Introduction
Hepatoblastoma (HB) is a malignant liver tumor
observed in pediatric patients, the incidence of which has
increased by 2.18% annually in patients <20 years of age
(1). A total of 90% of patients with
liver malignancies and <5 years of age were diagnosed with HB
(1). Previously, the main treatment
for HB was surgical resection. However, complete tumor resection
may only be achieved in a small proportion of patients (1). However, since cisplatin (DDP) was
introduced into the treatment regimen, the survival rate had
improved markedly (1). DDP is a
platinum-based chemotherapeutic that belongs to a class of
alkylating agents widely used in the treatment of a variety of
pediatric malignancies (2). However,
as occurs with the majority of anticancer drugs, treatment
resistance and side effects (including hearing loss) in healthy
tissues are two major challenges in the use of DDP (2,3). The
DDP-associated toxicity is dose-dependent, but inhibition of cancer
cell proliferation requires a sufficient dose of DDP. Therefore,
there exists a contradiction between chemotherapy efficiency and
side effects, meaning that there is an urgent requirement to
enhance the sensitivity of DDP chemotherapy.
The anticancer mechanism of DDP is associated with
its ability to form inter- and intra-strand DNA crosslinks, which
perturb DNA replication and transcription, therefore inducing a
replication stress and DNA damage response, eventually resulting in
cell cycle arrest and apoptosis (4).
Apoptosis induced by DDP can be initiated through two main core
signaling pathways, the tumor protein 53-dependent transcription of
pro-apoptotic B-cell lymphoma-2 family members (p53 upregulated
modulator of apoptosis, phorbol-12-myristate-13-acetate-induced
protein 1 and Bcl-associated X) that trigger cell death via the
mitochondrial apoptotic pathway and the death receptors (DRs)
(5). DRs are receptors of tumor
necrosis factor-related apoptosis-inducing ligands (TRAILs), which
have five receptors. However, only two of the receptors, DR4
(TRAIL-R1) and DR5 (TRAIL-R2), are capable of effectively
transmitting the apoptotic signal (5). A number of studies reported that DDP
enhances the sensitivity of TRAIL to the cancer cells by increasing
the expression of DR4 and DR5 (6,7), which
triggers the relocalization of DR4 and DR5 to the cell membrane and
accelerates the internalization of TRAIL (2,6,8). However, DDP can also induce apoptosis
through the DR4 and DR5 signaling pathways, which is not dependent
on TRAIL and the mitochondrial apoptotic signaling pathway
(9).
Sphingomyelin synthase (SMS) is a key enzyme
involved in the generation of sphingomyelin (SM) and has two
isoforms, SMS1 and SMS2 (10). SMS
can participate in inflammation, atherosclerosis, proliferation,
apoptosis, differentiation and other functions (11,12).
However, the association between SMS2 with the expression of DR4
and DR5, and DDP-induced apoptosis is unclear in HepG2 cells.
Therefore, the present study constructed a SMS2
overexpression cell model to investigate the effects of SMS2 on the
expression of DR4 and DR5, and on apoptosis induced by DDP. HepG2
cells were previously misidentified as human hepatocarcinoma
(13); however, in the present study,
HepG2 cells were treated as HB, and the results were not affected
by this misidentification.
Materials and methods
Cell culture and transfection
HepG2 cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Hyclone; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Zhejiang Tianhang Biotechnology Co., Ltd., Huzhou, China) and
penicillin and streptomycin (100 U/ml and 0.1 mg/ml, respectively;
Beijing Solarbio Bioscience & Technology Co., Ltd., Beijing,
China), and incubated at 37°C in a humidified atmosphere containing
5% CO2.
Transfection was performed using LipoFiter
transfection reagent (Hanbio Biotechnology Co., Ltd., Shanghai,
China) according to the manufacturer's protocol. At 12 h before
transfection, the cells (7×105) were seeded into wells
of a 6-well plate that contained antibiotic-free DMEM. At the time
of transfection, cell confluence was 70–80%. The SMS2 plasmid (4
µg; provided by Dr Tingbo Ding, School of Pharmacy, Fudan
University, Shanghai, China) or negative plasmid (4 µg; provided by
Dr Tingbo Ding, School of Pharmacy, Fudan University) was diluted
with 250 µl DMEM (FBS- and antibiotic-free medium) or 12 µl
LipoFiter with 238 µl DMEM. The cells transfected with the SMS2
plasmid were termed the SMS2 group. The cells transfected with the
negative plasmid were termed the control group.
After 5 min, the dilutions were mixed together and
incubated at 37°C for 20 min, then dispensed into each well. After
6 h, the medium was replaced with DMEM containing 10% FBS and the
aforementioned antibiotics. These cells were cultured for 48 h. The
control and SMS2 groups were divided separately into two groups,
resulting in control, control + DDP, SMS2 and SMS2 + DDP groups.
The control + DDP and SMS2 + DDP groups were treated with 3.5 mg/l
DDP. After 24 h, the cells were harvested for analysis.
Western blot analysis
The proteins were extracted using
radioimmunoprecipitation assay buffer (CW2333S; Kangwei Century
Biotechnology Co., Ltd., Beijing, China), and the protein
concentration was measured using the DR4a bicinchoninic acid assay
(CW0014; Kangwei Century Biotechnology Co., Ltd.). Equal quantities
of cleared lysates (~50 µg protein) were separated by SDS-PAGE (10%
gel), and then transferred onto polyvinylidene fluoride membranes
(EMD Millipore, Billerica, MA, USA). Equal transfer was validated
by staining with Ponceau red for 15 min at room temperature. The
membranes were blocked with 10% skimmed milk in Tris-buffered
saline (TBS), and then incubated with primary antibodies in TBS
containing 0.05% Tween-20, 2% bovine serum albumin (A8010; Solarbio
Bioscience & Technology Co., Ltd.) and 0.05% sodium azide
overnight at 4°C. The following antibodies were used at the
indicated dilutions: SMS2 (1:1,000; sc-34048; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), caspase-3 (1:1,000;
19677-1-AP; ProteinTech Group, Inc., Chicago, IL, USA), avian
myelocytomatosis viral oncogene homolog (c-Myc) (1:1,000; AF0358;
Affinity Biosciences, Jiangsu, China), DR4 (1:1,000; AF0304;
Affinity Biosciences) and DR5 (1:1,000; DF6368, Affinity
Biosciences) GAPDH (1:10,000; 60004-1-Ig; ProteinTech Group, Inc.).
Secondary horseradish peroxidase-coupled antibodies [mouse
(SA00001-1) and rabbit (SA00001-2); ProteinTech Group, Inc.] were
used at 1:10,000 in 10% skimmed milk in TBS containing 0.05%
Tween-20. Signals were detected using an enhanced chemiluminescence
reagent (CW0049M; Kangwei Century Biotechnology Co., Ltd.) and an
autoradiography system (Chemiluminescence Imaging system; CLINX
Science Instruments Co., Ltd., Shanghai, China) (14). Each assay was repeated at least three
times.
Cell viability assays
For cell viability assays, the cells (control and
SMS2 groups; 1.5×104 cells/well) were plated into
flat-bottomed 96-well plates. Next, 3.5 mg/l DDP was added into
these cells, and the cells were cultured in 37°C to measure
viability. After incubation for 24 h, 20 µl Cell Counting kit-8
(CCK-8) solution (Beijing Zoman Biotechnology Co., Ltd., Beijing,
China) was added into each well at 37°C in the dark for 2 h. The
absorbance of each well was measured using a microplate reader at
an absorbance of 450 nm. Each assay was repeated at least three
times.
Flow cytometric analysis for
apoptosis
The proportion of apoptotic cells was determined by
flow cytometry, as described previously (15). In brief, cells from the control and
SMS2 groups (1×106 cells/ml) were seeded in 6-well
plates and cultured for 12 h, treated with DDP at 3.5 mg/l for 24 h
and collected. The cells were then washed twice and subsequently
analyzed apoptosis by flow cytometry (BD Biosciences, Franklin
Lakes, NJ, USA) according to the protocol of the apoptosis assay
kit (Promega Corporation, Madison, WI, USA). FlowJo Software
(version 7.6; FlowJo LLC, Ashland, OR, USA) was used to analyze
these data. Each assay was repeated in triplicate.
Statistical analysis
All data are reported as the mean ± standard
deviation. Student's t-test was used for single comparisons. For
multiple comparisons, one-way analysis of variance with Tukey's or
Games-Howell post hoc analysis was used. P<0.05 was considered
to indicate a statistically significant difference.
Results
Identification of SMS2 overexpression
cell model
To construct a cell model where SMS2 is
overexpressed, HepG2 cells were transfected with the SMS2 plasmid.
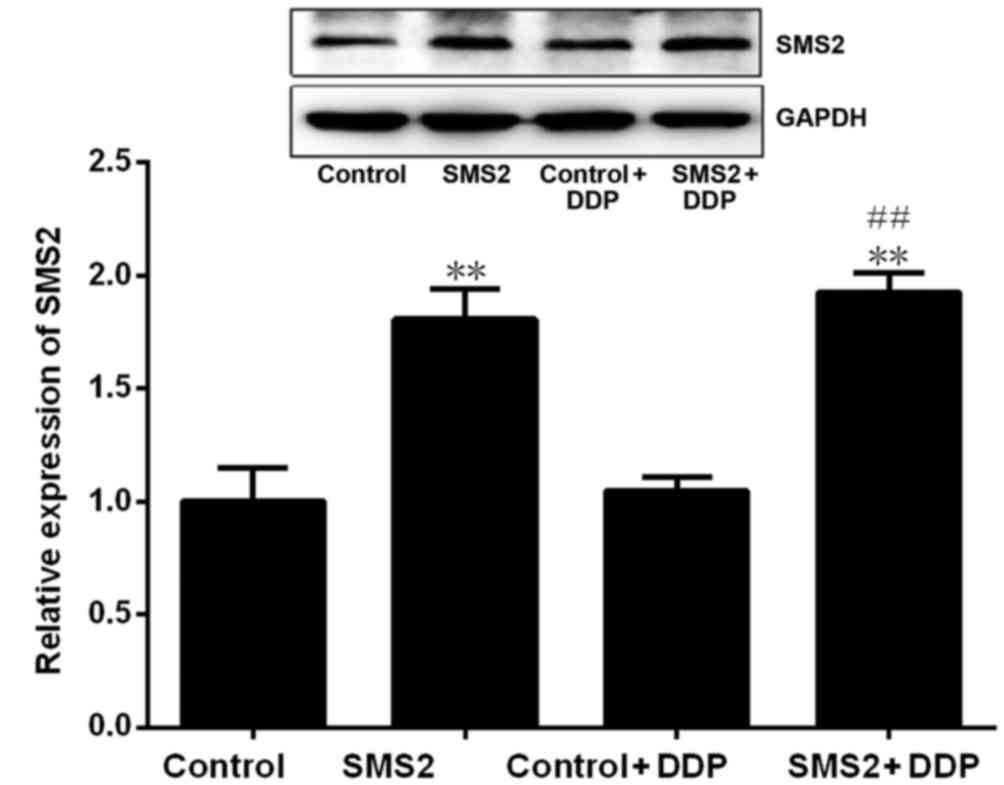
Western blotting revealed that SMS2 expression was significantly
upregulated in the SMS2 and SMS2 + DDP groups compared with control
and control + DDP group, respectively (P<0.001, n=3; Fig. 1). The expression of SMS2 in the SMS2
and SMS2 + DDP groups was increased 0.81- and 0.92-fold compared
with the control or control + DDP group, respectively. These
results demonstrated that transfection with the SMS2 plasmid was
able to effectively augment SMS2 protein expression.
Expression of DR4 and DR5
DR4 and DR5 are involved in DDP-induced apoptosis.
Therefore, the present study analyzed the expression of DR4 and DR5
by western blot analysis (Fig. 2).
The results indicated that upregulation of SMS2 was able to
modulate the expression of DR4 and DR5 compared with the control
group, respectively. The expression of DR4 and DR5 was increased
~0.48 and 0.27-fold in the SMS2 group compared with the control
group, respectively (P<0.05, n=3; Fig.
2B and C). However, when the control and SMS2 groups were
treated with DDP, the expression of DR4 and DR5 was also induced.
The expression of DR4 in the control + DDP group was significantly
increased 1.02-fold compared with the control group (P<0.001,
n=3; Fig. 2B). By contrast, the
expression of DR5 in the control + DDP group only increased
~0.81-fold compared with the control group (Fig. 2C). Notably, the expression of DR 4 and
DR 5 in SMS2 + DDP group compared with the control group was
increased 1.64 and 1.47-fold, respectively (P<0.001, n=3).
Additionally, the expression of DR4 and DR5 in the SMS2 + DDP group
was upregulated compared with the control group (P<0.05,
n=3).
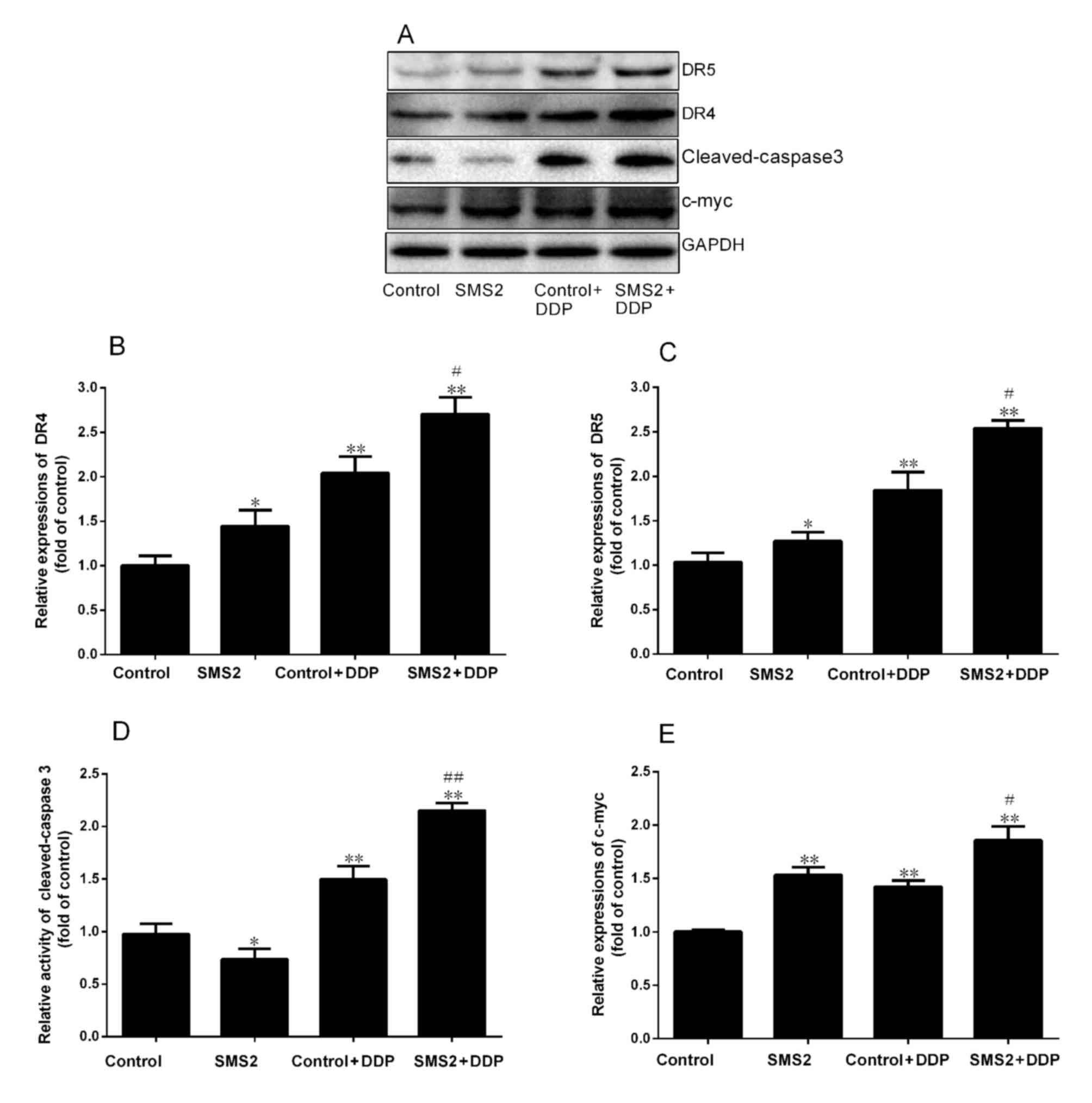 | Figure 2.Expression of DR4, DR5, cleaved
caspase-3 and c-Myc in HepG2 cells. (A) The protein expression of
DR4, DR5, cleaved caspase-3 and c-Myc was investigated using
western blotting. Quantification and statistical analysis was
performed on (B) DR4, (C) DR5, (D) cleaved caspase-3 and (E) c-Myc.
n=3, *P<0.05, **P<0.001 vs. control group;
#P<0.05, ##P<0.001 vs. control + DDP
group. DR. death receptor; DDP, cisplatin; SMS2, sphyngomyelin
synthase 2; c-Myc, avian myelocytomatosis viral oncogene
homolog. |
Effect of SMS2 expression on
apoptosis
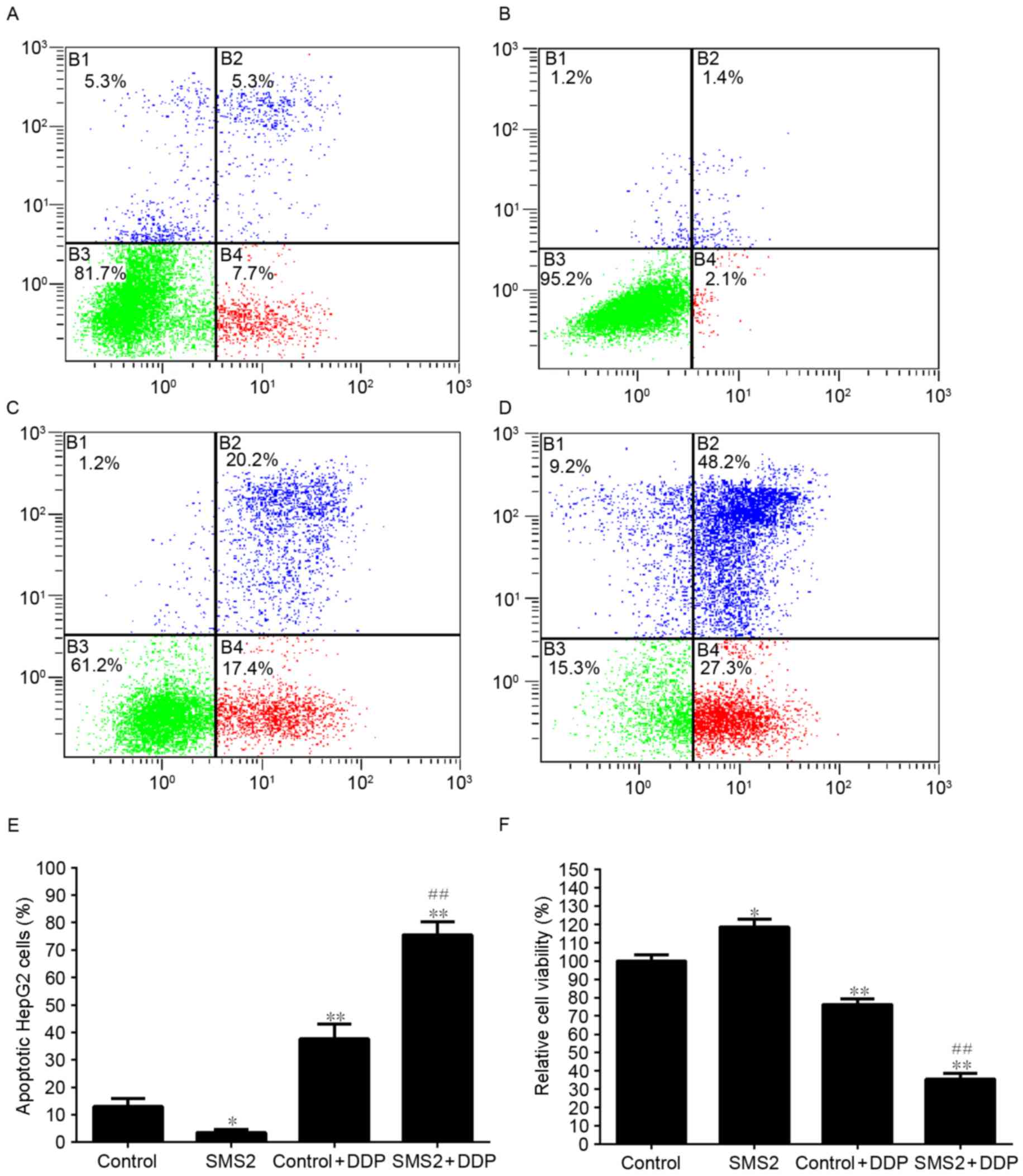
To analyze the effect of SMS2 expression on the
DDP-induced apoptosis of HepG2 cells, the viability of cells was
analyzed (Fig. 3). As shown in
Fig. 3F, the expression of SMS2 was
able to increase the viability of HepG2 cells by ~18.3% compared
with the control group (P<0.05, n=9). However, when the cells
were treated with DDP, the viability of the cells in the control +
DDP and SMS2 + DDP groups compared with the control group was
significantly decreased by ~23.7% (P<0.05, n=9) and 64.4%
(P<0.001, n=9), respectively. Moreover, compared with the
control + DDP group, the viability of the cells in the SMS2 + DDP
group significantly decreased, by ~40.7% (P<0.001, n=9). These
observations were confirmed by flow cytometric analysis. The
percentage of apoptotic cells in the control, SMS2, control + DDP
and SMS2 + DDP groups were 13.0, 3.5, 37.6 and 75.5%, respectively
(Fig. 3A-D). The proportion of
apoptotic cells in the SMS2 + DDP group increased by ~37.9%
compared with the control + DDP group (Fig. 3E, P<0.001, n=3). The levels of
cleaved caspase-3 and c-myc were assessed using western blotting
(Fig. 2A). The levels of cleaved
caspase-3 in the SMS2 + DDP group was increased by 115.3% compared
with the control + DDP group (P<0.001, n=3; Fig. 2D). The levels in the control + DDP
group were significantly increased compared with the control group
(P<0.001, n=3; Fig. 2D), where
expression was increased by 37.2% (P<0.001, n=3; Fig. 2D). Expression of SMS2 was able to
increase the sensitivity of HepG2 cells to DDP treatment.
Expression of c-Myc
Furthermore, the present study continued to
investigate the expression of c-Myc. As presented in Fig. 2E, the expression of c-Myc in the SMS2
group was increased 0.48-fold, compared with the control group
(P<0.01, n=3). However, when the HepG2 cells were treated with
DDP, the expression of c-Myc in the control + DDP and SMS2 + DDP
groups was increased compared with the control group (P<0.001,
n=3). Notably, the degree of increase in c-Myc expression in the
SMS2 + DDP group compared with the control was greater compared
with the increase in the control + DDP group compared with the
control (P<0.05, n=3).
Discussion
The results of the present study indicated that the
overexpression of SMS2 was able to slightly increase the viability
of HepG2 cells and increase the expression of DR4, DR5 and c-Myc.
Overexpression of SMS2 was also able to increase the sensitivity of
the HepG2 cells to DDP.
SMS2 is a key enzyme involved in the biosynthesis of
sphingomyelin and is also involved in the production of
diacylglycerol (DAG), which is a second messenger that can induce
cell proliferation (16).
Overexpression of SMS2 may therefore increase the cellular levels
of DAG and induce growth signal transmission.
Moreover, the proliferation of HepG2 cells may also
be associated with c-Myc. c-Myc is a proto-oncogene which is a key
regulator of cell proliferation, apoptosis and cellular
differentiation (17). However, in
the present study, the expression of SMS2 was able to upregulate
the expression of c-Myc, which would lead to the viability of HepG2
cells. A number of studies have reported that upregulation of SMS
is able to induce cell proliferation (18,19). For
example, chronic myelogenous leukemia K562 cells have high SMS
activity, and when SMS1 expression was downregulated using small
interfering RNAs, cell apoptosis was induced (18). This result indicates that maintaining
the SMS activity was necessary to proliferation of K562 cells
(18).
c-Myc not only modulates the expression of growth
genes, but also participates in the expression of DR4 and DR5,
which are associated with apoptosis (20,21). When
c-Myc was overexpressed, the expression of DR4 and DR5 were also
increased, which led to an increase in the sensitivity to tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL), as DR4
and DR5 are TRAIL receptors (20,21). This
may overcome multidrug resistance in human ovarian, breast and
gastric carcinoma cells (20,21).
Similar to TRAIL, DDP is also able to induce
apoptosis through the DR4 and DR5 signaling pathways (6,7). DDP is
able to trigger apoptosis through the DR4 and DR5 signal pathways
in H460 cells, independent of TRAIL and the mitochondrial apoptotic
pathway (9). Therefore, in the
present study, it was hypothesized that overexpression of SMS2 was
able to alter the expression of DR4 and DR5, and increased the
expression of c-Myc, as well as increasing the sensitivity of the
cells to DDP. However, DDP is also able to increase the expression
of DR4 and DR5 to induce cell apoptosis (7,21). In the
present study, it was demonstrated that treatment with DDP was able
to significantly increase the expression of DR4 and DR5 in HepG2
cells, and the increase in the expression of DR4 and DR5 was higher
in the SMS2 + DDP group compared with the control + DDP group.
Other studies reported that overexpression of SMS2 is able to
increase the sensitivity of cancer cells to antitumor drugs. For
example, Ding et al (22)
found that when SMS1 and SMS2 were overexpressed in THP-1 cells,
apoptosis was increased via tumor necrosis factor or
lipopolysaccharide. 2-hydroxyoleic acid (2OHOA) is a potent
antitumor compound. It has been demonstrated that 2OHOA is able to
regulate SMS activity in tumor cells, which is a critical upstream
event (23).
Moreover, the increase in apoptosis induced by DDP
may be associated with the lipid raft, which act as microdomains of
the plasma membrane and are enriched in cholesterol and
sphingolipids. Lipid rafts have an important function in signaling,
vesicular transporting, interaction with pathogens and viral
infection (24). SM is a type of
sphingolipid that can affect lipid raft structure and function. DR4
and DR5 localize to lipid rafts and can accelerate the
internalization of TRAIL (2,6,8). Certain
studies hypothesized that the localization of DR4 and DR5 to the
lipid raft was responsible for the transduction of intrinsic and
extrinsic apoptosis signaling pathways in response to DDP treatment
(8,9).
Furthermore, in the present study, SMS2 was overexpressed in HepG2
cells, which may alter the structure of the lipid raft, and the
relocalization of DR4 and DR5 to the lipid raft. Therefore,
overexpression of SMS2 not only increased apoptosis of HepG2 cells
by increasing the expression of DR4 and DR5, but may also have
caused the relocalization of DR4 and DR5 to the lipid raft.
To conclude, the present study found that
overexpression of SMS2 increased the sensitivity of HepG2 cells to
DDP through c-Myc and an increase in the expression of DR4 and DR5.
This increased sensitivity, if reflected in patients, would
decrease intrinsic and acquired resistance, and severe side effects
of HB chemotherapy in pediatric malignancies.
Acknowledgements
The present research was supported by grants from
the National Natural Science Foundation of China (grant no.
81560151) and Jiangxi Provincial Department of Science and
Technology (grant no. 20142BAB205014).
References
|
1
|
Yuan XJ, Wang HM, Jiang H, Tang MJ, Li ZL,
Zou X, Fang YJ, Pan C, Tou JF, Zhang KR, et al: Multidisciplinary
effort in treating children with hepatoblastoma in China. Cancer
Lett. 375:39–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van As JW, van den Berg H and van Dalen
EC: Medical interventions for the prevention of platinum-induced
hearing loss in children with cancer. Cochrane Database Syst Rev.
9:CD0092192016.PubMed/NCBI
|
|
3
|
Zhu S, Pabla N, Tang C, He L and Dong Z:
DNA damage response in cisplatin-induced nephrotoxicity. Arch
Toxicol. 89:2197–2205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujikawa Y, Kawanishi M, Kuraoka I and
Yagi T: Frequencies of mutagenic translesion DNA synthesis over
cisplatin-guanine intra-strand crosslinks in lacZ plasmids
propagated in human cells. Mutat Res Genet Toxicol Environ Mutagen.
770:23–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chipuk JE, Kuwana T, Bouchier-Hayes L,
Droin NM, Newmeyer DD, Schuler M and Green DR: Direct activation of
Bax by p53 mediates mitochondrial membrane permeabilization and
apoptosis. Science. 303:1010–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vondálová Blanárová O, Jelínková I, Szöor
A, Skender B, Soucek K, Horváth V, Vaculová A, Andera L, Sova P,
Szöllosi J, et al: Cisplatin and a potent platinum(IV)
complex-mediated enhancement of TRAIL-induced cancer cells killing
is associated with modulation of upstream events in the extrinsic
apoptotic pathway. Carcinogenesis. 32:42–51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu X, Zhang K, Wang Q, Chen S, Gou Y, Cui
Y and Li Q: Cisplatin-mediated c-Myc overexpression and cytochrome
c (cyt c) release result in the up-regulation of the death
receptors DR4 and DR5 and the activation of caspase-3 and caspase
9, likely responsible for the TRAIL-sensitizing effect of
cisplatin. Med Oncol. 32:1332015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song JH, Tse MC, Bellail A, Phuphanich S,
Khuri F, Kneteman NM and Hao C: Lipid rafts and non-rafts mediate
tumor necrosis factor related apoptosis-inducing ligand induced
apoptotic and nonapoptotic signals in non small cell lung carcinoma
cells. Cancer Res. 67:6946–6955. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paul I, Chacko AD, Stasik I, Busacca S,
Crawford N, McCoy F, McTavish N, Wilson B, Barr M, O'Byrne KJ, et
al: Acquired differential regulation of caspase-8 in
cisplatin-resistant non-small-cell lung cancer. Cell Death Dis.
3:e4492012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yeang C, Ding T, Chirico WJ and Jiang XC:
Subcellular targeting domains of sphingomyelin synthase 1 and 2.
Nutr Metab (Lond). 8:892011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taniguchi M and Okazaki T: The role of
sphingomyelin and sphingomyelin synthases in cell death,
proliferation and migration-from cell and animal models to human
disorders. Biochim Biophys Acta. 1841:692–703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Slotte JP: Biological functions of
sphingomyelins. Prog Lipid Res. 52:424–437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
14
|
Yan N, Ding T, Dong J, Li Y and Wu M:
Sphingomyelin synthase overexpression increases cholesterol
accumulation and decreases cholesterol secretion in liver cells.
Lipids Health Dis. 10:462011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang CR, Jin ZX, Dong L, Tong XP, Yue S,
Kawanami T, Sawaki T, Sakai T, Miki M, Iwao H, et al: cisplatin
augments FAS-mediated apoptosis through lipid rafts. Anticancer
Res. 30:2065–2071. 2010.PubMed/NCBI
|
|
16
|
Luberto C and Hannun YA: Sphingomyelin
synthase, a potential regulator of intracellular levels of ceramide
and diacylglycerol during SV40 transformation. Does sphingomyelin
synthase account for the putative phosphatidylcholine-phospholipase
C. J Biol Chem. 273:14550–14559. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sumi T, Tsuneyoshi N, Nakatsuji N and
Suemori H: Apoptosis and differentiation of human embryonic stem
cells induced by sustained activation of c-Myc. Oncogene.
26:5564–5576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burns TA, Subathra M, Signorelli P, Choi
Y, Yang X, Wang Y, Villani M, Bhalla K, Zhou D and Luberto C:
Sphingomyelin synthase 1 activity is regulated by the BCR-ABL
oncogene. J Lipid Res. 54:794–805. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wesley UV, Hatcher JF and Dempsey RJ:
Sphingomyelin synthase 1 regulates Neuro-2a cell proliferation and
cell cycle progression through modulation of p27 expression and Akt
signaling. Mol Neurobiol. 51:1530–1541. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim DY, Kim MJ, Kim HB, Lee JW, Bae JH,
Kim DW, Kang CD and Kim SH: Suppression of multidrug resistance by
treatment with TRAIL in human ovarian and breast cancer cells with
high level of c-Myc. Biochim Biophys Acta. 1812:796–805. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kondo K, Yamasaki S, Sugie T, Teratani N,
Kan T, Imamura M and Shimada Y: Cisplatin-dependent upregulation of
death receptors 4 and 5 augments induction of apoptosis by
TNF-related apoptosis-inducing ligand against esophageal squamous
cell carcinoma. Int J Cancer. 118:230–242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding T, Li Z, Hailemariam T, Mukherjee S,
Maxfield FR, Wu MP and Jiang XC: SMS overexpression and knockdown:
Impact on cellular sphingomyelin and diacylglycerol metabolism, and
cell apoptosis. J Lipid Res. 49:376–385. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barceló-Coblijn G, Martin ML, de Almeida
RF, Noguera-Salvà MA, Marcilla-Etxenike A, Guardiola-Serrano F,
Lüth A, Kleuser B, Halver JE and Escribá PV: Sphingomyelin and
sphingomyelin synthase (SMS) in the malignant transformation of
glioma cells and in 2-hydroxyoleic acid therapy. Proc Natl Acad
Sci. 108:pp. 19569–19574. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simons K and Ikonen E: Functional rafts in
cell membranes. Nature. 387:569–572. 1997. View Article : Google Scholar : PubMed/NCBI
|