Introduction
Plants synthesize a wide array of compounds with
structurally complex molecular scaffolds. Several of these
compounds and their derivatives such as alkaloids and flavonoids
exhibit a diversity of medicinal properties (1). Among plant derived secondery metabolites
alkaloids are biologically active found across plant kingdom. They
have been found to exhibit several pharmacological properties such
as anticancer and antimicrobial. Cancer is considered one of the
most lethal diseases and due to the dearth of operative drugs,
lavish cost of chemotherapeutic agents and the side effects, there
is tremendous need for exploration of novel molecules for their
anticancer activities (2). Among all
cancers, oral squamous cell carcinoma (OSCC) accounts for more 2.5
lakh new cases and about 1.3 lakh deaths each year around the globe
(3). If OSCC is detected at an early
stage, treatment with surgery or radiotherapy or the combination of
both and has a five-year survival rates varying between 70 to 90%
(3–6).
However, 2/3 of OSCC patients are diagnosed at advanced stages of
the diseases (6,7). In the present study we evaluated the
anticancer activity of a plant derived natural alkaloid
mecambridine against squamous cell carcinoma HSC-3 oral cell line.
Results indicated that mecambridine exhibited an IC50
value of 50 µM and exerted its cytotoxic effects in a dose
dependent manner on OSCC HSC-3 cell line. Moreover, it was observed
that the mecambridine lessens cell viability and induces autophagy
dose dependently. The underlying mechanism for the induction of
autophagy was found to be ROS mediated in mitochondrial membrane
potential and changes in the expression mTOR/PI3K/Akt signalling
pathway proteins in HSC-3 at the IC50 concentration of
mecambridine. These results strongly stress that mecambridine may
prove to be an anticancer lead molecule for the treatment and of
OSCC.
Materials and methods
Chemicals and regents and cell culture
conditions
The chemicals used in this study include; triton
X-100, dimethyl and sulfoxide (DMSO), RNase A purchased from from
Sigma-Aldrich Co., (St. Louis, MO, USA), primary and secondary
antibodies purchased from Santa Cruz Biotechnology Inc., (Santa
Cruz, CA, USA) and fetal bovine serum (FBS), RPMI-1640 medium,
L-glutamine, antibiotics procured from Invitrogen Life Technologies
(Carlsbad, CA, USA). Squamous cell carcinoma HSC-3 oral cell line
was procured from Cancer Research Institute of Beijing, China, and
it was maintained in DMEM and was supplemented with 10% FBS and
antibiotics (100 µg/ml streptomycin and 100 U/ml penicillin G) in
an incubator at 37°C (5% CO2 and 95% air). Mecambridine
was purchased from Chemical Land21 Company, South Korea.
Determination of IC50 by
MTT assay
The anti-proliferation effect of the mecambridine on
cancer cell line squamous cell carcinoma HSC-3 oral cell line was
evaluated by MTT assay. HSC-3 cells were grown at 1×106
cells per well in 96-well plates for a time period of 12 h and then
exposed to 0, 10, 25, 50, 100, 150 and 200 µM of mecambridine dose
for 24 h. To each well, MTT solution (20 µl of 2.5 mg/ml stock) was
added. Prior to the addition of 500 µl of DMSO, the medium was
completely removed. To solubilize MTT formazan crystals, 500 µl
DMSO was added. ELISA plate reader was used for the determination
of optical density at 570 nm.
Detection of autophagy
Cells were plates at a density of 1.5×105
cells/well treated with either DMSO or 50 µM (IC50), of
mecambridine for 24 h and successively stained with
monodansylcadaverine (MDC) or acridine orange (AO). All cell
samples were observed under microscope and Images were captured for
at least three independent experiments.
Expression of autophagy related
proteins and inhibitor treatment
HSC-3 cells were seeded in 6-well plates at the
density of 1.5×105 cells/well and kept for 24 h. The
cells were then administrated with the mecambridine at
IC50 concentration. Untreated cells were included as
control. Following 24 h of treatment, cells were collected and
lysed for quantification of proteins and expression analysis. For
inhibitor treatment, HSC-3 cells were seeded at the density of
1.5×105 cells/well in 6-well plates and permitted to
adhere for 24 h. Cells were then administrated with 15 µg/ml of the
liposomal inhibitor pepstatin A for 1 h with and then administrated
IC50 concentration of mecambridine for 24 h. untreated
cells were kept as control. Protein expression analysis was carried
out by Western blot analysis.
Evaluation of ROS and MMP
HSC-3 cells were platted at a density of
2×105 cells/well in a 6-well plate and kept for 24 h and
treated with 0, 25, 50 and 100 µM mecambridine for 72 h at 37°C in
5% CO2 and 95% air. Thereafter cells from all samples
were collected, washed 2 times by PBS and re-suspended in 500 µl of
DCFH-DA (10 µM) for ROS estimation and DiOC6 (1 µmol/l)
for MMP at 37°C in dark room for 30 min. The samples were then
examined instantly using flow cytometer as described previously in
literature (8).
Protien expression by western blot
analysis
The mecambridine administrated cells were harvested
and lysed. The protein concentrations of the lysates were
quantified by BCA assay using specific antibodies. β-actin was used
as a control. From each sample equal amounts of protein were loaded
and separated by electrophoresis on a 12% denaturing SDS gel.
Afterwards, the proteins were electroblotted on polyvinylidene
difluoride membranes (0.45 m pore size).
Statistical analysis
All experiments were carried out in triplicates and
presented as representative images or average values ± SD. Results
were considered significant at *P<0.01, **P<0.001,
***P<0.0001.
Results
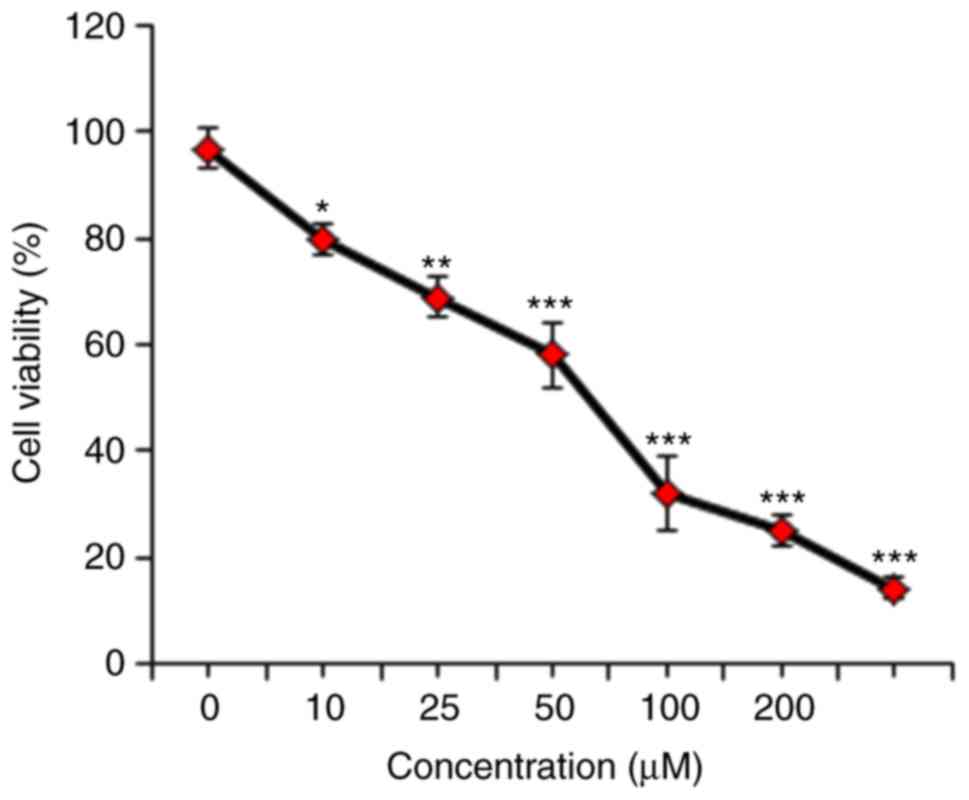
Cytotoxic potential of mecambridineon
HSC-3 cell line
The growth inhibitory role of mecambridine on HSC-3
cells was detected by treatment of these cells with varied
concentrations of mecambridine. Mecambridine displayed the potent
anti-proliferative effect against HSC-3 cells with an
IC50 of 50 µM (Fig. 1).
The anti-proliferative activity of the mecambridine was found be
concentration-dependent.
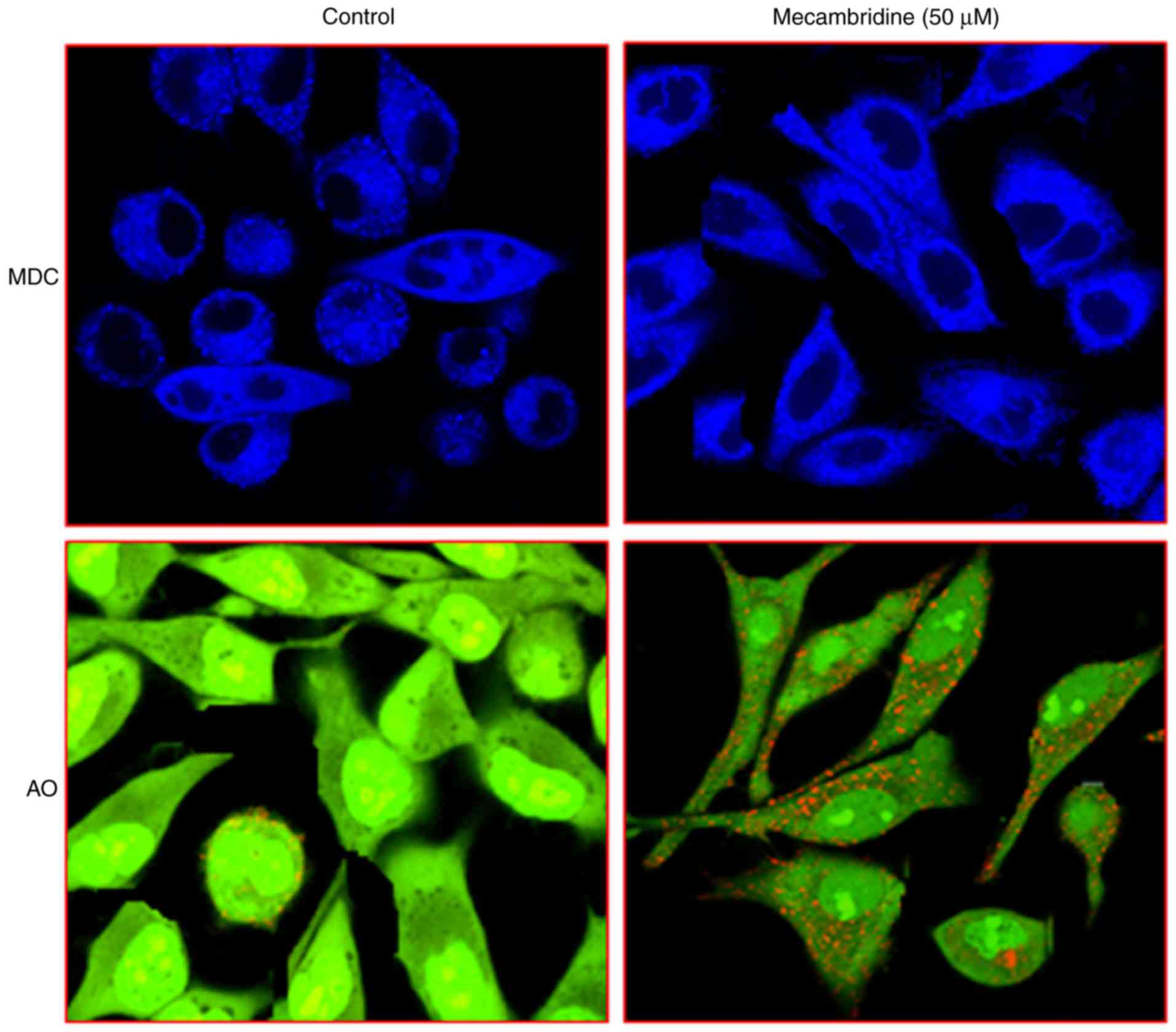
Mecambridine induces autophagy in
HSC-3 cells
In order to confirm that mecambridine induces
autophagy in HSC-3 cells, mecambridine treated cells were stained
with MDC. Vital staining of mecambridine-treated HSC-3 cells with
MDC (monodansylcadaverine, an autophagolysosome marker) and AO
(acridine orange), indicated an increased buildup of the dye as
compared to the control cells. In control the dye is scattered and
comparatively fainter than treatment (Fig. 2). These observations provided strong
clue that mecambridine induces autophagy in HSC-3 cells. To
quantify the increase of the acidic vesicular organelles,
mecambridine-treated cells were treated with acridine orange dye
and it was revealed that there was accumulation of acridine orange
in the mecambridine-treated cells.
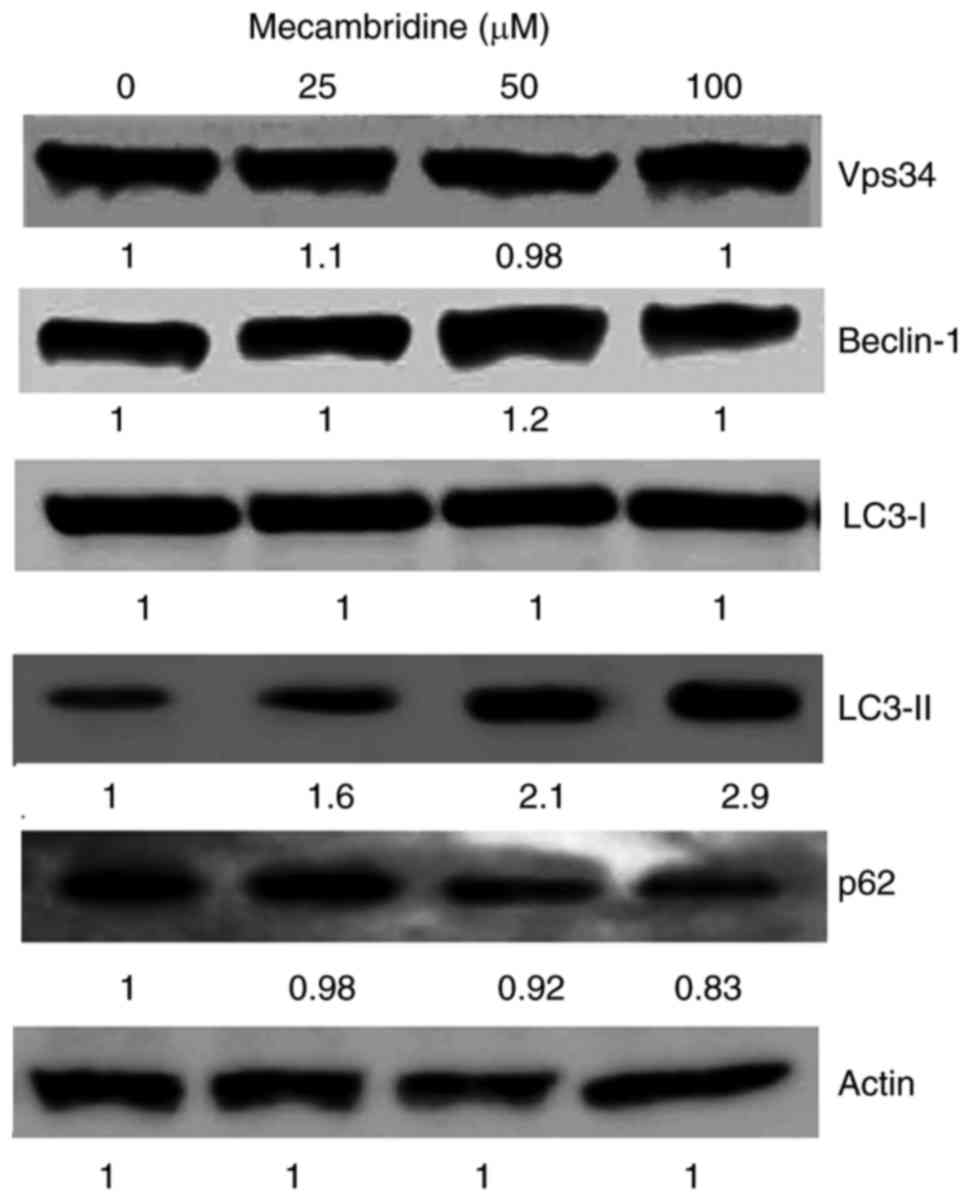
To confirm autophagy, we evaluated the expression of
several autophagy associated proteins. The results indicated that
the treatment with the extract induced the expression of several
autophagy associated proteins (Fig.
3). It was observed there was no change in the expression of
several proteins which include Vps34, Beclin-1, and LC3-I. However
expression of LC3-II was significantly increased in a concentration
dependent manner while as slight reduction in the expression of
together with a slight reduction in the levels of p62 was also
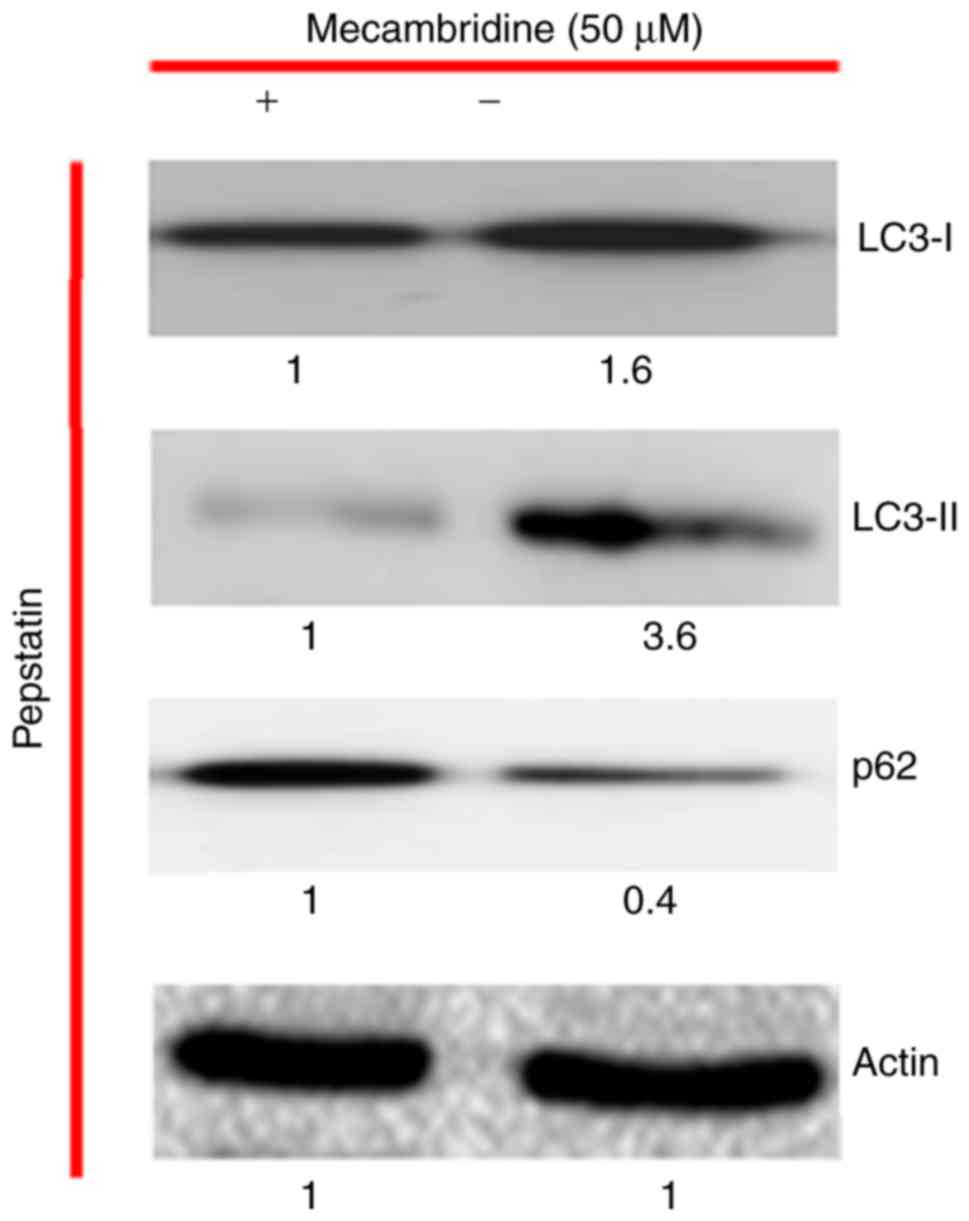
observed. The capacity of the mecambridine to induce autophagy was
further confirmed by the use of autophagy inhibitor, pepstatin. The
results indicated that mecambridine abridged the effect of the
inhibitors (Fig. 4).
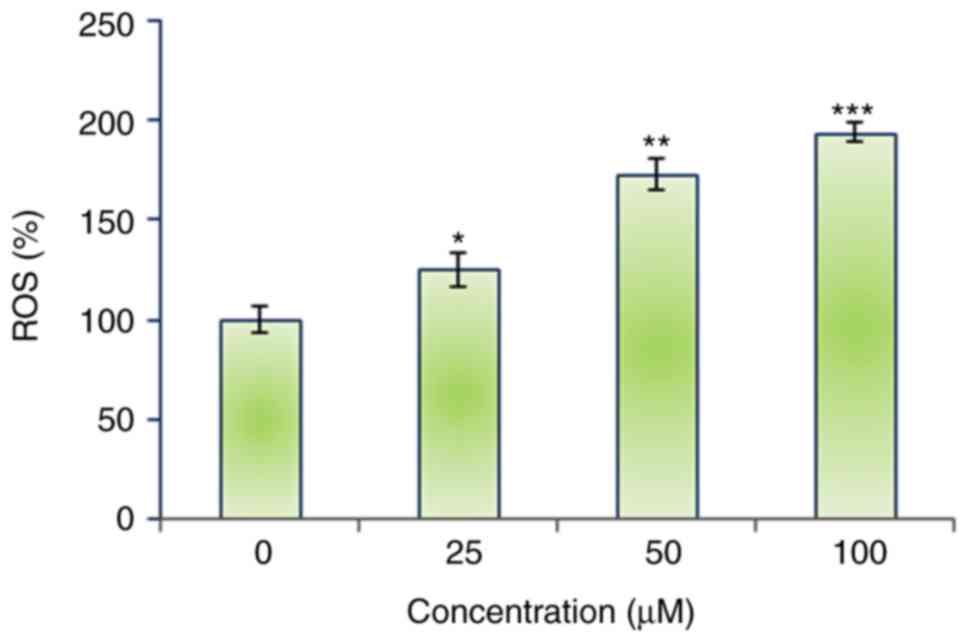
Mecambridine induces the ROS
accretions and MMP reduction in HSC-3 cells
The autophagic potential of mecambridine indicated
that it might induce generation of intracellular ROS. Therefore, we
calculated the ROS level at varied concentrations of mecambridine
for 24 h. The results showed that the intracellular ROS levels of
treated cells increased to 194% at 100 µM as compared to untreated
cells (Fig. 5). Our result suggested
that mecambridine a potent molecule for activating ROS in squamous
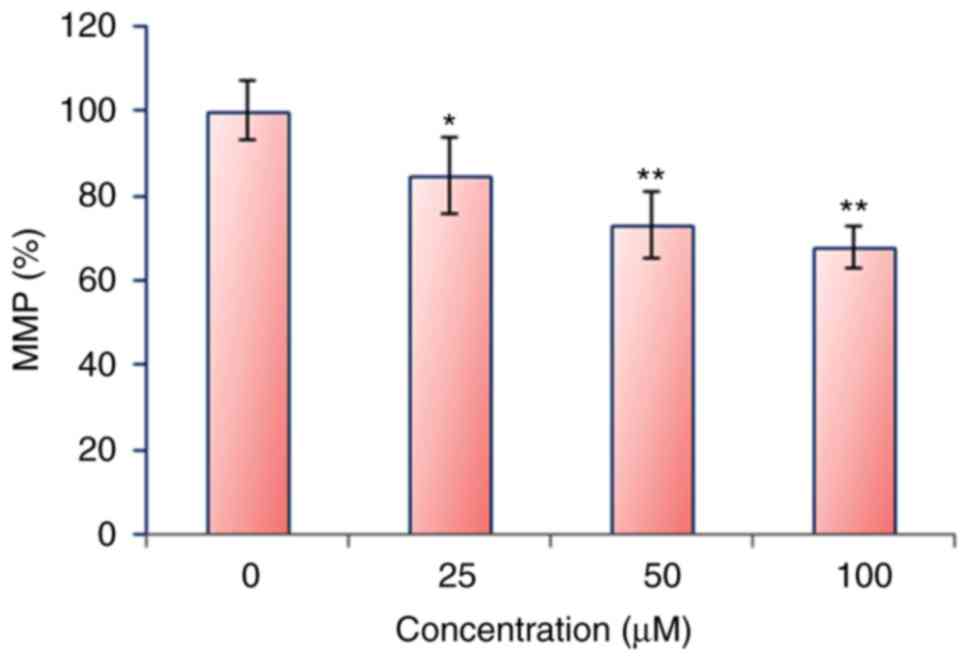
oral carcinoma HSC-3 cells to trigger the autophagy. ROS generation
causes mitochondrial dysfunction. It disrupts the outer
mitochondrial potential to release the death-promoting proteins
(9). Therefore, we examined whether
mecambridine reduces the MMP in squamous oral carcinoma HSC-3 cells
in a concentration dependent manner. Treated squamous oral
carcinoma HSC-3 cells showed a significant reduction in MMP in a
dose-dependent manner. The MMP reduced by 68% at 100 µM of
mecambridine as compared to untreated control (Fig. 6).
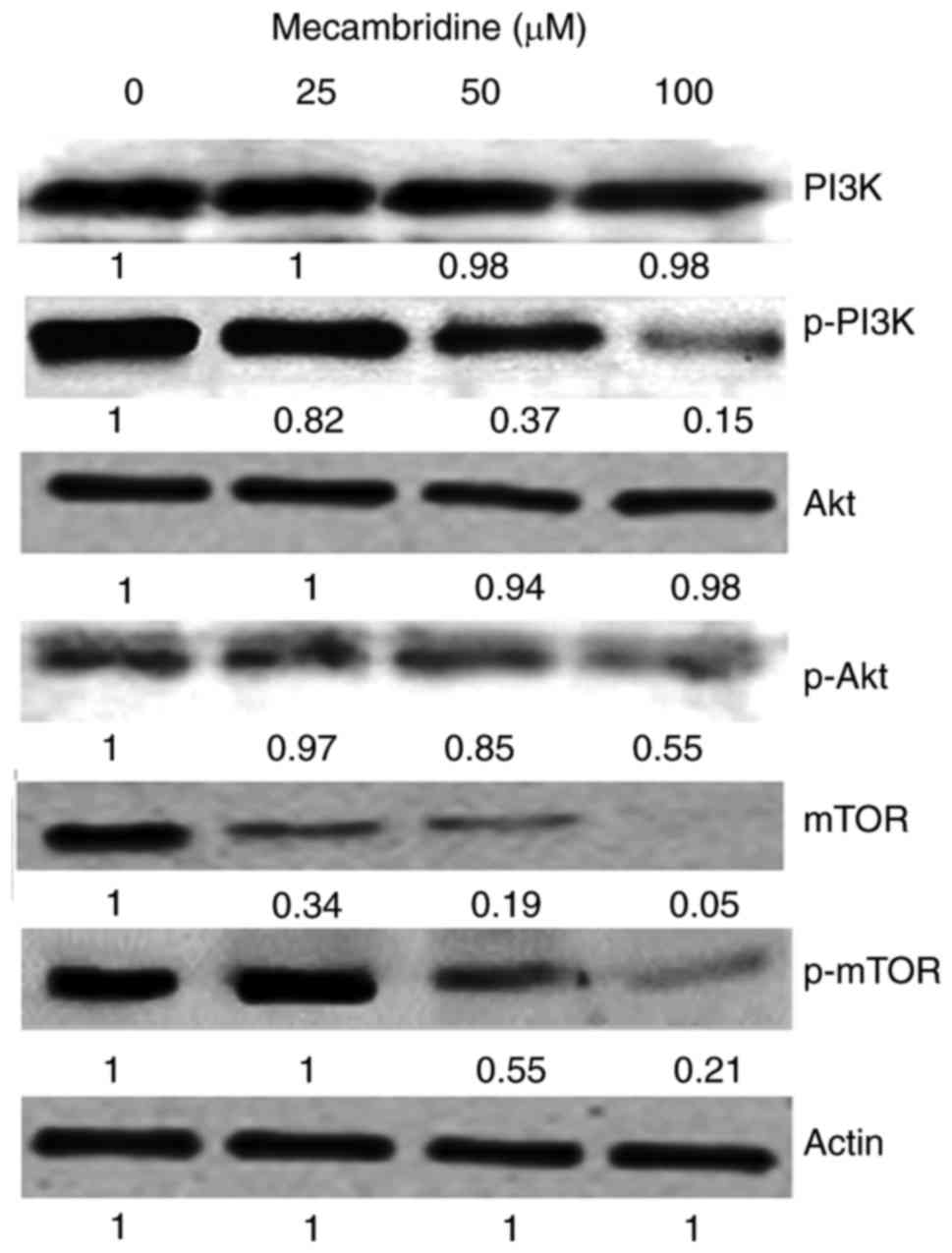
Mecambridine targets m-TOR/PI3K/Akt
signalling pathway
The fact that mecambridine could modulate the
protein expressions of m-TOR/PI3K/Akt signalling pathway was
evaluated by using western blot analysis (Fig. 7). Compared to the untreated control
cells, mecambridine treated HSC-3 cells showed a
concentration-dependent downregulation of m-TOR and pm-TOR
proteins. It also caused downregulation of PI3K/Akt protein
expressions. Thus it may be concluded that mecambridine induced
anticancer and autophagy inducing effects via m-TOR/PI3K/Akt
signalling pathway.
Discussion
Oral squamous cell carcinoma is of the leading
causes of cancer related mortality and about 1.3 lakh new patients
are diagnosed for OSCC every year across the world. Additionally,
treatment options for this type of cancer are limited and currently
available treatments have severe side effects and badly affects the
quality line of the patients (4,5).
Therefore, the present study aimed at determining the anticancer
activity of the mecambridine (a natural alkaloid) against OSCC
HSC-3 cells. The results indicated that the test molecule
mecambridine exerted significant anticancer activity against OSCC
HSC-3 cells in a dose dependent manner with an IC50 of
50 µM. These results suggest that the mecambridine is a potential
source of cytotoxic agents. The cytotoxic effect of mecambridine
was later on reported to be due to the induction of autophagy, as
evident from the accumulation of MDC and acridine orange dyes in
mecambridine treated OSCC HSC-3 cells. Expression of several of the
autophagy associated proteins was evaluated and it was found that
the expression of only LC3-II was highly induced by the
mecambridine in OSCC HSC-3 cells. Furthermore mecambridine
exhibited a strong potential to abridge the expression of autophagy
inhibitors, providing a strong clue towards the role of this
molecule in the execution of autophagy.
Moreover, results indicated that mecambridine
treated cells displayed ROS mediated MMP reduction. Therefore, the
results suggest that the mecambridine may induce autophagy through
increasing intracellular ROS and reduction in MMP. Our results are
in agreement with studies wherein several anti-tumor agents have
been reported to target cancer cells partly by accretion of high
levels of ROS (10–15). Finally, effects of mecambridine on the
expression levels of various proteins including m-TOR, pm-TOR,
PI3K, p-PI3K and Akt were studied using western blot assay. Results
showed mecambridine-treated OSCC HSC-3 cells showed a
concentration-dependent downregulation of m-TOR and pm-TOR
proteins. It also caused downregulation of PI3K/Akt protein
expressions. Therefore, inhibitory effect of mecambridineon OSCC
HSC-3 cells may prove crucial in the treatment and management of
OSCC.
In conclusion, the present results suggest that
mecambridine induces anticancer and autophagy effects via the
m-TOR/PI3K/Akt signalling pathway. The mechanism involved will be
further studies in the future.
Acknowledgements
The study was supported by Science and Technology
Plan Project of Mudanjiang (grant no. Z2016s0077).
References
|
1
|
McChesney JD, Venkataraman SK and Henri
JT: Plant natural products: Back to the future or into extinction?
Phytochem. 68:2015–2022. 2007. View Article : Google Scholar
|
|
2
|
George S, Bhalerao SV, Lidstone EA, Ahmad
IS, Abbasi A, Cunningham BT and Watkin KL: Cytotoxicity screening
of Bangladeshi medicinal plant extracts on pancreatic cancer cells.
BMC Complement Altern Med. 10:522010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: Cancer Incidence and Mortality Worldwide: IARC Cancer Base
No. 11 [Internet]. Lyon, France: International Agency for Research
on Cancer; 2013, GLOBOCAN 2012 v1.0, 2012. http://globocan.iarc.fr
|
|
4
|
Scully C and Bagan JV: Recent advances in
Oral Oncology 2007: Imaging, treatment and treatment outcomes. Oral
Oncol. 44:211–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagao T, Chaturvedi P, Shaha A and
Sankaranarayanan R: Prevention and early detection of head and neck
squamous cell cancers. J Oncol. 2011:3181452011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rousseau A and Badoual C: Head and Neck:
Squamous cell carcinoma: An overview. Atlas Gen Cytogenetics Oncol
Haematol. 16:145–155. 2012.
|
|
8
|
Hissin PJ and Hilf R: A fluorometric
method for determination of oxidized and reduced glutathione in
tissues. Anal Biochem. 74:214–226. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Azuma M, Tamatani T, Ashida Y, Takashima
R, Harada K and Sato M: Cisplatin induces apoptosis in oral
squamous carcinoma cells by the mitochondria-mediated but not the
NF-kappa B-suppressed pathway. Oral Oncol. 39:282–289. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shoemaker RH: The NCI60 human tumour cell
line anticancer drug screen. Nat Rev Cancer. 6:813–823. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sreelatha S, Jeyachitra A and Padma PR:
Antiproliferation and induction of apoptosis by Moringa oleifera
leaf extract on human cancer cells. Food Chem Toxicol.
49:1270–1275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rejiya CS, Cibin TR and Abraham A: Leaves
of Cassia tora as a novel cancer therapeutic-An in vitro study.
Toxicol In Vitro. 23:1034–1038. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding H, Han C, Guo D, Chin YW, Ding Y,
Kinghorn AD and D'Ambrosio SM: Selective induction of apoptosis of
human oral cancer cell lines by avocado extracts via a ROS mediated
mechanism. Nutr Cancer. 61:348–356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kowaltowski AJ, de Souza-Pinto NC,
Castilho RF and Vercesi AE: Mitochondria and reactive oxygen
species. Free Radic Biol Med. 47:333–343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aadil K, Manzoor AR and Rafiya R:
Plant-based natural compounds and herbal extracts as promising
apoptotic agents: Their implications for cancer prevention and
treatment. Advan Biomed Pharma. 3:245–269. 2016.
|