Introduction
Gastric cancer (GC) is one of the most common types
of malignant tumors of the digestive system (1). Among the incident diagnosed cases of
cancer each year, GC ranked fourth, and ranked third in
cancer-associated mortalities in 2015 (1). Although the global incidence of GC has
declined slightly, it remains one of the most common malignant
tumors in China, with no notable decrease in mortality. The reason
may be attributed to the low early diagnosis rate of ~10% (the
majority of stomach cancer cases are diagnosed at late stage) and
the low 5-year survival rate (7–34%), leading to high morbidity and
mortality rates (2). There is
currently an urgent requirement to improve the survival rate of
advanced GC.
Folkman (3) first
confirmed that ‘both tumor growth and metastasis are dependent on
angiogenesis’. In 1997, Asahara et al (4) isolated cluster of differentiation
(CD)34+/vascular endothelial growth factor receptor
(VEGFR)-2+ endothelial progenitor cells (EPCs) from
peripheral blood by magnetic-activated cell sorting. EPCs are the
precursor cells of endothelial cells (ECs), which have a stronger
proliferative capacity compared with mature ECs and are involved in
tumor angiogenesis (5). It is
recognized that EPCs are initially primarily present in the bone
marrow, where they express CD34, CD133 (AC133) and kinase domain
insert receptor (KDR; also termed VEGFR-2 or Flk-1), but no CD144
(vascular endothelial-cadherin) or Von Willebrand factor (vWF).
Following the release of EPCs into the peripheral blood, CD133 is
not expressed, CD34 expression is gradually reduced and expression
of KDR continues (6). This is
associated with several mature EC markers, including CD144, CD31,
vWF and endothelial nitric oxide synthase, as well as low density
lipoprotein and Ulex europaeus agglutinin-1 (7). The identification of vWF may be a
landmark of the differentiation of EPCs into mature ECs (7).
Tumor growth, invasion and metastasis depend on the
formation of new tumor blood vessels, occurring by vasculogenesis
and angiogenesis (8). The former
refers to the in situ differentiation of EPCs into blood
vessels, while the latter refers to the formation of new blood
vessel branches and capillary plexus from the existing blood
vessels via budding (8). The two
processes are complementary. Tumor growth requires blood vessels to
maintain tumor cells; tumor volume is usually <3 mm3
when lacking new blood vessels (9).
Only tumors completing vascularization can achieve a rapid increase
in cell number and volume (10). The
stomach has abundant blood vessels, thus providing a good material
foundation for tumor growth, metastasis and invasion. Therefore,
studies on angiogenesis are necessary for understanding the growth,
metastasis, tumor infiltration and prognosis of gastric cancer.
Formation of tumor blood vessels is a continuous,
uncontrolled and complex multi-step process, including capillary
basement membrane degradation, endothelial cell migration,
proliferation, formation of a tubular structure, basement membrane
formation and blood flow patency, which is regulated by
angiogenesis-promoting factors and angiogenesis inhibitory factors
(11). Thus far, over 30 types of
angiogenic factors have been reported, and VEGF, considered the
most important and potent one among them, can promote the division,
proliferation, migration and vascular construction of ECs in
vivo (12). VEGF is highly
expressed in numerous types of malignant tumors such as ovarian and
prostatic cancer and gastrointestinal adenocarcinomas (13–15), and
its overexpression is considered to be associated with increased
angiogenesis, proliferation and metastasis (16,17).
Tumor angiogenesis can be assessed quantitatively by
microvessel density (MVD), which is calculated as the number of
microvessels per unit area using specific antibodies (such as VIII
factor antibody, CD31 and CD34) to label vascular ECs by an
immunohistochemical method (18).
CD34 is the most sensitive tumor blood vessel marker. Numerous
malignant tumors have a significantly larger MVD when compared with
normal tissue, and tumors with a higher MVD are also prone to
metastasis, recurrence and poor prognosis (19). MVD has become an important indicator
for forecasting tumor metastasis, recurrence and prognosis
(20). It has been reported that MVD
is a good indicator of prognosis in gastric cancer, particularly
for early-stage gastric cancer. VEGF and MVD can be prognostic
factors for GC (21). Kido et
al (22) investigated VEGF
expression in GC tissues, and showed that high expression of VEGF
is associated with poor prognosis. In addition, they identified
that the VEGF-positive tissues have a significantly larger MVD
value compared with VEGF-negative tissues.
A previous study demonstrated that EPCs are more
likely to be involved in tumor angiogenesis than in common
granulation tissue- and growth factor-mediated angiogenesis, which
can account for 5–25% of new blood vessels in common tissues, and
as much as 35–45% in tumors (16).
Whether growth, metastasis and invasion of gastric tumor cells
depend on EPC mobilization and incorporation into the tumor
vasculature has not yet been elucidated. In the present study,
through measuring the number of EPCs and ECs in the peripheral
blood of patients with GC, detecting VEGF expression and
calculating MVD value in GC tissue, the association between either
EPCs or ECs and tumor growth, angiogenesis, invasion and metastasis
was investigated, aiming to provide a basis for GC diagnosis,
prognosis and targeted therapy.
Materials and methods
Patients and clinicopathological
classification
Between December 2008 and March 2011, a total of 42
patients with GC confirmed by pathological examination who were
subject to surgical resection at Shanghai Gongli Hospital were
recruited in the present study (approved by the Ethical Committee
of Shanghai Gongli Hospital, Secondary Military Medical University,
Shanghai, China). Written informed consent was obtained from each
patient prior to the study. Patients were excluded if they met any
of the following criteria: Diagnosis with any other severe
syndrome, such as upper gastrointestinal bleeding and diffuse
peritonitis; taking non-steroidal anti-inflammatory drugs,
corticosteroids or statins; history of acute myocardial infarction,
angina pectoris, limb ischemia, trauma or surgery; or if the
patient had received chemotherapy and radiotherapy. Finally, 42
patients with GC, with a mean age of 55.2±1.8 years, including 28
males (66.7%) and 14 females (33.3%) were enrolled. The tumor size
and depth (T) of primary lesion, and lymph node metastasis status
(N) were determined by a pathologist, and whether there was a
distant metastasis (M) was determined by pathological examination.
The tumor diameter was <5 cm in 29 cases and ≥5 cm in 13 cases.
Tumors had not invaded into the serosa in 11 cases and had
penetrated into the serosa in 31 cases. Tumors were highly
differentiated in 14 cases, moderately differentiated in 15 cases
and poorly differentiated in 13 cases. Local lymph node metastasis
was detected in 20 cases and not in 22 cases, while distant
metastasis was detected in 8 cases and not in 34 cases. According
to the tumor-node-metastasis (TNM) staging system developed by the
Union for International Cancer Control (6th edition; 2002)
(23), 9 cases were at stage I, 15
cases at stage II, 10 at stage III and 8 cases at stage IV. The
normal gastric tissues adjacent to gastric tumor tissue were also
collected as a control. All the pathological specimens were fixed
in 10% neutral formalin at room temperature overnight once
collected.
Immunohistochemical staining and
scoring
For tissues collected from each case, one
paraffin-embedded section was prepared for routine hematoxylin and
eosin (H&E) staining, and the rest were used for
immunohistochemical staining.
All the collected tissues were first fixed in 10%
neutral formalin at room temperature overnight, embedded in
paraffin and finally prepared into 4-µm thick serial sections.
Subsequently, the sections were heated at 60°C for 60 min, and then
were dewaxed using xylene (purity, >95%) twice for 10 min each
time, following by de-benzolization and hydration in serial alcohol
for 5 min (100, 95, 80 and 70%). H&E staining was performed as
follows: The sections were stained with 0.5 g/100 ml of hematoxylin
for 10 min to stain the nuclei at room temperature, followed by
color separation using 1% hydrochloric acid solution and 1% ammonia
for 5 sec. Subsequent to washing under tap water for 1 h, the
sections were briefly immersed in distilled water, followed by
dehydration in 70 and 90% ethanol successively for 10 min each,
followed by cytoplasmic staining using ethanol eosin (0.5 g/100 ml)
for 3 min at room temperature. Finally, the stained sections were
dehydrated using absolute ethyl alcohol, xylene clearing and
mounting. When the gum became somewhat dry, the sections were
labelled. VEGF and CD34 expression in gastric carcinoma and normal
gastric tissues was detected by the streptomycin avidin-peroxidase
(SP) method using S-P kits (Fuzhou Maixin Biotech Co., Ltd.,
Fuzhou, China) following the manufacturer's protocol (24). Primary rabbit anti-human monoclonal
antibody against VEGF (cat. no. MAB-0243; Fuzhou Maixin Biotech
Co., Ltd.) was diluted 100 times, and was incubated at the room
temperature for 60 min with known colorectal cancer-positive
sections as a positive control. In addition, primary mouse
anti-human antibody against CD34 (cat. no. MAB-0034-P, Fuzhou
Maixin Biotech Co., Ltd.) was diluted 200 times, and was incubated
at 4°C overnight. Known GC-positive sections were used as a
positive control, and PBS buffer instead of primary antibody was
used for the negative control. Subsequently, 50 µl of biotin
labeling sheep anti-rabbit or rabbit anti-mouse secondary
antibodies (cat. no. KIT-9706 or KIT-9710; 1:1,000; Fuzhou Maixin
Biotech Co., Ltd.) were added into the above sections at the room
temperature for 10 min, respectively. Finally, VEGF and
CD34-positive cells in the sections were stained using 100 µl DAB
for 3–10 min at room temperature. Degree of histological
differentiation was then determined under the light microscope
(BX41; Olympus Corporation, Tokyo, Japan) at ×400
magnification.
VEGF-positive cells in tumor tissue were stained
brown-yellow or brown in the cytoplasm. The tissue sections were
scored according to the staining intensity and positive cell
proportion. Staining intensity was scored as follows: Negative, 0;
weakly-positive, 1; positive, 2; and strongly-positive, 3. No
positive cells was scored as 0; containing <25% of positive
cells as 1, containing 25–50% of positive cells as 2 and containing
>50% of positive cells as 3. For each section, the final score
was calculated as the sum of the staining intensity and positive
cells proportion, with a final score of 0–3 as VEGF-negative, and
3–6 as VEGF-positive.
MVD calculation
The tumor MVD was calculated by the method presented
by Weidner with certain modifications (25). First, five fields with the highest
blood vessels densities were identified at ×100 magnification under
a light microscope; microvessels were then counted on the monitor
of a computer image analysis system (Image Pro Plus 4; Media
Cybernetics, Rockville, MD, USA) under a light microscope at ×200
magnification, and the mean microvessel number was calculated as
the MVD value. No matter whether a tube was formed, any single EC
or multiple ECs arranged compactly were assumed to be a blood
vessel. When a tubular structure was not continuous phase, its
branches were also viewed as a blood vessels. Cells that were
difficult to distinguish or dimly stained, as well as thick-walled
muscular vessels or lumen of >50 µm (equivalent to ≥1 cm at
magnification, ×200) were not counted.
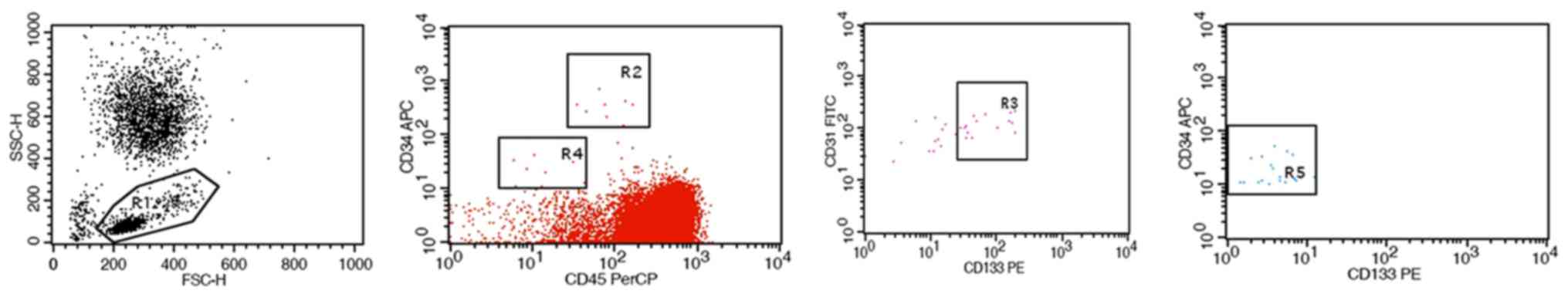
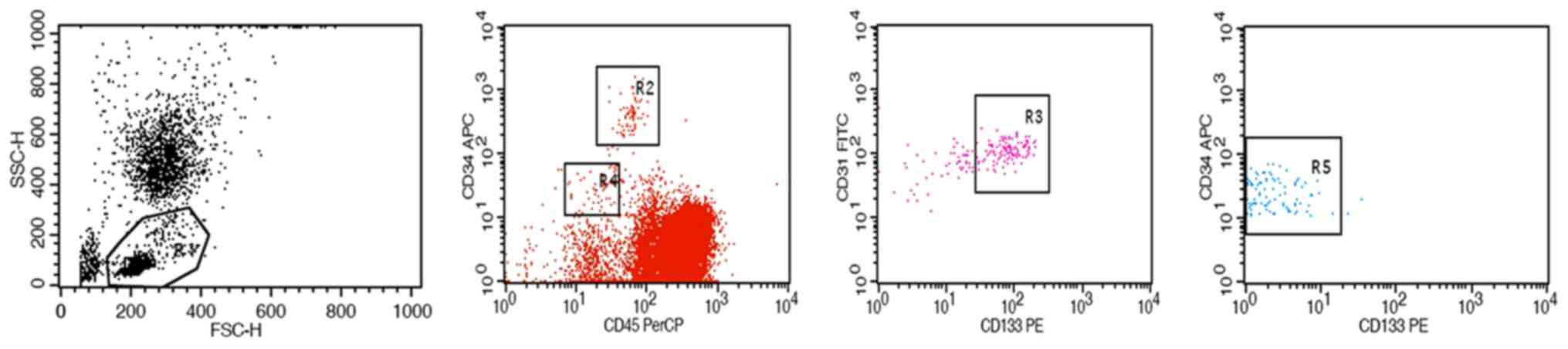
Detection of EPCs/ECs in peripheral
blood
For each patient, 6 ml peripheral blood with added
anticoagulant was collected, followed by determination of the
number of EPCs and ECs by flow cytometry (BD Biosciences, Franklin
Lakes, NJ, USA) (26). In brief, EPCs
were defined as
CD34brightCD133+CD31+CD45dim
cells, and ECs were defined as
CD34dimCD133−CD31brightCD45−cells,
as described previously (24).
Statistical analysis
SPSS19.0 software (IBM Corp., Armonk, NY, USA) was
used for statistical analysis. Measurement data were denoted as
mean ± standard deviation, and the nominal data were denoted as
percentages. The difference in measurement data between two
independent samples was determined using Student's t-test, and that
between two sets of categorical data was determined using the
χ2 test. The correlation between EPCs/ECs and VEGF/MVD
was investigated by Spearman's correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Association between EPCs/ECs and
clinicopathological factors in GC
The number of EPCs and ECs gradually increased in
patients with GC between stages I and III, with stages II and III
each have a significantly higher number of EPCs and ECs compared
with stage I (P<0.05). The number of EPCs in stage IV patients
was reduced, while there were significantly more ECs than in either
stage I, II or III patients (P<0.05; Figs. 1 and 2).
There were significantly fewer EPCs in patients with
GC with tumors that had not invaded into serosa compared with EPCs
in patients with GC with tumors that had invaded into the serosa
(P<0.01), while there were significantly more ECs in patients
with tumors that had not invaded into serosa compared with ECs in
patients with GC with tumors that had invaded into serosa
(P<0.01). The number of EPCs in patients with distant metastasis
was significantly smaller than that in patients without distant
metastasis (P<0.01), while the number of ECs in patients with
distant metastasis was significantly larger than that in patients
without distant metastasis (P<0.01). There were significantly
more EPCs and ECs in patients with lymph node metastasis compared
with those in patients without lymph node metastasis (P<0.05).
No significant association was observed between the EPC/EC ratio
and either sex, age, tumor size or differentiation degree
(P>0.05; Table I).
 | Table I.Association between either EPCs/ECs,
VEGF expression or MVD and clinicopathological factors in gastric
cancer. |
Table I.
Association between either EPCs/ECs,
VEGF expression or MVD and clinicopathological factors in gastric
cancer.
|
|
|
|
| VEGF
expression |
|
|---|
|
|
|
|
|
|
|
|---|
| Clinicopathological
factors | Patients, n | EPCs/mononuclear
cells | ECs/mononuclear
cells | VEGF+
(n=28) | VEGF-(n=14) | MVD |
|---|
| Age, years |
|
|
| 58.179±11.112 | 56.643±10.043 |
|
|
≤60 | 32 |
0.038±0.030 |
0.181±0.072 | 22 (68.75) | 10 (31.25) |
33.5±6.0 |
|
>60 | 10 |
0.030±0.017 |
0.111±0.091 | 6
(60.00) | 4
(40.00) |
30.1±4.5 |
| Sex |
|
|
|
|
|
|
|
Male | 28 |
0.041±0.024 |
0.146±0.084 | 18 (64.29) | 10 (35.71) |
30.7±4.9 |
|
Female | 14 |
0.047±0.001 |
0.133±0.002 | 10 (71.43) | 4
(28.57) |
31.2±4.8 |
| Tumor size, cm |
|
|
|
|
|
|
| ≤5 | 29 |
0.029±0.022 |
0.138±0.090 | 20 (68.97) | 9
(31.03) |
31.5±6.4 |
|
>5 | 13 |
0.056±0.030 |
0.198±0.031 | 8
(61.54) | 5
(38.46) |
29.7±5.2 |
| Histological
type |
|
|
|
|
|
|
| Highly
differentiated | 14 |
0.032±0.020 |
0.110±0.059 | 8
(57.14) | 6
(42.86) |
31.0±7.5 |
|
Moderately differentiated | 15 |
0.043±0.027 |
0.141±0.071 | 10 (66.67) | 5
(33.33) |
29.8±4.5 |
| Poorly
differentiated | 13 |
0.031±0.032 |
0.272±0.077 | 10 (76.92) | 3
(23.08) |
30.6±5.1 |
| Invasion depth |
|
|
|
|
|
|
|
Sub-serosa | 11 |
0.057±0.020 |
0.120±0.002 | 3
(27.27) | 8
(72.73) |
29.4±4.2 |
|
Serosa | 31 |
0.030±0.021a |
0.150±0.088a | 25
(80.65)a | 6
(19.35) |
33.8±4.4b |
| Lymph node
metastasis |
|
|
|
|
|
|
| No | 22 |
0.030±0.023 |
0.133±0.067 | 10 (45.45) | 12 (54.55) |
25.8±5.7 |
|
Yes | 20 |
0.040±0.020b |
0.181±0.070b | 18
(90.00)a | 2
(10.00) |
32.2±4.1a |
| Distant
metastasis |
|
|
|
|
|
|
| No | 34 |
0.040±0.026 |
0.136±0.072 | 22 (64.71) | 12 (35.29) |
30.3±7.5 |
|
Yes | 8 |
0.011±0.0002a |
0.297±0.002a | 6
(75.00) | 2
(25.00) |
32.4±8.9 |
| TNM staging |
|
|
|
|
|
|
| I | 9 |
0.023±0.010 |
0.085±0.058 | 4
(44.44) | 5
(55.56) |
28.1±4.7 |
| II | 15 |
0.043±0.019b |
0.168±0.036b | 9
(60.00)b | 6
(40.00) |
29.4±4.6a |
|
III | 10 |
0.049±0.039b |
0.142±0.107b | 8
(80.00)b | 2
(20.00) |
36.9±4.3a |
| IV | 8 |
0.011±0.001 |
0.298±0.001b | 7
(87.50)b | 1
(12.50) |
38.8±4.0a |
Association between VEGF and
clinicopathological factors in GC
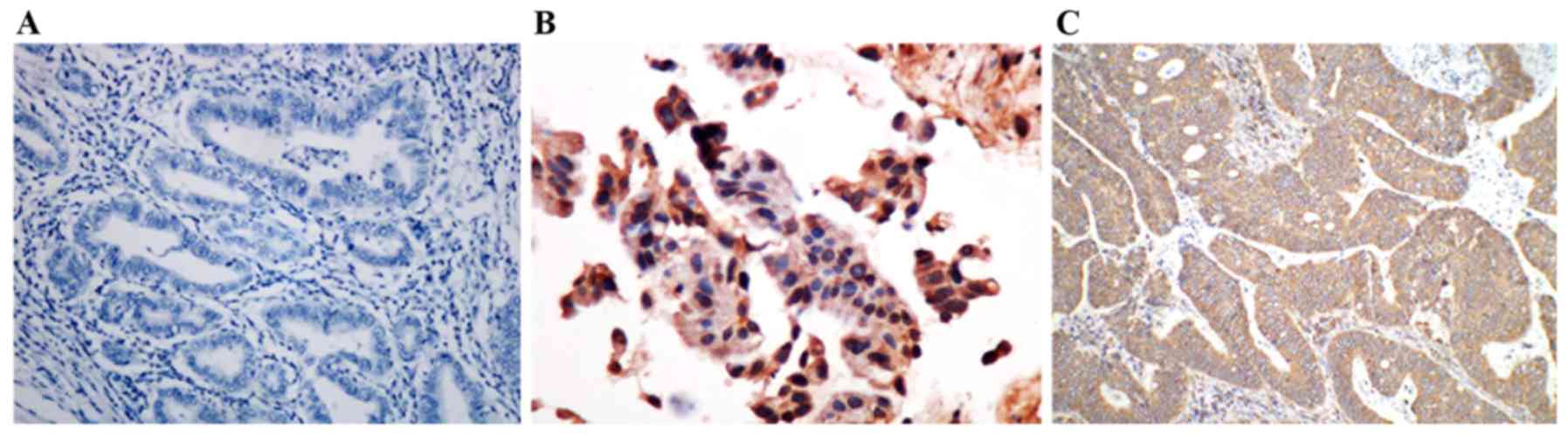
VEGF expression was mainly observed in tumor cell
cytoplasm, and also somewhat in the nuclei. VEGF was not expressed
in the majority of normal gastric mucosal epithelia (Fig. 3A). Comparatively, VEGF-positive cells
were identified in tumors in 66.67% of patients with GC (Fig. 3B).
VEGF-positive cells accounted for 44, 60, 80 and
87.5% in patients with GC at TNM stages I, II, III and IV,
respectively, with significant differences between each other
(P<0.05). VEGF-positive cells increased significantly from
27.27% in patients with GC with tumors that had not invaded into
the serosa to 80.65% in patients with GC with tumors that had
invaded into the serosa (P<0.01). The VEGF expression rate in
patients with GC with lymph node metastasis was 90%, which was
significantly higher than that in patients without lymph node
metastasis (45.45%) (P<0.01). VEGF expression level was not
significantly associated with the age, sex, tumor size,
histological grade or distant metastasis of patients (P>0.05)
(Table I).
Association between VEGF and
clinicopathological factors in GC
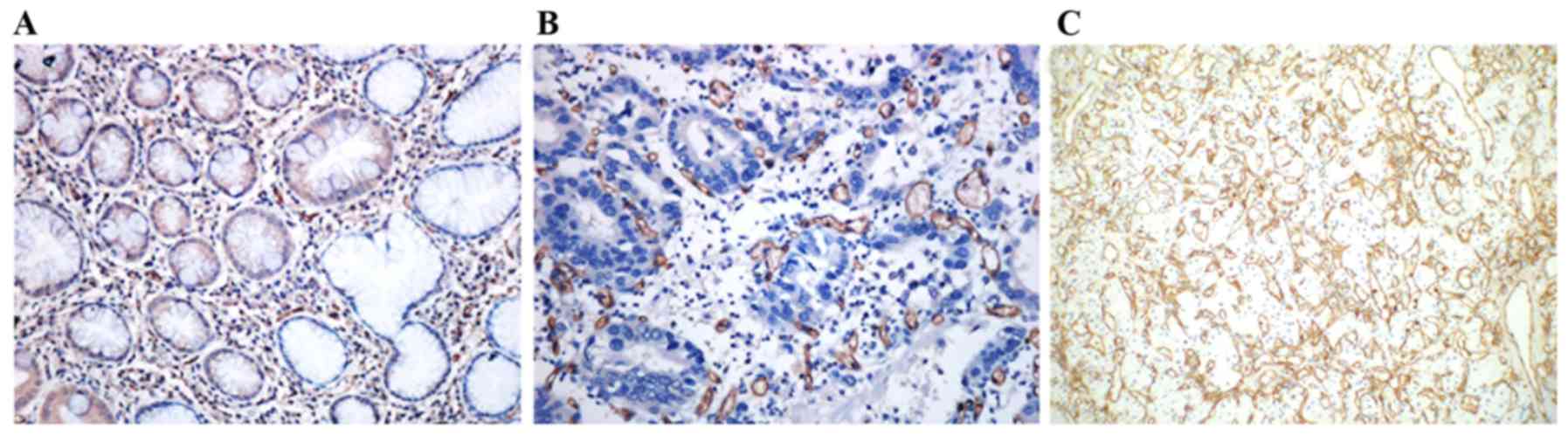
CD34 was expressed in the majority of capillaries.
Areas with the highest CD34 density were mostly the leading edges
infiltrated by tumor cells. The vascular endothelial cells were
evenly stained in tumor tissues, and the new vessels were expanded,
narrowed or deformative. Microvascular distribution was
heterogeneous, with disordered branches, and the vascular
endothelial cells had varied shapes. The MVD (34.48±5.96) was
significantly larger than that of normal gastric tissues adjacent
to tumor tissues (17.76±5.63) (P<0.01; Fig. 4A and B). MVD was significantly
associated with TNM stage, invasion depth and lymph node metastasis
in GC (P<0.01 or P<0.05), while not with the age, sex, tumor
size or histological type of the patients (P>0.05; Table I).
Correlation between EPCs/ECs and
VEGF/MVD in gastric cancer tissues
Both the EPC and EC number were positively
correlated with VEGF level in gastric cancer tissues (P<0.01),
with correlation coefficients of 0.535 and 0.433, respectively. The
number of EPCs was positively correlated with MVD (P<0.05;
r=0.332), and the number of ECs was positively correlated with MVD
(P<0.01; r=0.669).
Discussion
The present study determined the numbers of EPCs and
ECs in the peripheral blood, and also detected MVD distribution and
VEGF expression in gastric tissues.
Although EPCs perform an important role in tumor
angiogenesis, their role in gastric cancer angiogenesis remains
unclear. Furthermore, there are few studies on EPCs in patient
peripheral blood. Kim et al (27) reported no increase in the number of
circulating EPCs in patients with cancer (including GC), although
the plasma VEGF level was found to be elevated. However, a
significant increase was found in EPC number during tumor
angiogenesis (28). The present study
revealed that the number of EPCs and ECs in patients with stage III
GC was higher than the numbers in stage I and II patients. The
number of EPCs in stage IV patients was reduced, while the number
of ECs was significantly increased compared with those in either
stage I, II or III patients, which is consistent with our previous
findings (29). Furthermore, the
number of EPCs decreased in patients with tumors that had not
invaded into the serosa or in those without distant metastasis
compared with that in those patients with tumors that had
penetrated into the serosa or with distant metastasis EPCs, while
the number of ECs increased; this is consistent with previous
findings that ECs are involved in the invasion of tumors into the
serosa or in an angiogenesis occurring during a distant metastasis
(30), not EPCs in angiogenesis
(31). The number of EPCs and ECs in
patients with lymph node metastasis was significantly increased
compared with that in patients without lymph node metastasis,
indicating that EPCs may be involved in GC lymph node metastasis,
which requires validation. In addition, invasion of tumors into the
serosa and/or distant metastasis was observed in the majority of
patients with stage III and/or stage IV GC. Therefore, considering
the aforementioned factors, it was hypothesized that EPCs are
involved in angiogenesis at stages I and II, ECs and EPCs are
involved in angiogenesis at stage III, and ECs may be the main cell
type involved in angiogenesis at stage IV. The role of EPCs was
found to vary following release into the blood and differentiation
into ECs at different clinical stages of GC, and EPCs were also
found to be involved in angiogenesis in varied ways.
In the present study, it was also observed that EPCs
are found less in the peripheral blood of patients with stage IV GC
compared with other stages, and thus, it was speculated that EPCs
are more likely to gather around the tumor tissues to differentiate
into ECs. It is currently known that recruitment of EPCs is closely
associated with hypoxia, angiogenesis-associated factors and cell
adhesion molecules (32). Studies
using embryonic EPC tumor angiogenesis show higher MVD around the
tumor and more EPCs homing to the tumor surroundings. EPC adhesion
receptors associated with vascular endothelial adhesion molecules
and laminin are highly expressed in the tumor periphery. As solid
tumors grow, a relative hypoxic microenvironment is produced,
promoting the secretion of growth factors, including VEGF and
platelet-derived growth factor-BB, which promote the activation of
EPCs and mobilization in the bone marrow, and then migrate into the
ischemic tumor tissues (33). In
addition, these factors also activate matrix metalloproteinases
(MMPs), particularly MMP-9, inducing the release of a soluble kit
ligand, accordingly facilitating the proliferation and emigration
of EPCs from the bone marrow microenvironment (34). Thus, it is inferred that the decrease
in EPCs in the peripheral blood of patients with stage IV GC is
possibly due to an increased number of EPCs gathering around the
tumors.
In the present study, VEGF expression was found to
be significantly associated with MVD, TNM stage, invasion depth and
lymph node metastasis in GC, while not with age, sex, histological
type and tumor size, which is consistent with previous data
(35). Furthermore, VEGF was
positively associated with either EPC or EC level, indicating that
VEGF, as a major angiogenesis regulator, promotes the involvement
of EPCs in GC occurrence and development. EPC or EC levels, VEGF
expression and MVD value are associated with angiogenesis, tumor
growth, invasion and metastasis in GC.
EPCs have demonstrated their promising value as
tumor diagnosis markers in renal cell carcinoma (36) and lung adenocarcinoma (37). It has been found that adrenomedullin
receptor antagonists achieve their targeted therapy of pancreatic
and kidney tumors in mice via inhibition of the mobilization of
tumor endothelial cells and EPCs (38). As more becomes known about EPCs and
angiogenesis-associated angiogenic factors, endogenous angiogenesis
inhibitory factors and synthetic exogenous angiogenesis inhibitors,
angiogenesis inhibition therapy may be a promising anticancer
treatment. Therefore, a full understanding of the important role of
EPCs in the angiogenesis of GC occurrence, development and
metastasis will provide a novel insight in anti-angiogenic therapy
against GC. Our future studies will investigate factors effecting
the mobilization, migration and differentiation of EPCs at
different clinical stages.
Acknowledgements
The present study was supported by the Shanghai
Pudong New Area Health and Family Planning Commission Project
(PW2015A-16), the Shanghai Pudong New Area Health and Family
Planning Commission Project (PW2015A-18), the Academic Leader
Training Program of Pudong Health Bureau of Shanghai (PWRd2014-02)
and the Shanghai Sailing Program (15YF1410800).
References
|
1
|
Rugge M, Fassan M and Graham DY:
Epidemiology of gastric cancerGastric Cancer. Strong V: Springer;
Cham: pp. 23–34. 2015, View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Basile DP and Yoder MC: Circulating and
tissue resident endothelial progenitor cells. J Cell Physiol.
229:10–16. 2014.PubMed/NCBI
|
|
6
|
Ishige-Wada M, Kwon SM, Eguchi M, Hozumi
K, Iwaguro H, Matsumoto T, Fukuda N, Mugishima H, Masuda H and
Asahara T: Jagged-1 signaling in the bone marrow microenvironment
promotes endothelial progenitor cell expansion and commitment of
CD133+ human cord blood cells for postnatal vasculogenesis. PLoS
One. 11:e01666602016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salven P, Mustjoki S, Alitalo R, Alitalo K
and Rafii S: VEGFR-3 and CD133 identify a population of CD34+
lymphatic/vascular endothelial precursor cells. Blood. 101:168–172.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Folkman J, Browder T and Palmblad J:
Angiogenesis research: Guidelines for translation to clinical
application. Thromb Haemost. 86:23–33. 2001.PubMed/NCBI
|
|
9
|
Koch S, van Meeteren LA, Morin E, Testini
C, Weström S, Björkelund H, Le Jan S, Adler J, Berger P and
Claesson-Welsh L: NRP1 presented in trans to the endothelium
arrests VEGFR2 endocytosis, preventing angiogenic signaling and
tumor initiation. Dev Cell. 28:633–646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kushner EJ and Bautch VL: Building blood
vessels in development and disease. Curr Opin Hematol. 20:231–236.
2013.PubMed/NCBI
|
|
11
|
Szala S and Jarosz M: Tumor blood vessels.
Postepy Hig Med Dosw (Online). 65:437–446. 2011.(In Polish).
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Olson TA, Mohanraj D, Carson LF and
Ramakrishnan S: Vascular permeability factor gene expression in
normal and neoplastic human ovaries. Cancer Res. 54:276–280.
1994.PubMed/NCBI
|
|
14
|
Brown LF, Berse B, Jackman RW, Tognazzi K,
Manseau EJ, Senger DR and Dvorak HF: Expression of vascular
permeability factor (vascular endothelial growth factor) and its
receptors in adenocarcinomas of the gastrointestinal tract. Cancer
Res. 53:4727–4735. 1993.PubMed/NCBI
|
|
15
|
Joseph IB, Nelson JB, Denmeade SR and
Isaacs JT: Androgens regulate vascular endothelial growth factor
content in normal and malignant prostatic tissue. Clin Cancer Res.
3:2507–2511. 1997.PubMed/NCBI
|
|
16
|
Takahashi Y, Kitadai Y, Bucana CD, Cleary
KR and Ellis LM: Expression of vascular endothelial growth factor
and its receptor, KDR, correlates with vascularity, metastasis, and
proliferation of human colon cancer. Cancer Res. 55:3964–3968.
1995.PubMed/NCBI
|
|
17
|
Salven P, Ruotsalainen T, Mattson K and
Joensuu H: High pre-treatment serum level of vascular endothelial
growth factor (VEGF) is associated with poor outcome in small-cell
lung cancer. Int J Cancer. 79:144–146. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:9–19.
1995.PubMed/NCBI
|
|
19
|
Joo HJ, Oh DK, Kim YS, Lee KB and Kim SJ:
Increased expression of caveolin-1 and microvessel density
correlates with metastasis and poor prognosis in clear cell renal
cell carcinoma. BJU Int. 93:291–296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cavallaro U and Christofori G: Molecular
mechanisms of tumor angiogenesis and tumor progression. J
Neurooncol. 50:63–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Erenoglu C, Akin ML, Uluutku H, Tezcan L,
Yildirim S and Batkin A: Angiogenesis predicts poor prognosis in
gastric carcinoma. Dig Surg. 17:581–586. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kido S, Kitadai Y, Hattori N, Haruma K,
Kido T, Ohta M, Tanaka S, Yoshihara M, Sumii K, Ohmoto Y and
Chayama K: Interleukin 8 and vascular endothelial growth
factor-prognostic factors in human gastric carcinomas? Eur J
Cancer. 37:1482–1487. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wittekind C, Compton CC, Greene FL and
Sobin LH: TNM residual tumor classification revisited. Cancer.
94:2511–2516. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
An FQ, Matsuda M, Fujii H and Matsumoto Y:
Expression of vascular endothelial growth factor in surgical
specimens of hepatocellular carcinoma. J Cancer Res Clin Oncol.
126:153–160. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:9–19.
1995.PubMed/NCBI
|
|
26
|
Duda DG, Cohen KS, Scadden DT and Jain RK:
A protocol for phenotypic detection and enumeration of circulating
endothelial cells and circulating progenitor cells in human blood.
Nat Protoc. 2:805–810. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim HK, Song KS, Kim HO, Chung JH, Lee KR,
Lee YJ, Lee DH, Lee ES, Kim HK, Ryu KW and Bae JM: Circulating
numbers of endothelial progenitor cells in patients with gastric
and breast cancer. Cancer Lett. 198:83–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahn JB, Rha SY, Shin SJ, Jeung HC, Kim TS,
Zhang X, Park KH, Noh SH, Roh JK and Chung HC: Circulating
endothelial progenitor cells (EPC) for tumor vasculogenesis in
gastric cancer patients. Cancer Lett. 288:124–132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li BJ, Cao FF, Xu LM, Nie ZH, Wei TX,
Zhang LG and Zhang DH: Endothelial progenitor cells and endothelial
cells in gastric cancer. Shanghai Med J. 34:467–469. 2011.(In
Chinese).
|
|
30
|
Folkman J, Browder T and Palmblad J:
Angiogenesis research: Guidelines for translation to clinical
application. Thromb Haemost. 86:23–33. 2001.PubMed/NCBI
|
|
31
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin H, Aiyer A, Su J, Borgstrom P, Stupack
D, Friedlander M and Varner J: A homing mechanism for bone
marrow-derived progenitor cell recruitment to the neovasculature. J
Clin Invest. 116:652–662. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Francavilla C, Maddaluno L and Cavallaro
U: The functional role of cell adhesion molecules in tumor
angiogenesis. Semin Cancer Biol. 19:298–309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chakroborty D, Sarkar C, Basu B, Dasgupta
PS and Basu S: Catecholamines regulate tumor angiogenesis. Cancer
Res. 69:3727–3730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng H, Takahashi H, Murai Y, Cui Z,
Nomoto K, Niwa H, Tsuneyama K and Takano Y: Expressions of MMP-2,
MMP-9 and VEGF are closely linked to growth, invasion, metastasis
and angiogenesis of gastric carcinoma. Anticancer Res,.
26:3579–3583. 2006.
|
|
36
|
Yang B, Gu W, Peng B, Xu Y, Liu M, Che J,
Geng J and Zheng J: High level of circulating endothelial
progenitor cells positively correlates with serum vascular
endothelial growth factor in patients with renal cell carcinoma. J
Urol. 188:2055–2061. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maeda R, Ishii G, Ito M, Hishida T,
Yoshida J, Nishimura M, Haga H, Nagai K and Ochiai A: Number of
circulating endothelial progenitor cells and intratumoral
microvessel density in non-small cell lung cancer patients:
Differences in angiogenic status between adenocarcinoma histologic
subtypes. J Thorac Oncol. 7:503–511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsuchiya K, Hida K, Hida Y, Muraki C, Ohga
N, Akino T, Kondo T, Miseki T, Nakagawa K, Shindoh M, et al:
Adrenomedullin antagonist suppresses tumor formation in renal cell
carcinoma through inhibitory effects on tumor endothelial cells and
endothelial progenitor mobilization. Int J Oncol. 36:1379–1386.
2010.PubMed/NCBI
|