Introduction
Although the morbidity of malignant melanoma only
contributes to 1% of skin neoplasms, its mortality rate is the
highest of all skin neoplasms, mainly due to its high potential to
metastasize to vital organs in 2010 in China (1). Malignant melanoma accounted for 80% of
skin neoplasm-associated mortalities in 2012 in China (1,2). There is
a rising trend in the number of melanoma cases worldwide (3). Among the industrial cities in South
China, the morbidity of melanoma is much higher compared with the
Chinese average rate, due to complex environmental factors
(4).
Gupta recently reported that HOX transcript
antisense RNA (HOTAIR) performs an important role in breast cancer
metastasis with high expression in metastatic breast cancer tissues
(5). In addition, high HOTAIR
expression in primary breast cancer tissues can be used to predict
tumor metastasis and mortality with a good accuracy (6). These studies indicated that long
non-coding RNAs, such as HOTAIR, are involved in tumor formation
and progression (6).
Propofol is a fast and short-acting intravenous
anesthetic, which is widely used in the induction and maintenance
of various clinical surgeries (7,8). In
addition, since propofol is also used in intensive care patients
and with other clinical sedative treatment (9). Propofol has a number of characteristics
that make it advantageous as an anesthetic, including taking effect
quickly, short metabolic time, easy control of narcosis and slight
adverse reactions. However, propofol has a number of non-narcotic
effects (10), for example,
sub-hypnotic doses of propofol cause forgetfulness and inhibit
anxiety. When used for anesthetic introduction and maintenance,
propofol can reduce the incidence of nausea and vomiting following
surgery (11). Furthermore, small
doses of propofol can be directly used to treat nausea and vomiting
following surgery. Propofol can adjust the activity of cytotoxic T
lymphocytes to increase their antitumor activity and immune
regulation (12). In the present
study, the anticancer effects of propofol on cell apoptosis of
melanoma cells were investigated, and the underlying mechanism was
investigated.
Materials and methods
Cell culture
The murine melanoma B16F10 cell line was purchased
from the Cell bank of Chinese Academy of Sciences (Shanghai,
China). B16F10 cells were cultured in complete growth RPMI-1640
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented 10% heat-inactivated fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a humidified 5%
CO2 atmosphere.
Cell proliferation analysis by MTT
assay
Cell proliferation of murine melanoma B16F10 cells
was determined by MTT assay kit (Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer's
protocol. B16F10 cells (1×104) were plated on 96-well
culture plates and co-cultured with different concentrations (0–10
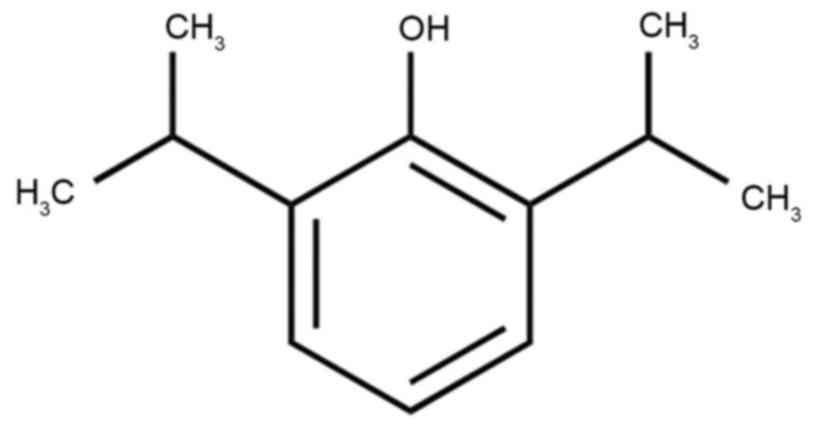
µM) of propofol (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany;
Fig. 1) for 24 or 48 h at 37°C. MTT
stock solution (20 µl; 5 mg/ml) was supplemented and cultured at
37°C in a humidified 5% CO2 atmosphere for 4 h. Dimethyl
sulfoxide (200 µl; Invitrogen; Thermo Fisher Scientific, Inc.) was
added to dissolve the precipitate. Absorbance was detected at 570
nm by Multiskan Spectrum (Thermo Fisher Scientific, Inc.).
Detection of cell apoptosis
B16F10 cells (1×106) were plated on
6-well culture plates and co-cultured with different concentrations
(0–10 µM) of propofol (Sigma-Aldrich; Merck KGaA) for 24 or 48 h at
37°C. Apoptosis of murine melanoma B16F10 cells was determined
using Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide
(PI) (Vybrant; Invitrogen; Thermo Fisher Scientific, Inc.). Annexin
V-FITC (100 µl) and 5 µl PI were added to each well for 15 min at
room temperature and analyzed by flow cytometry (FACSCanto II; BD
Biosciences, Franklin Lakes, NJ, USA), using FlowJo software 7.6.1
(Tree Star, Inc., Ashland, OR, USA).
B16F10 cells (1×104) were plated on
96-well culture plates and co-cultured with different
concentrations (0–10 µM) of propofol (Sigma-Aldrich; Merck KGaA;
Fig. 1) for 24 or 48 h at 37°C.
Caspase-3 substrate Ac-DEVD-pNA (10 µl; cat. no. C1115; Beyotime
Institute of Biotechnology) was added to each well of a 96-well
plate for 6 h at 37°C. The control group was the equivalent B16F10
cells treated with vehicle. The caspase-3 activity was detected at
405 nm by Multiskan Spectrum (Thermo Fisher Scientific, Inc.).
Plasmid constructs and
transfection
The pcDNA 3.1(−)-HOTAIR and pcDNA 3.1(−)-control
plasmids were prepared by Sangon Biotech Co. Ltd. (Shanghai,
China). The pcDNA 3.1(−)-HOTAIR (50 ng) and pcDNA 3.1(−)-control
plasmids (50 ng) were transfected using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Experiments were conducted following
transfection for 48 h in vitro at 37°C.
Western blot analysis
B16F10 cells (1×106) were incubated on
6-well culture plates and co-cultured with different concentrations
(0–10 µM) of propofol (Sigma-Aldrich; Merck KGaA) for 24 or 48 h.
The total protein from cervical cancer cells was isolated using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) with protease or phosphatase inhibitors (Beyotime
Institute of Biotechnology). The protein concentration was
quantified using bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). Total protein (50 µg) was loaded onto
10–12 10% SDS-PAGE and transferred to polyvinylidene fluoride
(PVDF) membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
PVDF membranes were blocked with 5% (w/v) skim milk in TPBS (0.05%
Tween-20) at 37°C for 1 h and incubated with primary antibodies:
Anti-HOTAIR, anti-phosphorylated (p)-mTOR (sc-101738, 1:500; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), anti-p-70 kDa ribosomal
protein S6 kinase (p70S6K, sc-9027, 1:500; Santa Cruz
Biotechnology, Inc.) and β-actin (sc-7210, 1:500; Santa Cruz
Biotechnology, Inc.) for 12 h at 4°C. Subsequent to washing with
TPBS, membranes were incubated with anti-IgG conjugated with
horseradish peroxidase antibody (sc-2004, 1:5,000; Santa Cruz
Biotechnology, Inc.) at 37°C for 1 h and detected under a
chemiluminescence detection system (UVItec, Cambridge, UK).
Statistical analysis
SPSS 17.0 performed the statistical analysis. (SPSS,
Inc., Chicago, IL, USA) and performed with one-way analysis of
variance followed by Scheffe's post hoc test. All values are
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Propofol suppresses melanoma cell
growth
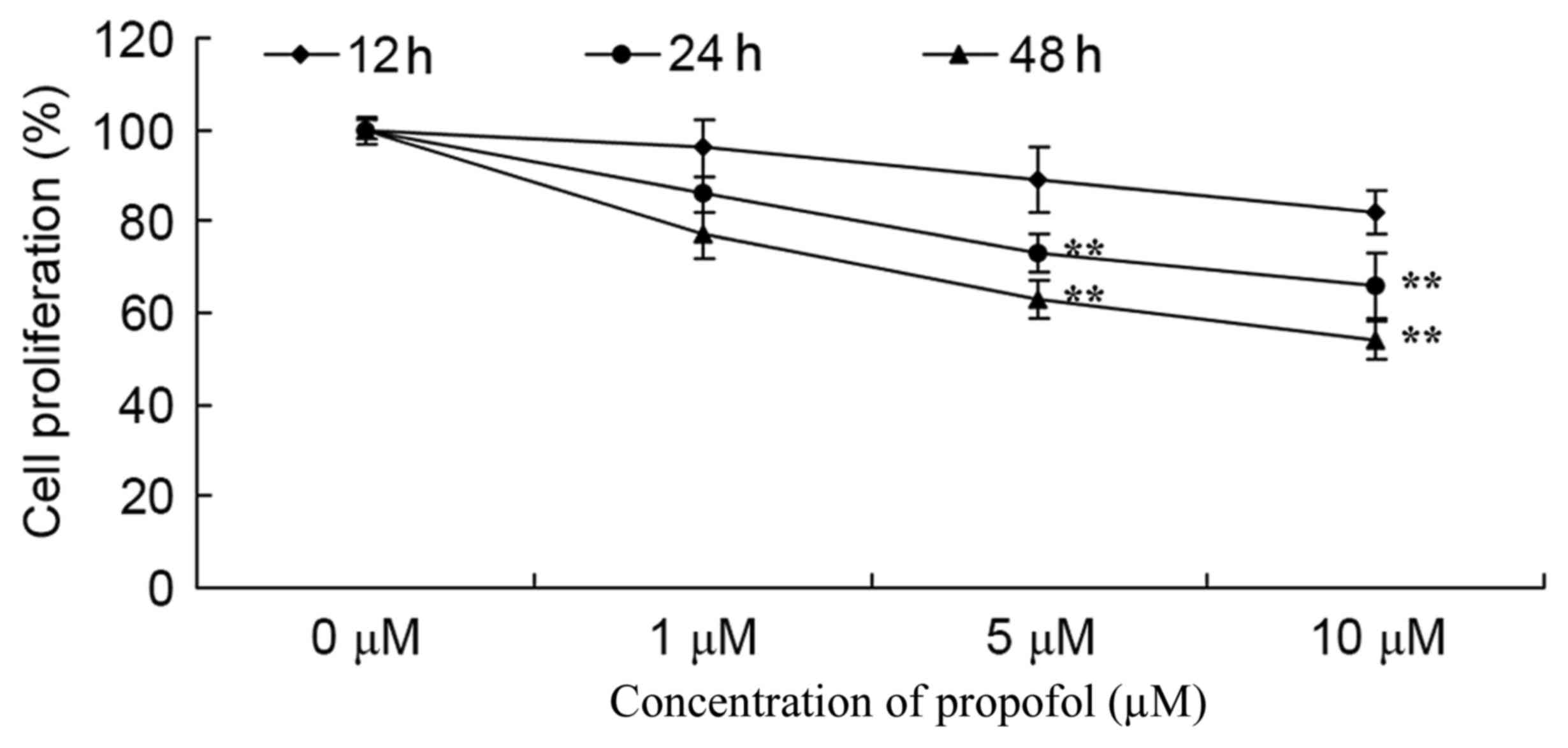
The effect of propofol on melanoma cell growth was
assessed using the recommended concentrations (0–10 µM), and cell
proliferation was analyzed by MTT assay. The results showed that
treatment with 5 or 10 µM of propofol significantly suppressed
melanoma cell growth in a dose- and time-dependent manner compared
with no treatment (Fig. 2).
Propofol promotes apoptosis of
melanoma cells
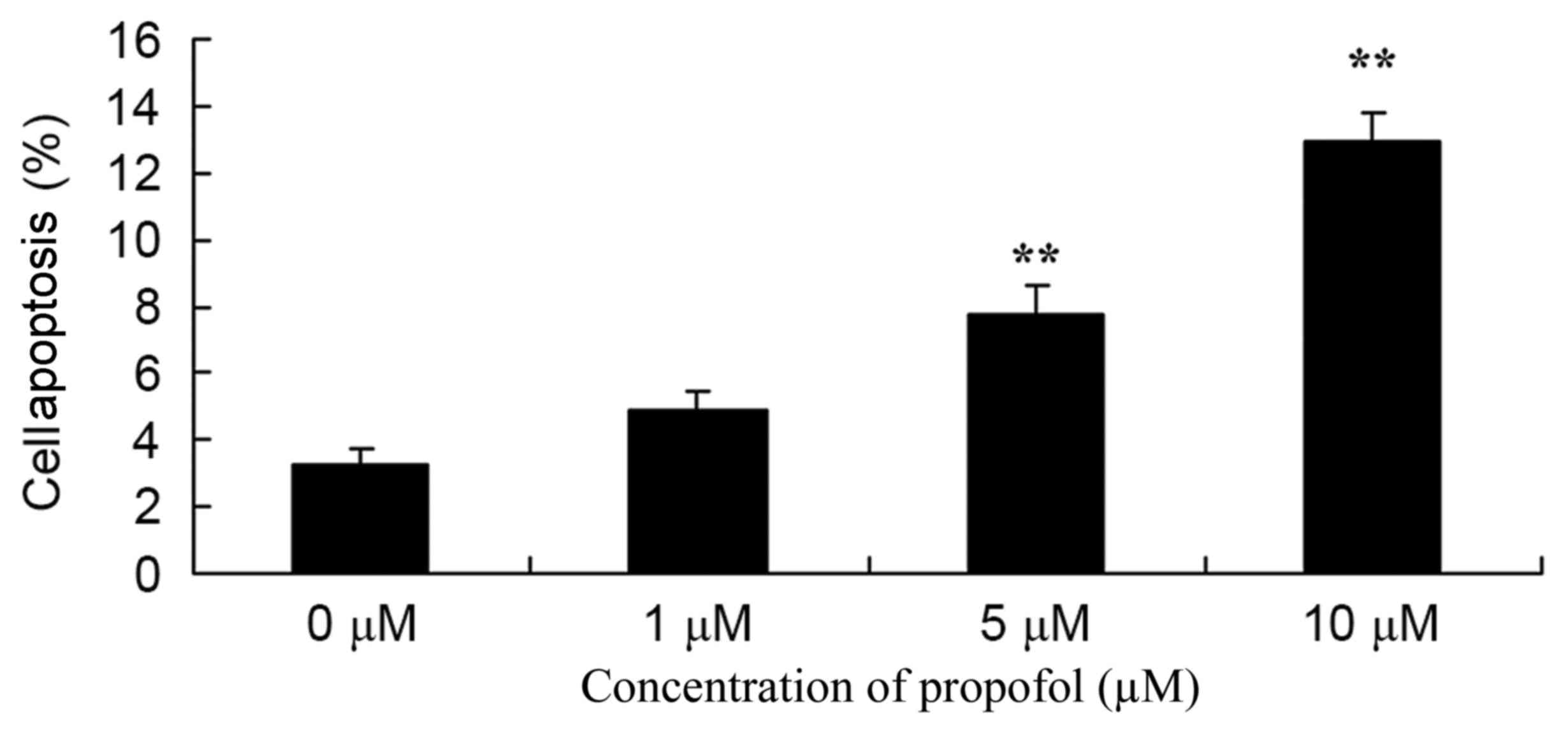
Melanoma B16F10 cells were treated with different
concentrations (0–10 µM) of propofol, and apoptosis was detected.
As shown in Fig. 3, treatment with 5
or 10 µM propofol significantly promoted apoptosis of melanoma
cells in a dose-dependent manner compared with no treatment.
Propofol promotes caspase-3 activity
in melanoma
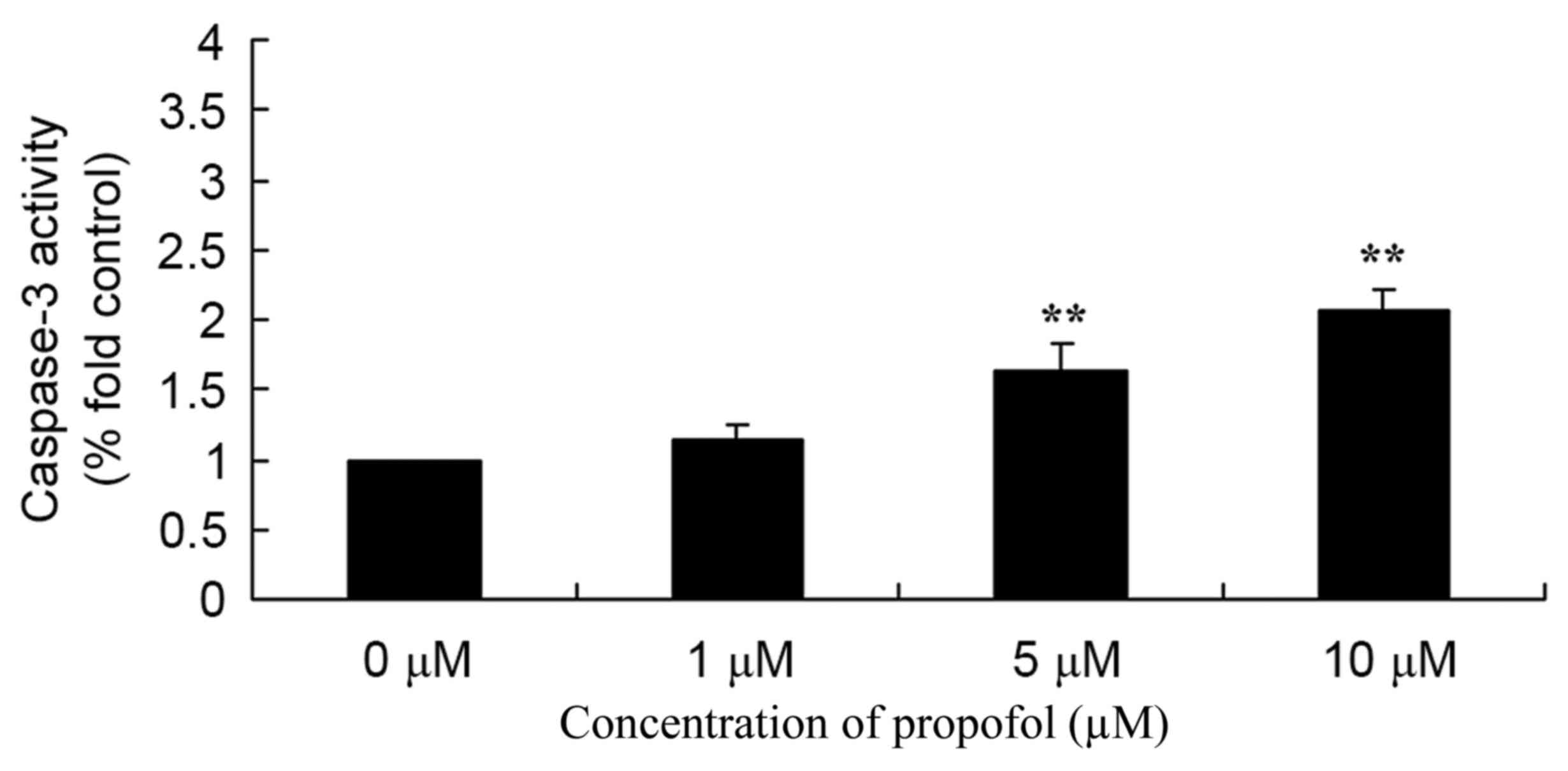
The antitumor activity of propofol on caspase-3
activity in melanoma was evaluated. Melanoma B16F10 cells were
incubated with 0–10 µM propofol, and caspase-3 activity was found
to be significantly increased in the 5 or 10 µM propofol groups
compared with the 0 µM propofol treatment (Fig. 4).
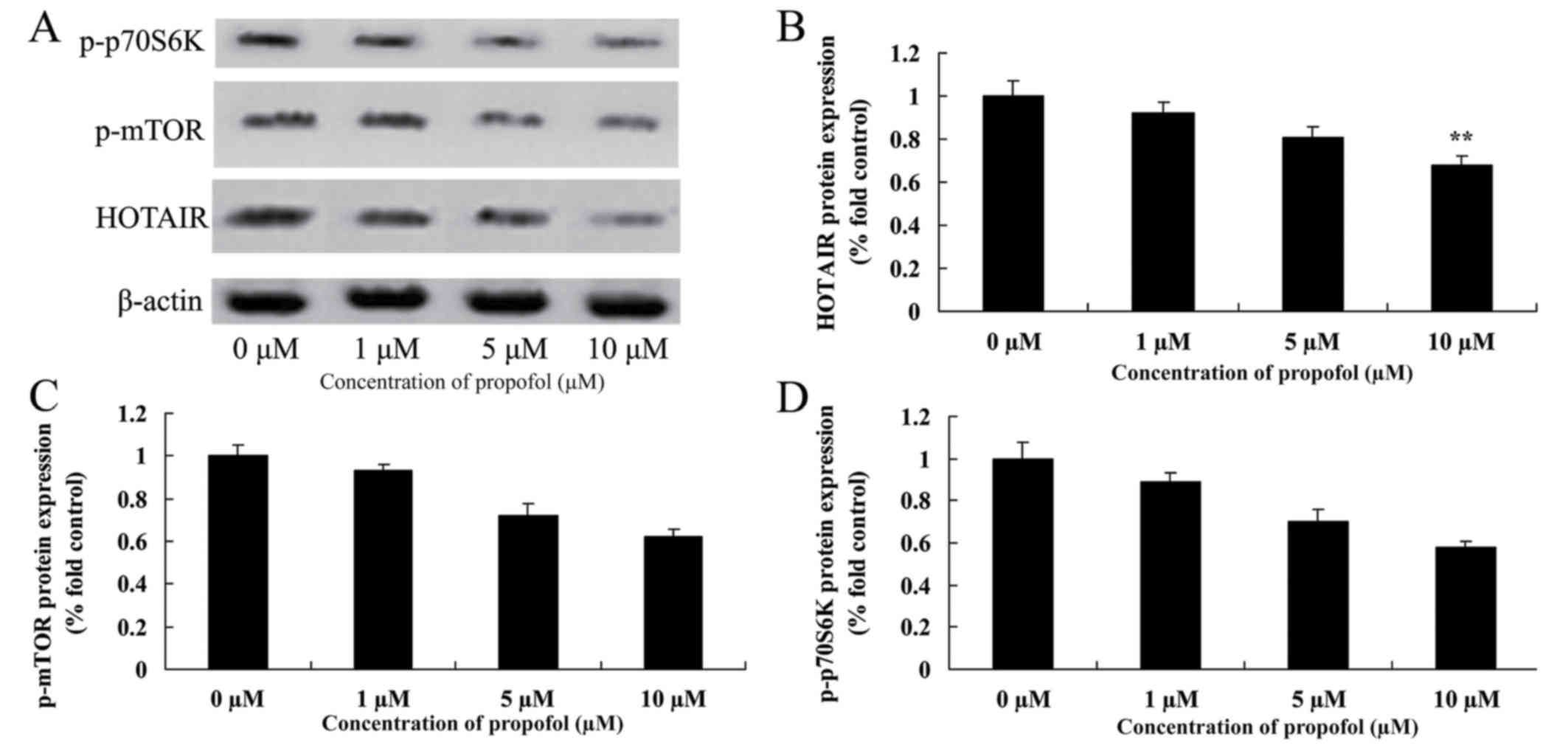
Propofol suppresses the level of
HOTAIR, p-mTOR and p-p70S6K protein expression in melanoma
It was examined whether propofol-induced apoptosis
is associated with alterations of HOTAIR signaling pathways in
melanoma cells. Data obtained from western blot analysis (Fig. 5) demonstrated that 5 or 10 µM propofol
suppressed the level of HOTAIR, p-mTOR and p-p70S6K protein
expression in B16F10 cells compared with no treatment.
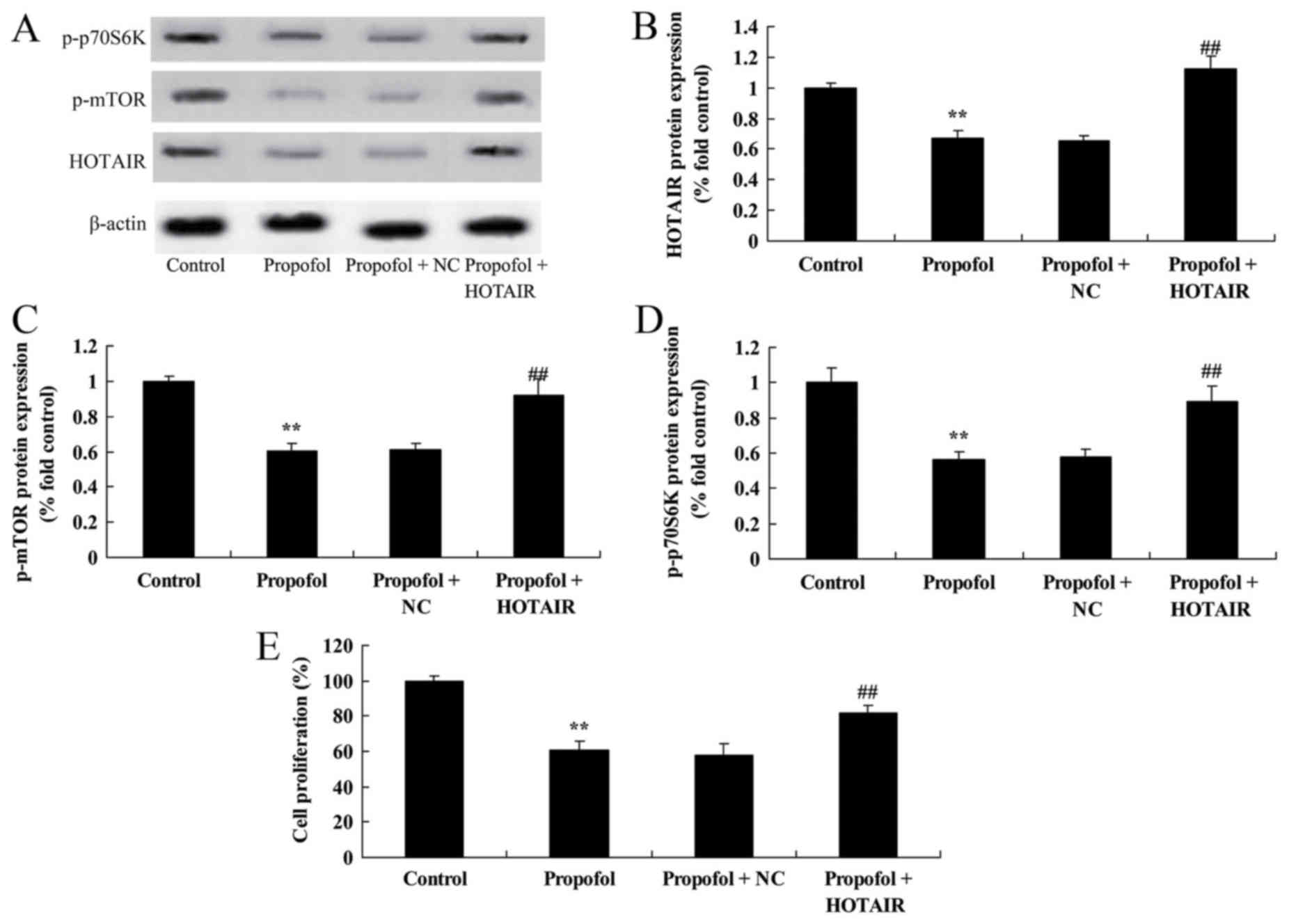
HOTAIR overexpression suppresses the
anti-cancer effect of propofol on melanoma cell growth by altering
the expression of HOTAIR, p-mTOR and p-p70S6K
To address the mechanism of how propofol triggers
the activation of HOTAIR signaling pathways, B16F10 cells were
transfected with HOTAIR plasmids. B16F10 cells transfected with
HOTAIR plasmids significantly decreased the level of protein
HOTAIR, p-mTOR and p-p70S6K expression in melanoma (Fig. 6A-D). Notably, HOTAIR overexpression
was able to suppress the anti-cancer effect of propofol (5 µM) on
B16F10 cell growth (Fig. 6E).
Discussion
Melanoma, also termed malignant melanoma, is a
common skin neoplasm caused by hyperplasia of abnormal melanocytes
(13). Although China is one of the
countries with a low incidence of melanoma, the growth rate of
malignant melanoma has continued to increase over previous years
(14). Due to the early metastasis,
fast progression, poor prognosis and high mortality rate of
malignant melanoma, there is still no favorable drug to cure
melanoma (15). With research and
development of Chinese herbal medicinal ingredients as anticancer
drugs, a number of active ingredients in Chinese herbs have reached
good efficacy in clinical anticancer experiments, which provided
new direction in melanoma drug therapy research (16). Zhang et al (17) reported that propofol significantly
inhibited viability and increased apoptosis of cervical cancer
cells through suppression of the HOTAIR-mediated mTOR signaling
pathway.
In the present study, the results showed that
treatment with propofol was able to significantly suppress growth,
and promote apoptosis and caspase-3 activity in melanoma cells.
Taken together, these data demonstrated the ability of propofol to
suppress melanoma, but the underlying mechanism requires additional
study.
HOTAIR is a non-coding RNA containing 231
nucleotides, which is associated with the human HOX locus and
promoted the metastasis of breast cancer (6). A study verified that HOTAIR has the
capacity to induce the metastasis of numerous tumors (18).
In order to verify whether HOTAIR is able to promote
melanoma progression, a series of in vitro functional
experiments were previously conducted by the present authors, with
the scratch test introducing HOTAIR-knockout to inhibit the
metastasis of melanoma, and Matrigel invasion test showing that the
invasion of melanoma cells is dependent on whether HOTAIR is
expressed (5). The expression of
HOTAIR in hepatocellular carcinoma is high and close to non-tumor
tissues, which indicates that the expression of HOTAIR in
hepatocellular carcinoma is associated with lymph node metastasis
(19). In a previous study, which
knocked out HOTAIR in hepatocellular carcinoma cells, it was found
that matrix metalloproteinase-9 (MMP-9) was also knocked out, which
indicated that MMP-9 is likely to be involved in the regulation of
hepatocellular carcinoma progression (20). In the present study, propofol
suppressed HOTAIR protein expression and overexpression of HOTAIR
suppressed the anticancer effect of propofol on melanoma cell
growth. The present study reported a potential role of HOTAIR in
the regulation of propofol-induced apoptosis of melanoma B16F10
cells.
The phosphoinositide 3-kinase (PI3K)/Akt/mTOR signal
transduction pathway consists of three proteases, including PI3K,
Akt and mTOR (21). The activation of
the PI3K/Akt/mTOR signal transduction pathway can inhibit triggers
of apoptosis by numerous types of stimuli, and promote cell cycle
progression, survival and proliferation of tumor cells (22,23). The
PI3K/Akt/mTOR signaling pathway also has several other functions,
including involvement in angiogenesis and in the occurrence,
development and drug resistance of malignant tumor, and also tumor
invasion and metastasis (22,23). The present study demonstrated that
propofol significantly suppressed the level of p-mTOR protein
expression, and overexpression of HOTAIR significantly increased
mTOR protein expression in melanoma B16F10 cells. Chang et
al (7) reported that propofol
induced autophagy and increased angiogenic capacity of human
umbilical vascular endothelial cells through PI3K/Akt and mTOR.
A previous study indicated that the Akt/mTOR/p70S6K
signal transduction pathway is aberrantly activated during tumor
generation and development (24).
Akt/mTOR is regarded as a main signal adjustment pathway of protein
synthesis, involved in cell proliferation, differentiation and
metastasis (25). mTOR is a
multi-functional kinase associated with the regulation of important
cellular processes (25).
Phosphorylation of p70S6K can promote mRNA translation and the cell
cycle (26). Restraining mTOR can
lead to blocked p70S6K phosphorylation and translation, thereby
causing cell cycle arrest and induction of apoptosis (27,28). Chang
et al (7) reported that
treatment with propofol induced autophagy and increased angiogenic
capacity of human umbilical vascular endothelial cells through
PI3K/Akt and mTOR. In the current study, propofol suppressed p70S6K
protein expression, and HOTAIR overexpression promoted the
anti-cancer effect of propofol on p70S6K protein expression in
melanoma cells. Taken together, the present findings indicate the
involvement of mTOR and p70S6K in the promotion of propofol-induced
apoptosis of melanoma.
The present results indicated that propofol
significantly suppresses cell growth, and promotes apoptosis and
caspase-3 activity in melanoma. Western blot analysis demonstrated
that HOTAIR overexpression suppressed mTOR and p70S6K expression in
B16F10 cells following treatment with propofol. Therefore, the main
findings of the present study were that propofol promotes apoptosis
and suppresses the HOTAIR-mediated mTOR/p70S6K signaling pathway in
melanoma cells.
References
|
1
|
Constantinescu R, Elm J, Auinger P, Sharma
S, Augustine EF, Khadim L and Kieburtz K: NET-PDInvestigators:
Malignant melanoma in early-treated Parkinson's disease: The NET-PD
trial. Mov Disord. 29:263–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hiniker SM, Reddy SA, Maecker HT,
Subrahmanyam PB, Rosenberg-Hasson Y, Swetter SM, Saha S, Shura L
and Knox SJ: A prospective clinical trial combining radiation
therapy with systemic immunotherapy in metastatic melanoma. Int J
Radiat Oncol Biol Phys. 96:578–588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hansson J, Aamdal S, Bastholt L, Brandberg
Y, Hernberg M, Nilsson B, Stierner U and von der Maase H; Nordic
Melanoma Cooperative Group, : Two different durations of adjuvant
therapy with intermediate-dose interferon alfa-2b in patients with
high-risk melanoma (Nordic IFN trial): A randomised phase 3 trial.
Lancet Oncol. 12:144–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Escudier B, Lassau N, Angevin E, Soria JC,
Chami L, Lamuraglia M, Zafarana E, Landreau V, Schwartz B, Brendel
E, et al: Phase I trial of sorafenib in combination with IFN
alpha-2a in patients with unresectable and/or metastatic renal cell
carcinoma or malignant melanoma. Clin Cancer Res. 13:1801–1809.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang L, Zhang W, Su B and Yu B: Long
noncoding RNA HOTAIR is associated with motility, invasion, and
metastatic potential of metastatic melanoma. Biomed Res Int.
2013:2510982013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan R, Cao J, Song C, Chen Y, Wu Z, Wang K
and Dai L: Polymorphisms in lncRNA HOTAIR and susceptibility to
breast cancer in a Chinese population. Cancer Epidemiol.
39:978–985. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang CY, Chen PH, Lu SC, Hsieh MC, Lin
CW, Lee HM, Jawan B and Kao YH: Propofol-enhanced autophagy
increases motility and angiogenic capacity of cultured human
umbilical vascular endothelial cells. Life Sci. 142:49–59. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Glen JB, Hunter SC, Blackburn TP and Wood
P: Interaction studies and other investigations of the pharmacology
of propofol (‘Diprivan’). Postgrad Med J. 61 Suppl 3:S7–S14.
1985.
|
|
9
|
Goudra BG, Singh PM, Gouda G, Borle A,
Gouda D, Dravida A and Chandrasekhara V: Safety of non-anesthesia
provider-administered propofol (NAAP) sedation in advanced
gastrointestinal endoscopic procedures: Comparative meta-analysis
of pooled results. Dig Dis Sci. 60:2612–2627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Orsini J, Nadkarni A, Chen J and Cohen N:
Propofol infusion syndrome: Case report and literature review. Am J
Health Syst Pharm. 66:908–915. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Habre C, Tramèr MR, Pöpping DM and Elia N:
Ability of a meta-analysis to prevent redundant research:
Systematic review of studies on pain from propofol injection. BMJ.
348:g52192014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Braz MG, Braz LG, Mazoti MA, Pinotti MF,
Pardini MI, Braz JR and Salvadori DM: Lower levels of oxidative DNA
damage and apoptosis in lymphocytes from patients undergoing
surgery with propofol anesthesia. Environ Mol Mutagen. 53:70–77.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang Q, Li J, Zhu H, Li P, Zou Z and Xiao
Y: Hmgb1-IL-23-IL-17-IL-6-Stat3 axis promotes tumor growth in
murine models of melanoma. Mediators Inflamm. 2013:7138592013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Yuan J, Yang Q, Cao W, Zhou X, Xie
Y, Tu H, Zhang Y and Wang S: Immunoliposome co-delivery of bufalin
and anti-CD40 antibody adjuvant induces synergetic therapeutic
efficacy against melanoma. Int J Nanomedicine. 9:5683–5700.
2014.PubMed/NCBI
|
|
15
|
Guo J, Zhu J, Sheng X, Wang X, Qu L, Han
Y, Liu Y, Zhang H, Huo L, Zhang S, et al: Intratumoral injection of
dendritic cells in combination with local hyperthermia induces
systemic antitumor effect in patients with advanced melanoma. Int J
Cancer. 120:2418–2425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen LG, Chang WL, Lee CJ, Lee LT, Shih CM
and Wang CC: Melanogenesis inhibition by gallotannins from Chinese
galls in B16 mouse melanoma cells. Biol Pharm Bull. 32:1447–1452.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang D, Zhou XH, Zhang J, Zhou YX, Ying
J, Wu GQ and Qian JH: Propofol promotes cell apoptosis via
inhibiting HOTAIR mediated mTOR pathway in cervical cancer. Biochem
Biophys Res Commun. 468:561–567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lv XB, Lian GY, Wang HR, Song E, Yao H and
Wang MH: Long noncoding RNA HOTAIR is a prognostic marker for
esophageal squamous cell carcinoma progression and survival. PLoS
One. 8:e635162013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding C, Cheng S, Yang Z, Lv Z, Xiao H, Du
C, Peng C, Xie H, Zhou L, Wu J and Zheng S: Long non-coding RNA
HOTAIR promotes cell migration and invasion via down-regulation of
RNA binding motif protein 38 in hepatocellular carcinoma cells. Int
J Mol Sci. 15:4060–4076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Z, Zhou L, Wu LM, Lai MC, Xie HY,
Zhang F and Zheng SS: Overexpression of long non-coding RNA HOTAIR
predicts tumor recurrence in hepatocellular carcinoma patients
following liver transplantation. Ann Surg Oncol. 18:1243–1250.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong M, Yang G, Liu H, Liu X, Lin S, Sun D
and Wang Y: Aged black garlic extract inhibits HT29 colon cancer
cell growth via the PI3K/Akt signaling pathway. Biomed Rep.
2:250–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mediani L, Gibellini F, Bertacchini J,
Frasson C, Bosco R, Accordi B, Basso G, Bonora M, Calabrò ML,
Mattiolo A, et al: Reversal of the glycolytic phenotype of primary
effusion lymphoma cells by combined targeting of cellular
metabolism and PI3K/Akt/mTOR signaling. Oncotarget. 7:5521–5537.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ochi E, Ishii N and Nakazato K: Time
course change of IGF1/Akt/mTOR/p70s6k pathway activation in rat
gastrocnemius muscle during repeated bouts of eccentric exercise. J
Sports Sci Med. 9:170–175. 2010.PubMed/NCBI
|
|
24
|
Li J, Li X, Xu W, Wang S, Hu Z, Zhang Q,
Deng X, Wang J, Zhang J and Guo C: Antifibrotic effects of luteolin
on hepatic stellate cells and liver fibrosis by targeting
AKT/mTOR/p70S6K and TGFbeta/Smad signalling pathways. Liver Int.
35:1222–1233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pratheeshkumar P, Budhraja A, Son YO, Wang
X, Zhang Z, Ding S, Wang L, Hitron A, Lee JC, Xu M, et al:
Quercetin inhibits angiogenesis mediated human prostate tumor
growth by targeting VEGFR- 2 regulated AKT/mTOR/P70S6K signaling
pathways. PLoS One. 7:e475162012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saraswati S, Kumar S and Alhaider AA:
α-santalol inhibits the angiogenesis and growth of human prostate
tumor growth by targeting vascular endothelial growth factor
receptor 2-mediated AKT/mTOR/P70S6K signaling pathway. Mol Cancer.
12:1472013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li SY, Fang CX, Aberle NS II, Ren BH,
Ceylan-Isik AF and Ren J: Inhibition of PI-3 kinase/Akt/mTOR, but
not calcineurin signaling, reverses insulin-like growth factor
I-induced protection against glucose toxicity in cardiomyocyte
contractile function. J Endocrinol. 186:491–503. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Wang H, Xu J, Zhu J and Ding K:
Inhibition of cathepsin S induces autophagy and apoptosis in human
glioblastoma cell lines through ROS-mediated PI3K/AKT/mTOR/p70S6K
and JNK signaling pathways. Toxicol Lett. 228:248–259. 2014.
View Article : Google Scholar : PubMed/NCBI
|