II.Description of response to
chemotherapy for metastatic HAC reported cases. |
Table II.
Description of response to
chemotherapy for metastatic HAC reported cases.
| Case | Chemotherapy
regimen | BOR | BPR | BMR | BioR | AFP
nadira | Surgery | Local surgery | Metastasis
alive | OS |
|---|
| 3 |
Cisplatin/paclitaxel | PR | PR | PR | Yes | 155.9 (3.8) | Yes | No | No | 14 |
| 4 | Weekly
paclitaxel | CR | Na | CR | Yes | 700 (2) | No | No | Yes | 8+ |
| 5 |
Cisplatin/Etoposide/5FU | CR | CR | CR | Yes | <10 (9) | Yes | No | Yes | 106+ |
| 6 |
Doxorubicin/mitomycin/5FU | PR | CR | PR | Yes | <10 (945) | Yes | No | No | 20 |
| 7 |
Cisplatin/capecitabine | SD | SD | SD | Yes | NA | No | No | Yes | 8+ |
| 8 | FOLFIRI (5FU+
irinotecan) bevacizumab | PD | Na | PD | Na | NA | No | No | No | 3 |
| 9 | Sorafenib | SD | SD | SD | Na | NA | No | No | No | 8 |
| 10 |
Paclitaxel/capecitabine | PD | Na | PD | No | 8431 | No | No | No | 13 |
| 11 | FOLFOX (5FU+
oxaliplatin) | PD | PD | PD | No | >50000 | No | No | No | 8 |
| 12 |
Cisplatin/capecitabine | PD | Na | PD | Na | NA | No | No | No | 15 |
| 13 | Gemcitabine | PD | PD | PD | Na | NA | No | No | No | 3 |
| 14 | Cisplatin/S1 | PR | Na | PR | Na | NA | No | No | Yes | 24+ |
| 15 | FOLFOX
(5fu+oxaliplatin)/bevacizumab | SD | Na | SD | Yes | 260 (18) | No | No | Yes | 6+ |
| 16 |
Carboplatin/paclitaxel/Sorafenib | PR | PR | PR | Yes | 25 (7) | No | No | No | 11 |
| 1 |
Cisplatin/Etoposide | PR | CR | PR | Yes | <10 (260) | Yes | Yes | Yes | 120+ |
| 2 |
Cisplatin/Etoposide | PR | CR | PR | Yes | 1751 (43) | No | No | No | 17 |
Second case report
A 60 year-old French woman was admitted to the
emergency ward in December 2014 for insomnia-related pains in her
right hypochondrias, associated with anorexia, asthenia and a lose
of weight of 3 kg in one month. Gastro-duodenal ulcer was notified
in her digestive antecedents. The physical examination found a
hepatomegaly and epigastric pains on the palpation. The laboratory
investigation found ASAT 2 ULN, LDH to 7 ULN, and a non-icteric
cholestasis. CT scans showed a tumor in the cardia extended to the
low-oesophagus and greater curvature, numerous liver metastasis, up
to 7 cm, with hypervascularization and necrotic center in arterial
phase. In Magnetic Resonance Imaging (MRI), cardia tumor and
hepatic metastasis appeared hyper-intense in T2 and diffusion
sequence, with peripheral arterial enhancement (ring-shape) without
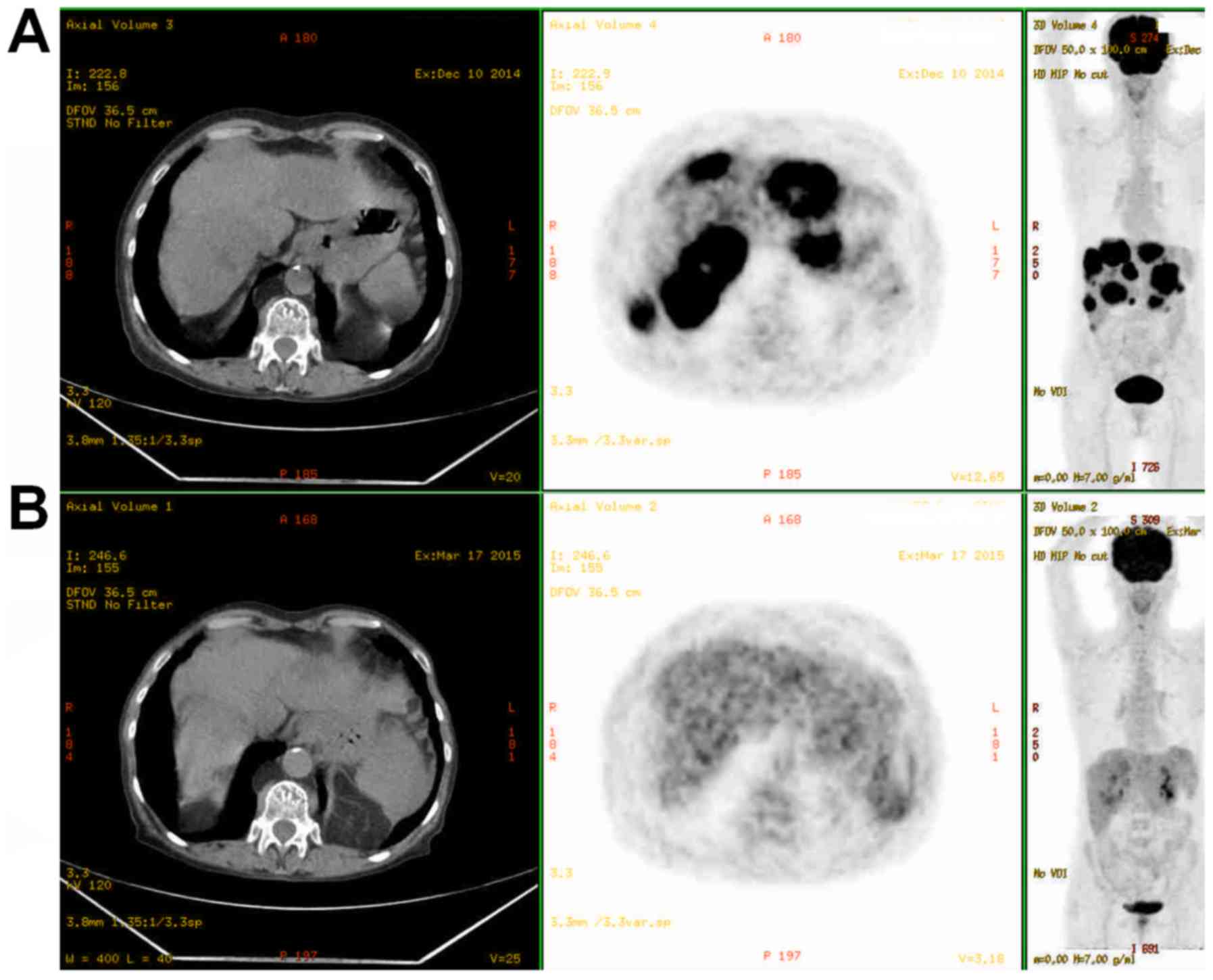
washout. PET revealed an intense hypermetabolism of the liver
metastasis and primary lesion (Fig.
2). With particularly features in CT scans, tumoral markers
have been analysed: 76,000 ng/ml for AFP, 176 IU/ml for CEA, and
normal CA19-9. Oesogastroduodenoscopy showed an infiltrated
ulceration of 4 cm on low-oesophagus and cardia on the third of the
curvature. The histopathologic examination confirmed a HAC: round
cells with big nuclei not much nucleolated, occasionally associated
with a very dense chromatin, an eosinophile cytoplasm, but without
intracytoplasmic hyaline globules as evaluated by PAS. As revealed
by IHC studies, tumors were found KL1 positive (+), Glypican+,
Glutamine Synthetase+, CK5-6 low, Hep Par antibodies low, CK20
negative (−), CK5-, AFP negative, Betacatenine-, Synaptophysin-,
and P63 or Oestrogen receptors negative.
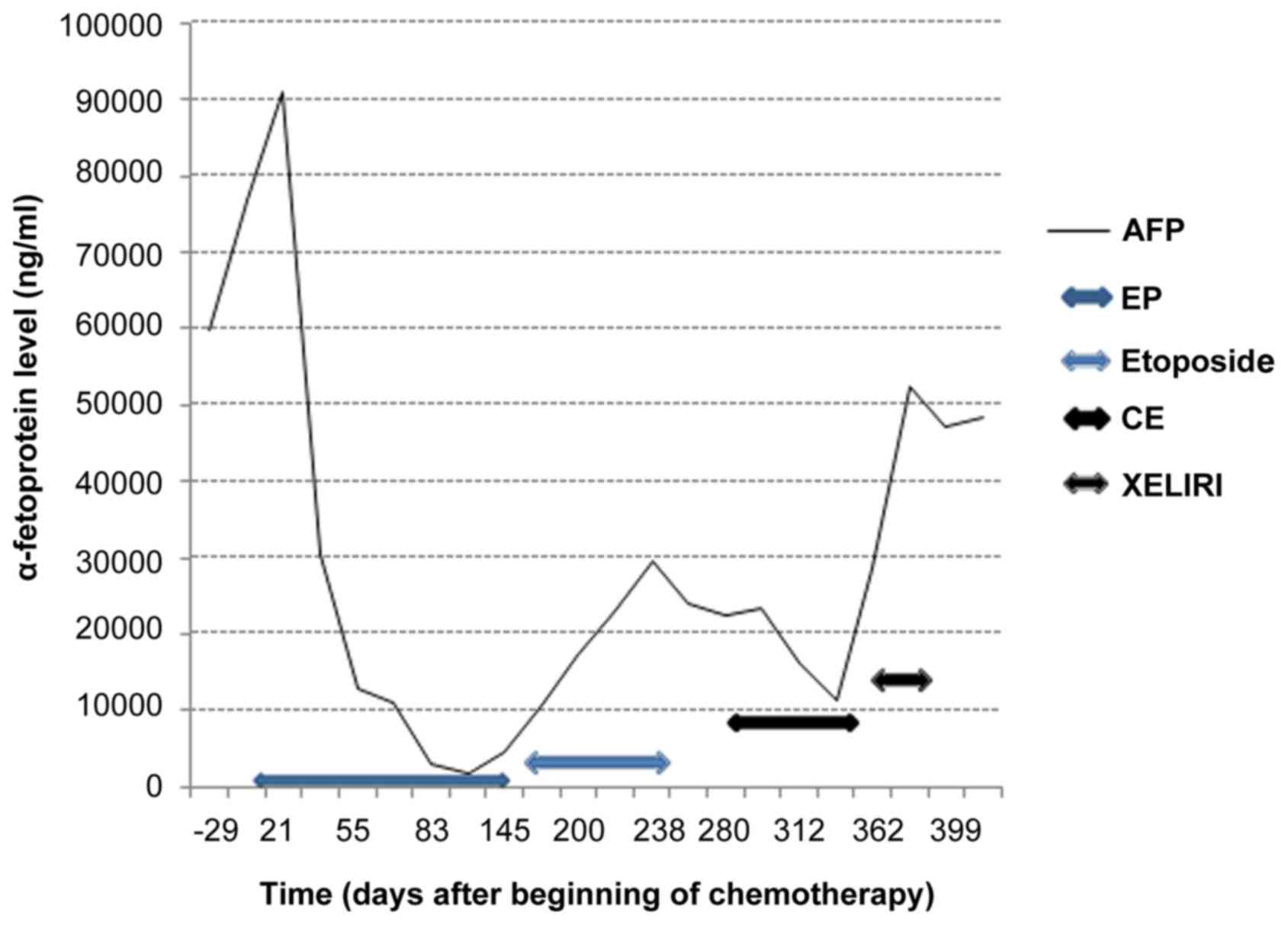
A systemic chemotherapy was delivered associating
cisplatin 25 mg/m2 with etoposide 100 mg/m2
each day, day 1 to day 3, every 3 weeks, with a rapid decrease of
pain, after the first cycle, although an initial increase of
tumoral markers was observed (Fig.
3). The first evaluation after 3 cycles by PET showed a
metabolic complete response on the primary oesogastric lesion and
metabolic partial response on the metastatic liver lesions
(Fig. 2). After 6 cycles, persistence
of complete response was confirmed on the primary lesion, but an
increase in size and intensity of liver metastasis was observed. A
maintenance therapy by oral etoposid was administrated, but stopped
3 months later due to marker's increase. Nowadays, PET scan
confirmed a hepatic progression with appearance of hypermetabolism
on the left lower paratracheal nodes, without any relapse in
oesogastric junction. A second line treatment with carboplatin and
etoposid was carried out leading to a novel decrease of AFP levels
before observing any progression (Fig.
3). Finally, a third treatment by XELIRI (capecitabin and
irinotecan) was administrated, without any efficiency. The patient
died after 23 months-follow-ups (Tables
I and II; case 2).
Results
Only one retrospective Korea cohort of 13 mHAC was
found in our study with: i) one partial response reported with
5-fluoruracil (5-FU) associated with cisplatin; and ii) a second
one with cisplatin-paclitaxel in second line (8). Nevertheless, the others patients
received different chemotherapy regimens. Therefore, as the nature
of the treatment for each patients remained unknown, this cohort
was excluded for the final analysis. Moreover, we have reported 2
colon, 2 lung, 2 pancreas, 1 bladder, 1 peritoneal, 1 mediastinum
mHACs and 11 gastric mHACs (Tables I
and III, especially with sorafenib
treatment). Including our two case reports, the median age of the
diagnosis was 56 years. Patients (73%) were male, 63% (14/22) and
72% (16/22) exhibited liver metastasis and synchrone metastasis,
respectively. IHC AFP study was positive in 75% of cases (15/20).
When increased, the average AFP serum level was of 16,597 ng/ml. An
initial surgery was carried out in 41% of cases (9/22). 11/22 BOR
were observed with chemotherapy. Among them, 8/22 were partial
responses and 3/22 complete ones. The complete response concerned 3
Japanese male patients with gastric HAC and liver metastasis. 55%
(12/22) of the patients received a cisplatin-based regimen, whose
9/12 had partial or complete responses. Among partial or complete
response of all case reports, 9/11 (81%) had a cisplatin-based
chemotherapy. One another partial response was observed with a
triple combined therapy: mitomycin-C, doxorubicin and
5-fluorouracil (Table II; case
8).
 | Table III.Characteristic and response to
sorafenib for metastatic HAC reported cases. |
Table III.
Characteristic and response to
sorafenib for metastatic HAC reported cases.
| Case | Year | First author | Nationality | Sex | Age | Primitive site | Metastasis
site(s) | IHC AFP | Initial
AFPa | Chemotherapy
regimen | BOR | BioR | AFP
nadirb | Alive | OS | (Refs.) |
|---|
| 19 | 2010 | Metzgeroth | European | M | 21 | Peritoneal | Peritoneal | – | ULN | Sorafenib | SD | NA | NA | No | 6 | (14) |
| 20 | 2011 | Karayiannakis | European | F | 60 | Bladder | Nodes | + | 62 | Sorafenib | SD | Yes | <10 (6) | No | 20 | (25) |
| 21 | 2012 | Petrelli | European | M | 37 | Pancreas | Liver lung
nodes | NA | 11 | Sorafenib | SD | NA | NA | No | 8 | (26) |
| 22 | 2015 | Gavrancic | American | M | 64 | Lung | Bones nodes | + | 181 |
Carboplatin/paclitaxel/sorafenib | PR | Yes | 25 (7) | No | 11 | (12) |
Three patients were still alive with a follow-up
superior to one year; these 3 patients were under Cisplatin-based
chemotherapy regimen (Table II;
cases 1, 7 and 14). Finally, BioR was observed in 14 of 16
evaluable patients and AFP serum levels were normalized in 4 of 15
included patients of our review (Table
II; cases 1, 4, 7 and 8).
In addition, 4 patients were treated by sorafenib in
literature (Table III). One of
them, presenting a lung mHAC, also received an additional
lung-chemotherapy regimen (Table
III; case 22) (12). Three other
patients were treated in monotherapy, which permitted a greater
stability of the disease (Table
III; cases 19, 20 and 21).
Discussion
Based on our investigation, we assume to describe
the first two case reports of mHAC treated by cisplatin-etoposid as
first-line chemotherapy leading to major partial and complete
responses considering the primitive lesion. Indeed, we report a
complete biological response: after a nine-year follow-up, we
consider the patient to be completely treated from mHAC (described
as the first case report). The treatment efficiency seems to be
mostly due to cisplatin as we could detect a recurrence on the
liver metastasis under oral etoposid in the case 2. As the maximal
dose of administrated cisplatin had to be taken into account, this
was preventing an additional cisplatin regimen in case 2. As
described in case 2, other chemotherapy regimens appeared as
inefficient as second and third lines treatment.
The review of literature is very limited and very
few articles concerning the treatment of mHACs were reported. Lots
of articles had to be excluded because no data regarding neither
the kind of used chemotherapy nor its response were described.
Therefore, these results had to be carefully interpreted and
analysed. Indeed, as it is only an analysis of retrospective cases
report, we cannot avoid some publication bias. In addition, the
dose and schedules of chemotherapy administration were often not
described in literature. Our results revealed a partial or complete
response in 11 cases reports whom 81% had a Cisplatin-based
regimen. Only one case, treated by cisplatin-etoposide-5-FU, can be
identified as an efficient treatment with a long follow-up.
In our sense, cisplatin is probably the most
interesting regimen to treat mHACs. These results were strongly
correlated with ex vivo studies. Indeed, in a xenograft model of
AFP-producing gastric cancer, mitomycin-C and cisplatin treatments
might be effective to induce suppression of tumor growth, whereas
5-FU, doxorubicin, and epirubicin were shown as inefficient
(13). In our review, two complete
responses have been observed with a cisplatin-based regimen or
mitomycin-C regimen. Other usual chemotherapies carried out in
digestive cancer as irinotecan, oxaliplatin, gemcitabine or 5-FU,
are appearing as inefficient in mHAC (Table II; cases 11, 13, 15 and 17).
Concerning targeted therapies, we do not possess
full information. We can suppose that sorafenib which is the
reference treatment in non-operable hepatocellular carcinoma could
be a treatment option in HAC since they have similar histological
features. An interesting progression free survival was reported in
our review (Table III). In
addition, an analysis of peritoneal mHAC in a 21-year-old man,
showed a strong activation of the epidermal growth factor, and of
the kinases ERK1 and AKT1 (14).
Performing genome sequencing could be interesting in order to
understand the molecular biology underlying mHAC (not described in
the present time). In our cases, histopathological material was old
or little biopsy materials, which is a strong limit to carry out a
next sequencing generation.
Concerning non-gastric mHAC, we have stated only 2/7
partial responses in a lung and mediastinum primitive, and no
objective responses in the two cases of colorectal HACs treated by
a combined therapy composed of FOLFOX (oxaliplatin and 5-FU) plus
bevacizumab, or FOLFIRI (irinotecan and 5-FU) plus bevacizumab
(Table II; cases 11 and 17,
respectively). No objective responses were related in a pancreatic
HAC treated by gemcitabine. Consequently, traditional regimens seem
to be not efficiency in mHAC. Cisplatin-based regimen seems to be
the best option in non-gastric primitive HAC. Moreover radical
surgery of primitive lesion or residual lesion could be ever
considered and discussed again in case of good and durable response
in mHACs as reported in the case 1.
In conclusion, HAC represents a very rare and
aggressive subtype of cancer defined as extrahepatic tumor with
hepatocyte differentiation and potentially an increased AFP
secretion. Currently, the treatment of mHAC remains not elucidated.
We reported two cases of patients with mHAC successfully treated by
chemotherapy with cisplatin and etoposide as first line leading to
complete response. One of them is still alive after more than 9
years. In the absence of prospective trials in this rare cancer, we
assume that cisplatin-based chemotherapy regimen could be the best
first-line treatment in mHAC and considering as well our real-life
experience as in view of literature, with 81% of response. Since
the data of genome-wide analysis in cancers, it might be useful to
provide some information with next generation sequencing in HAC, in
order to find efficient target therapies.
References
|
1
|
Qu BG, Bi WM, Qu BT, Qu T, Han XH, Wang H,
Liu YX and Jia YG: PRISMA-Compliant Article: Clinical
characteristics and factors influencing prognosis of patients with
hepatoid adenocarcinoma of the stomach in China. Medicine
(Baltimore). 95:e33992016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ishikura H, Ishiguro T, Enatsu C, Fujii H,
Kakuta Y, Kanda M and Yoshiki T: Hepatoid adenocarcinoma of the
renal pelvis producing alpha-fetoprotein of hepatic type and bile
pigment. Cancer. 67:3051–3056. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fornasa F: Soft-Tissue Localization of
Hepatoid Adenocarcinoma: First case report. Case Rep Oncol.
3:212–217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang J, Wang R, Zhang W, Zhuang W, Wang M
and Tang C: Clinicopathological and prognostic characteristics of
hepatoid adenocarcinoma of the stomach. Gastroenterol Res Pract.
2014:1405872014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagai E, Ueyama T, Yao T and Tsuneyoshi M:
Hepatoid adenocarcinoma of the stomach. A clinicopathologic and
immunohistochemical analysis. Cancer. 72:1827–1835. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiao C, Wu F, Jiang H, Teng L, Song F,
Wang Q and Yang H: Hepatoid adenocarcinoma of the stomach: Nine
case reports and treatment outcomes. Oncol Lett. 10:1605–1609.
2015.PubMed/NCBI
|
|
7
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate healthcare
interventions: Explanation and elaboration. BMJ. 339:b27002009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baek SK, Han SW, Oh DY, Im SA, Kim TY and
Bang YJ: Clinicopathologic characteristics and treatment outcomes
of hepatoid adenocarcinoma of the stomach, a rare but unique
subtype of gastric cancer. BMC Gastroenterol. 11:562011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimada S, Hayashi N, Marutsuka T, Baba Y,
Yokoyama S, Iyama K and Ogawa M: Irinotecan plus low-dose cisplatin
for alpha-fetoprotein-producing gastric carcinoma with multiple
liver metastases: Report of two cases. Surg Today. 32:1075–1080.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeyama H, Sawai H, Wakasugi T, Takahashi
H, Matsuo Y, Ochi N, Yasuda A, Sato M, Okada Y, Funahashi H, et al:
Successful paclitaxel-based chemotherapy for an
alpha-fetoprotein-producing gastric cancer patient with multiple
liver metastases. World J Surg Oncol. 5:792007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi T, Kochi M, Kanamori N, Kaiga T,
Funada T, Fujii M and Takayama T: Complete remission with FLEP
chemotherapy for multiple liver metastasis from
alpha-fetoprotein-producing gastric cancer-report of a case. Gan To
Kagaku Ryoho. 36:1885–1888. 2009.(In Japanese). PubMed/NCBI
|
|
12
|
Gavrancic T and Park YH: A novel approach
using sorafenib in alpha fetoprotein-producing hepatoid
adenocarcinoma of the lung. J Natl Compr Cancer Netw. 13:387–391.
2015. View Article : Google Scholar
|
|
13
|
Chang YC, Nagasue N, Kohno H, Ohiwa K,
Yamanoi A and Nakamura T: Xenotransplantation of
alpha-fetoprotein-producing gastric cancers into nude mice.
Characteristics and responses to chemotherapy. Cancer. 69:872–877.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Metzgeroth G, Ströbel P, Baumbusch T,
Reiter A and Hastka J: Hepatoid adenocarcinoma-review of the
literature illustrated by a rare case originating in the peritoneal
cavity. Onkologie. 33:263–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chiba N, Yoshioka T, Sakayori M, Mikami Y,
Miyazaki S, Akiyama S, Otsuka K, Yamaura G, Shibata H, Kato S, et
al: AFP-producing hepatoid adenocarcinoma in association with
Barrett's esophagus with multiple liver metastasis responding to
paclitaxel/CDDP: A case report. Anticancer Res. 25:2965–2968.
2005.PubMed/NCBI
|
|
16
|
Lin CW, Hsu CC, Chang HC, Sun YC, Sun PL,
Hsu CY and Perng DS: Hepatoid adenocarcinoma of the stomach with
liver metastasis mimicking hepatocellular carcinoma: A case report.
Cases J. 2:63172009.PubMed/NCBI
|
|
17
|
Gálvez-Muñoz E, Gallego-Plazas J,
Gonzalez-Orozco V, Menarguez-Pina F, Ruiz-Maciá JA and Morcillo MA:
Hepatoid adenocarcinoma of the stomach-a different histology for
not so different gastric adenocarcinoma: A case report. Int Semin
Surg Oncol. 6:132009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cappetta A, Bergamo F, Mescoli C, Lonardi
S, Rugge M and Zagonel V: Hepatoid adenocarcinoma of the colon:
What should we target? Pathol Oncol Res. 18:93–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye MF, Tao F, Liu F and Sun AJ: Hepatoid
adenocarcinoma of the stomach: A report of three cases. World J
Gastroenterol. 19:4437–4442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahn JS, Jeon JR, Yoo HS, Park TK, Park CK,
Sinn DH and Paik SW: Hepatoid adenocarcinoma of the stomach: An
unusual case of elevated alpha-fetoprotein with prior treatment for
hepatocellular carcinoma. Clin Mol Hepatol. 19:173–178. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Majumder S and Dasanu CA: Hepatoid variant
of pancreatic cancer: Insights from a case and literature review.
JOP. 14:442–445. 2013.PubMed/NCBI
|
|
22
|
Nagai Y, Kato T, Harano M, Satoh D, Choda
Y, Tokumoto N, Kanazawa T, Matsukawa H, Ojima Y, Idani H, et al: A
case of AFP-producing esophagogastric junction cancer with liver
metastases with a good response to chemotherapy. Gan To Kagaku
Ryoho. 41:2349–2351. 2014.(In Japanese). PubMed/NCBI
|
|
23
|
Chen Y, Schaeffer DF and Yoshida EM:
Hepatoid adenocarcinoma of the colon in a patient with inflammatory
bowel disease. World J Gastroenterol. 20:12657–12661. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu CH, Li QL, Li HP, Fan SQ, Zhang HX, Liu
XL, He Y, Huang M, Lu M, Wang SS and Wu F: Rare coexistence of
mediastinal hepatoid adenocarcinoma, idiopathic azoospermia and
horseshoe kidney: A case report and review of the literature. Int J
Clin Exp Pathol. 8:11741–11746. 2015.PubMed/NCBI
|
|
25
|
Karayiannakis AJ, Kakolyris S,
Giatromanolaki A, Courcoutsakis N, Bolanaki H, Chelis L, Sivridis E
and Simopoulos C: Hepatoid Adenocarcinoma of the Gallbladder: Case
Report and Literature Review. J Gastrointest Cancer. 43:139–144.
2012. View Article : Google Scholar
|
|
26
|
Petrelli F, Ghilardi M, Colombo S,
Stringhi E, Barbara C, Cabiddu M, Elia S, Corti D and Barni S: A
rare case of metastatic pancreatic hepatoid carcinoma treated with
sorafenib. J Gastrointest Cancer. 43:97–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mokrim M, Belbaraka R, Allaoui M,
Kairaouani M, Mahassini N, Tahri A and Errihani H: Hepatoid
Adenocarcinoma of the Lung: A Case Report and Literature Review. J
Gastrointest Cancer. 43:125–127. 2012. View Article : Google Scholar
|