Introduction
Esophageal carcinoma is one of the most aggressive
types of malignant tumor (1).
Multimodal treatments, including neoadjuvant chemotherapy and
chemoradiotherapy (CRT), have been demonstrated to improve the
survival rate of patients with locally advanced esophageal
carcinoma (2). Cisplatin, a
representative platinum-containing drug, is a key drug in the
chemotherapy of esophageal squamous cell carcinoma (ESCC) and a
number of other types of malignancy in Japan (3–5). Cisplatin
dose-dependently exerts potent antitumor effects; however, it also
induces dose-dependent nephrotoxicity. Cisplatin-induced
nephrotoxicity is mild and reversible in the majority of cases, but
it can also be severe and irreversible, resulting in the
discontinuation of chemotherapy (3).
Hypomagnesemia and renal magnesium (Mg) wasting are
established side effects of patients receiving cisplatin-containing
chemotherapy (6,7). This Mg depletion has been demonstrated
to further enhance renal platinum accumulation and
cisplatin-induced nephrotoxicity. Therefore, oral or intravenous Mg
supplementation can confer renal protective effects against
cisplatin-induced nephrotoxicity (8–12).
In normal conditions, the kidney is the principal
organ for regulating Mg homeostasis, and serum Mg concentrations
are maintained within normal ranges by excreting excess Mg from the
serum into urine, a rapid process (13). Due to this strict regulation by the
kidney, no hormone is particularly associated with the maintenance
of Mg homeostasis; however, large amounts of parathyroid hormone
(PTH) influence serum Mg concentrations by affecting loop of Henle
and bone resorption (13). Therefore,
PTH or parathyroid hormone-related protein (PTH-rP), which was
previously reported to be secreted by certain types of tumor cell,
including ESCC (14), may influence
the physiological regulation of Mg homeostasis and
cisplatin-induced nephrotoxicity in patients receiving
chemotherapy.
In the present study, Mg was administered
intravenously to patients with ESCC treated with a high-dose
cisplatin-containing regimen, and the protective effects of Mg
supplementation against cisplatin-induced nephrotoxicity were
prospectively examined in relation to PTH and PTH-rP levels.
Materials and methods
Patients and treatment courses
A total of 55 patients, including 43 males and 12
females with primary ESCC receiving high-dose cisplatin-containing
chemotherapy as neoadjuvant therapy (age range, 47–84 years;
median, 66 years), were investigated between January 2013 and
December 2014 at the University Hospital, Kyoto Prefectural
University of Medicine (Kyoto, Japan). The concentrations of high
sensitive PTH, PTH-rP, creatinine and Mg were measured using
in-hospital biochemical tests available for clinical use in all
patients before any treatments. The concentrations of creatinine
and Mg were monitored until the next cycle of chemotherapy
commenced (~3 weeks). No patients exhibited malnutrition or
dehydration during treatment. Clinical and pathological staging
were performed according to the criteria of the Japanese
Classification of Esophageal Cancer, tenth edition (4), and the Tumor-Node-Metastasis
Classification System of the International Union Against Cancer,
seventh edition (15).
The present study was conducted in accordance with
the principles of the Declaration of Helsinki, and written informed
consent for the treatments and data collection was obtained from
all patients. Ethical approval from the Facility of Science
Committee at the Kyoto Prefectural University of Medicine was not
required as the study was an observational study without
interpositions with the medical practice necessary for therapeutic
purpose.
Cisplatin-containing chemotherapy and
Mg supplement regimens
The regimens for the majority of the patients were
standard FP [5-fluorouracil (5FU; 800 mg/m2/day, days
1–5) and cisplatin (80 mg/m2/day, day 1)] (5) or DCF [5FU (700 mg/m2/day,
days 1–5), cisplatin (70 mg/m2/day, day 4) and docetaxel
(70 mg/m2/day, day 1)] (16). The modified FP regimen of 5FU (700
mg/m2/day, days 1–5) and cisplatin (70
mg/m2/day, day 1) was combined with radiotherapy for
patients treated with CRT, as previously described (17). The eligibility criteria for these
chemotherapy regimens were performance status 0 or 1, creatinine
level ≤1.5 mg/dl, and adequate organ function; no age criteria was
imposed at the University Hospital, Kyoto Prefectural University of
Medicine. Patients treated with <60 mg/m2 of
cisplatin were excluded from the study.
The regimens additionally included pre-hydration
with 1,000 ml of saline, a 5HT3 receptor antagonist
(palonosetron, 0.75 mg/day, day 1–5), aprepitant (125 mg/day, day
1; 80 mg/day, day 2–3) and dexamethasone (6.6 mg/day, day 1–5); 8
mEq of magnesium sulfate (day 1) was simultaneously administered
within 2–3 h. Following these pre-treatments, high-dose cisplatin
was administrated; post-hydration with 2,000 ml of electrolyte
liquid, 60 g of D-mannitol and 20 mg of furosemide (day 1) were
also performed. The electrolyte liquid included sodium, potassium
and calcium ions in the external solution.
Mg supplementation for the patients was not
determined at random; patients treated at the Division of Digestive
Surgery typically received chemotherapy with Mg supplementation;
however, patients treated at the Division of Gastrointestinal
Medicine frequently received chemotherapy without Mg
supplementation. The regimens excluding Mg supplementation,
including hydration or other administrations, were confirmed to be
the same between divisions. Appropriate treatments were performed
for all hematological and non-hematological adverse events.
Evaluation of nephrotoxicity due to
cisplatin
According to a previous study (18), the extent of nephrotoxicity was
evaluated by increases in serum creatinine concentrations. A
>1.1-fold increase in post-therapeutic creatinine concentrations
from pre-therapeutic concentrations was defined as an increase. The
grade of creatinine increase was indicated according to the
National Cancer Institute Common Terminology Criteria for Adverse
Events version 4.0 (19), as follows:
Grade 1 indicated creatinine concentrations from above the upper
limit of normal (ULN) to ≤1.5× ULN; grade 2, >1.5× and ≤3× ULN;
grade 3, >3× and ≤6× ULN; and grade 4, >6× ULN. The most
elevated creatinine concentration within the 3 weeks following the
first chemotherapy cycle was compared with the pre-therapeutic
concentration.
Statistical analysis
Statistical analyses were performed using StatView
5.0J software (SAS Institute, Inc., Cary, NC, USA). The Wilcoxon
signed-rank test or Mann-Whitney U test was used to analyze the
associations between various biochemical measurements. Spearman's
correlation test was used to determine the correlation between
pre-therapeutic creatinine concentrations and alterations in
creatinine concentration. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
Patient characteristics are included in Table I. All the patients in the present
study were diagnosed with SCC. A total of 37 out of the 55 patients
received Mg supplementation; the remaining 18 patients did not. FP
was administered to 46 patients, including 5 patients receiving
CRT, whereas DCF was administered to 9 patients. FP therapy was
more frequent for patients receiving Mg supplementation (P=0.02).
The majority of the patients were clinically diagnosed with stage
II or III disease. No significant differences were observed in
clinicopathological features between the 2 groups with and without
Mg supplementation, except for the types of chemotherapy.
 | Table I.Characteristics of patients with
esophageal squamous cell carcinoma with and without Mg
supplementation. |
Table I.
Characteristics of patients with
esophageal squamous cell carcinoma with and without Mg
supplementation.
|
| Mg
supplementation |
|---|
|
|
|
|---|
| Characteristics | Absent | Present |
|---|
| Total | 18 | 37 |
| Age, median
(range) | 65 (56–75) | 67 (47–84) |
| Sex |
|
Male | 15 | 28 |
|
Female | 3 | 9 |
| Performance
status |
| 0 | 14 | 29 |
| 1 | 4 | 8 |
| Chemotherapy |
|
|
| FP
alone | 11 | 30 |
| FP
chemoradiotherapy | 1 | 4 |
|
Docetaxel, cisplatin and
5-fluorouracil | 6 | 3 |
| Location |
|
Cervical or upper thoracic
esophagus | 3 | 6 |
| Middle
thoracic esophagus | 11 | 21 |
| Lower
thoracic or abdominal esophagus | 4 | 10 |
| T stage |
| 1 | 1 | 1 |
| 2 | 1 | 4 |
| 3 | 11 | 28 |
| 4 | 5 | 4 |
| Lymph node
metastasis |
|
Absent | 5 | 13 |
|
Present | 13 | 24 |
| Disease stage |
| I | 0 | 1 |
| II | 4 | 11 |
|
III | 11 | 23 |
| IV | 3 | 2 |
Effects of Mg supplementation
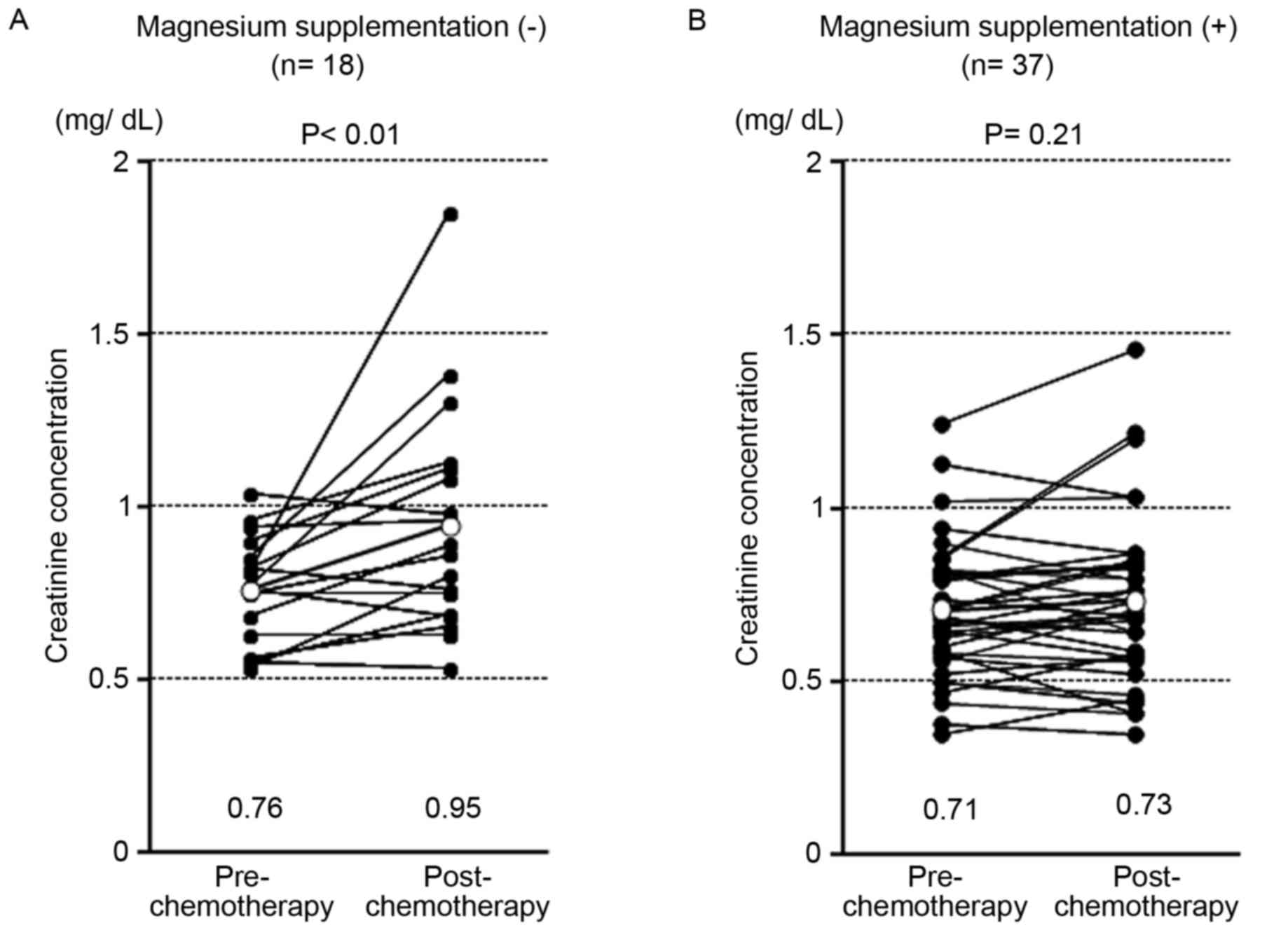
Post-chemotherapeutic creatinine concentrations were
significantly higher in patients without Mg supplementation
(P<0.01; Fig. 1A) than with Mg
supplementation (P=0.21; Fig. 1B).
The frequency of creatinine increases due to chemotherapy is
included in Table II. In patients
without Mg supplementation, 22.2% experienced grade 1 and 5.6%,
grade 2 creatinine increases, whereas 8.1% of patients receiving Mg
supplementation experienced grade 1 creatinine increases.
 | Table II.Patients in the Mg absent and present
groups experiencing an increase in creatinine. |
Table II.
Patients in the Mg absent and present
groups experiencing an increase in creatinine.
|
| Mg
supplementation |
|---|
|
|
|
|---|
| Increase in
creatinine, n (%) | Absent | Present |
|---|
| Total | 18 | 37 |
| Grade 1 | 4
(22.2) | 3 (8.1) |
| Grade 2 | 1 (5.6) | 0 |
| Grade 3 | 0 | 0 |
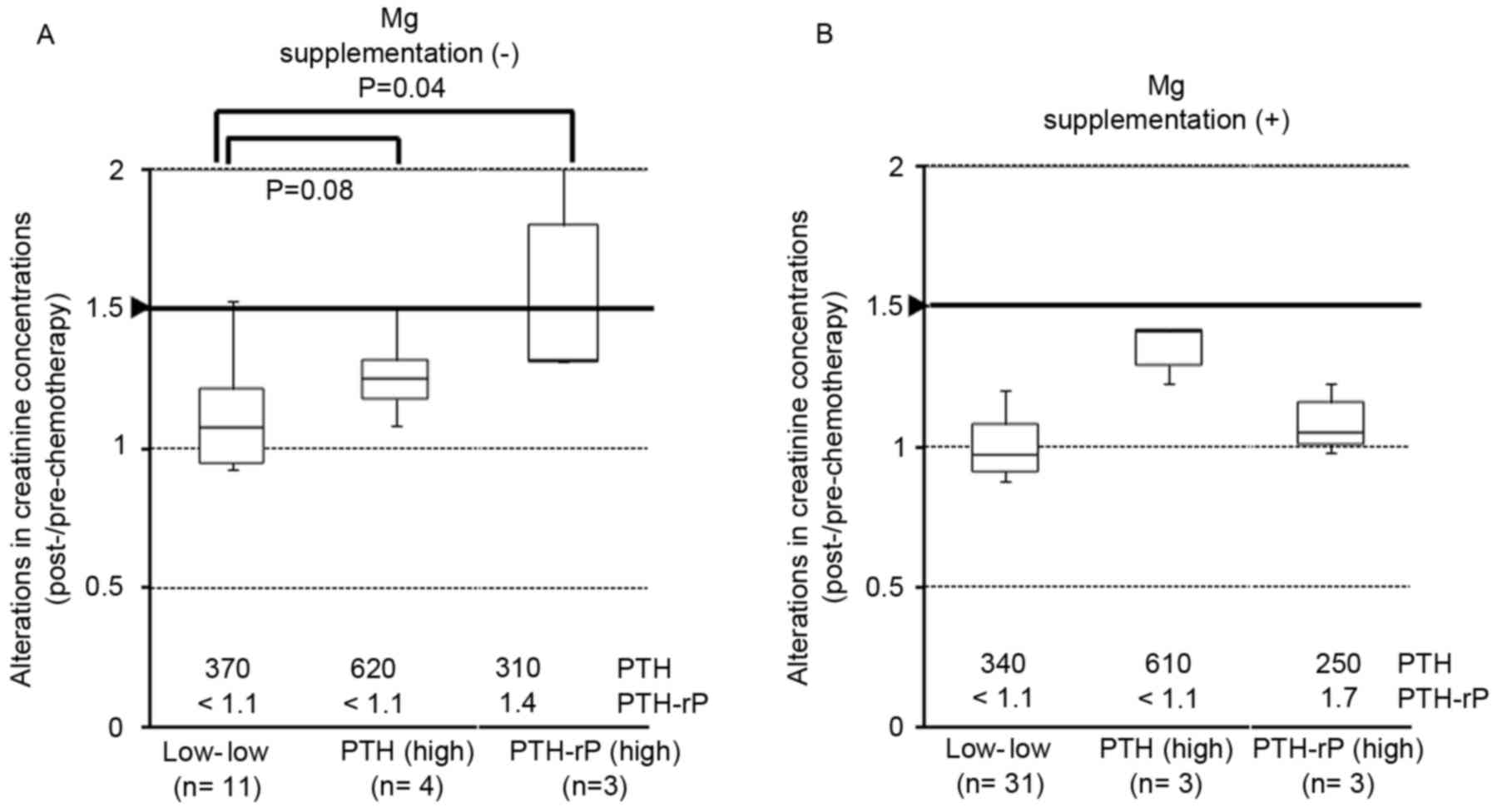
Increases in PTH or PTH-rP and the
influence on creatinine concentrations
PTH and PTH-rP were high in 8 (14.5%) and 6 (10.9%)
patients, respectively (Table III).
Alterations in creatinine concentration following high-dose
cisplatin-containing regimen tended to be higher in the high of PTH
or PTH-rP level groups than in the low level groups (P=0.08 and
P=0.04, respectively; Fig. 2A). These
alterations were reduced in patients receiving Mg supplementation,
particularly in the low PTH and PTH-rP level group and the high
PTH-rP level group (Fig. 2B).
 | Table III.Frequency of increases in PTH or
PTH-rP. |
Table III.
Frequency of increases in PTH or
PTH-rP.
|
| PTH-rP level, n
(%) |
|
|---|
|
|
|
|
|---|
| PTH level,
pg/ml | Low (<1.1
pmol/l) | High (≥1.1
pmol/l) | Total, n (%) |
|---|
| Low (<520) | 42 (76.4) | 5 (9.1) | 47 (85.5) |
| High (≥520) | 7
(12.7) | 1 (1.8) | 8
(14.5) |
| Total | 49 (89.1) | 6
(10.9) |
|
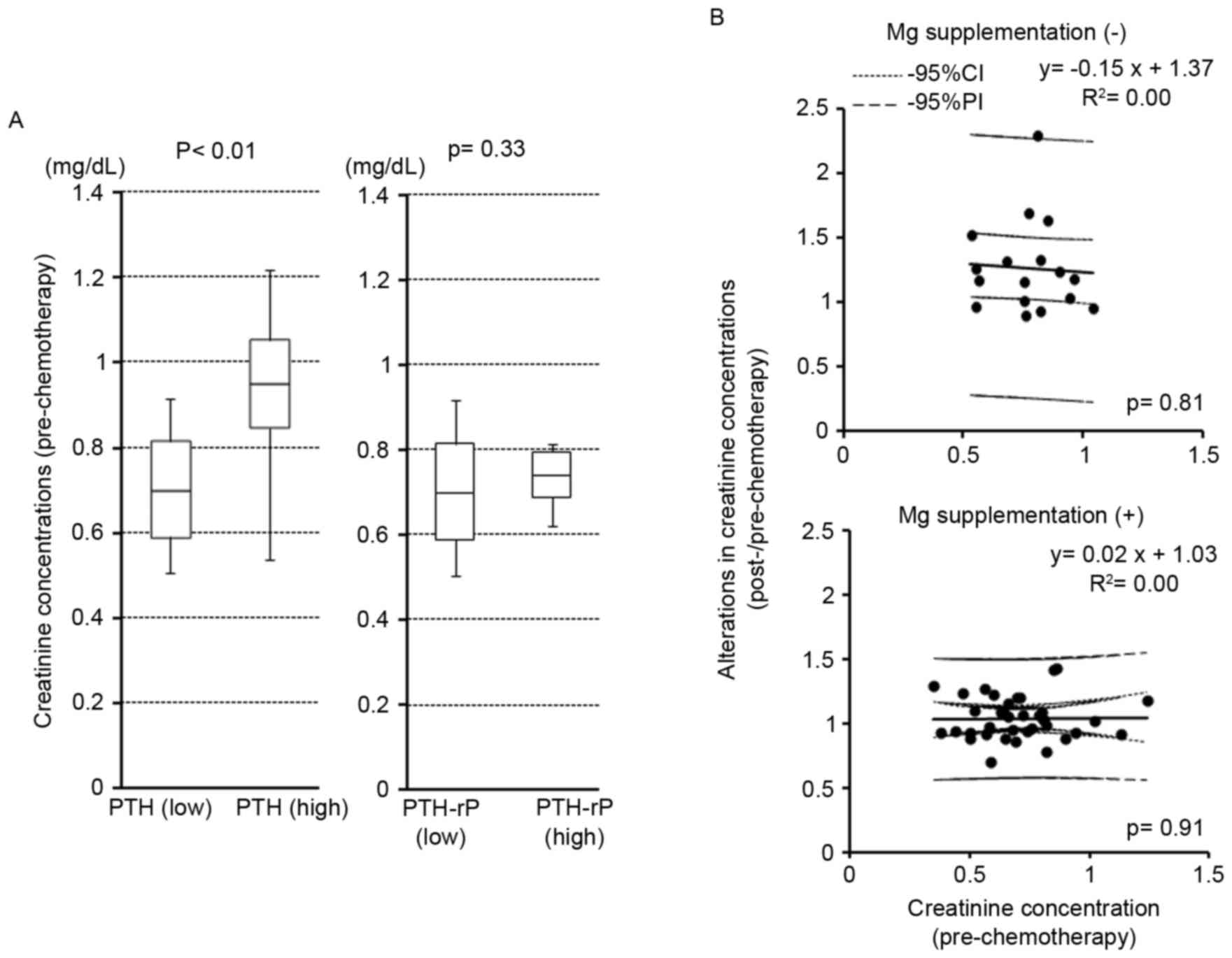
Association of pre-therapeutic
creatinine with PTH or PTH-rP levels and creatinine
alterations
Pre-therapeutic creatinine concentrations were
significantly higher in patients with high levels of PTH
(P<0.01; Fig. 3A); however, they
did not differ between high and low PTH-rP groups (P=0.33; Fig. 3A). Pre-therapeutic creatinine
concentrations did not correlate with the alterations to creatinine
concentrations following cisplatin-containing chemotherapy in the
patients with or without Mg supplementation (P=0.81 and P=0.91,
respectively; Fig. 3B).
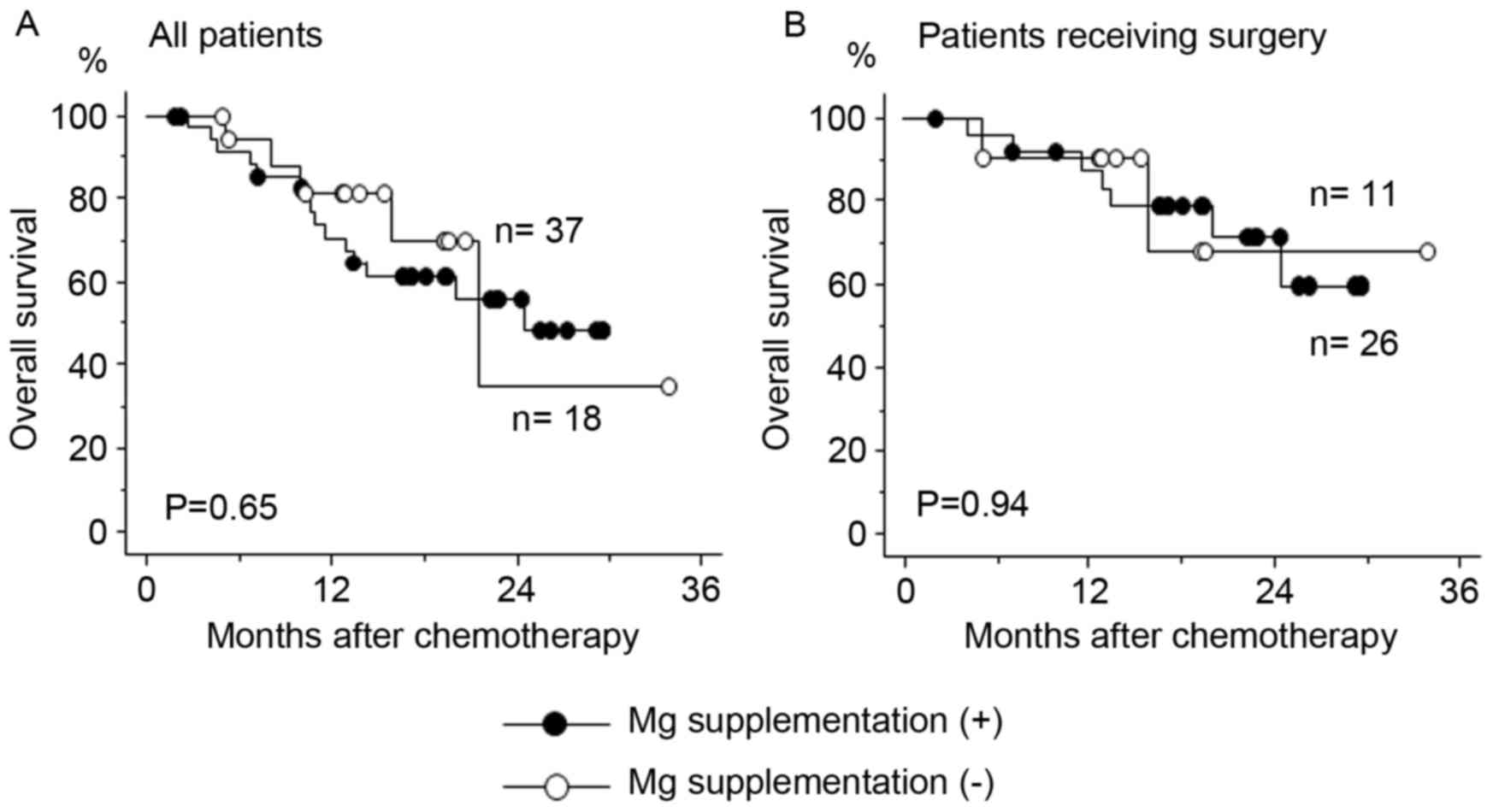
Survival analysis of the patients with
or without Mg supplementation
Mg supplementation did not affect the efficacy of
high-dose cisplatin-containing chemotherapy, as the overall
survival rate was similar between the patients with or without Mg
supplementation regardless of whether surgery was performed
subsequent to chemotherapy (Fig.
4).
Discussion
Cisplatin has been used in combination with other
drugs as chemotherapy for various types of malignancy, including
digestive tumors (3). The efficacy of
administering high-dose cisplatin has been confirmed; however,
severe adverse events, including hematotoxicity, anorexia, nausea
and nephrotoxicity have been also reported (3,6,7). Large amounts of hydration with saline,
mannitol and furosemide are accepted as the standard of care for
patients treated with regimens containing high-dose (≥60
mg/m2) cisplatin (3,10,11). Previous studies have demonstrated that
Mg supplementation also confers protective effects against
cisplatin-induced nephrotoxicity (10,20–22).
In the present study, the focus was on intravenous
Mg supplementation for patients with ESCC treated with high-dose
cisplatin. The results obtained confirmed that increases in
post-chemotherapeutic creatinine concentrations were significantly
suppressed by magnesium supplementation (Fig. 1 and Table
II). The protective effects of oral or intravenous Mg
supplementation against cisplatin-induced nephrotoxicity have been
reported in various types of malignancy, including in lung
(10–12), head and neck (10,23,24),
digestive (8,10), testicular (25), ovarian (9,24) and
bladder (24) cancer. However, just a
limited number of clinical reports have demonstrated the protective
effects of intravenous Mg supplementation against cisplatin-induced
nephrotoxicity in ESCC (10).
PTH or PTH-rP levels in patients with ESCC were also
considered; an association between increases in post-therapeutic
creatinine concentrations and high levels of PTH was identified
(Fig. 2). Previous studies reported
that PTH-rP was expressed in a range of types of cancer,
particularly ESCC and lung cancer, and mimicked the effects of PTH
(14,26,27). In
normal conditions, PTH is not an important physiological regulator
of Mg homeostasis as renal regulation is, relatively, much more
efficacious. However, it has been suggested that a large amount of
PTH regulates serum Mg concentrations by decreasing urinary Mg
excretion through influences on loop of Henle and bone resorption
(13).
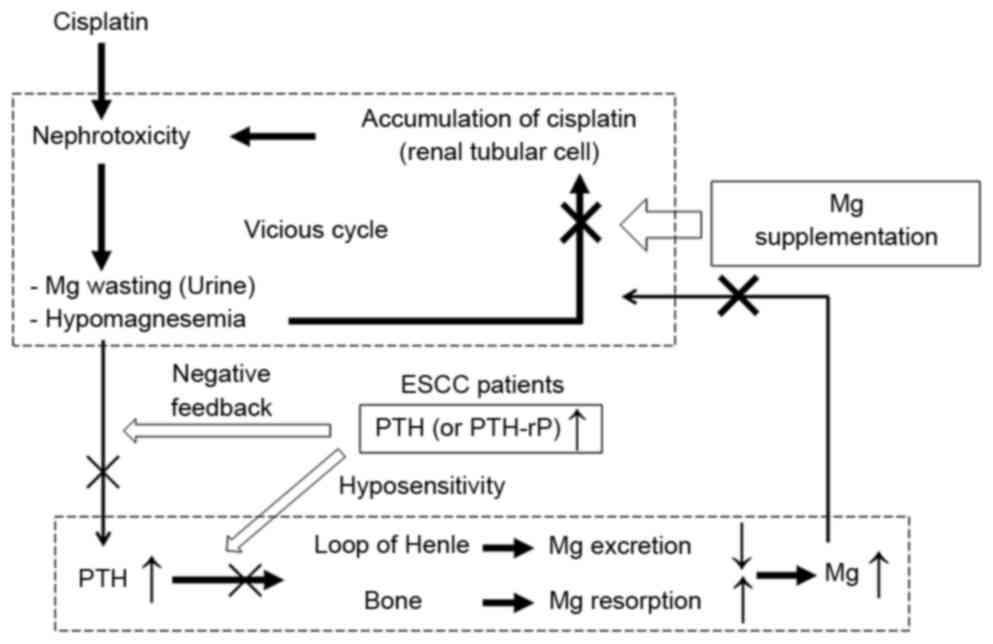
Fig. 5 is a schema for
the rationale of the present study. Cisplatin induces renal Mg
wasting and hypomagnesemia through its renal toxicity by directly
injuring the mechanisms of Mg reabsorption (6,7,28). Hypomagnesemia reduces the expression
of certain transporters in renal tubules in order to maintain serum
Mg concentrations. This reduction in tubular transporters also
amplifies the renal accumulation of cisplatin, which is accompanied
by further nephrotoxicity (20–22). In
this vicious cycle, we hypothesize that Mg supplementation protects
against nephrotoxicity by blocking the augmented accumulation of
cisplatin.
Alternatively, reductions in serum Mg levels due to
cisplatin will induce increases in parathyroid hormone and serum Mg
in order to resolve nephrotoxicity (Fig.
5). In patients with ESCC with high levels of PTH or PTH-rP, we
hypothesize that rapid serum Mg increase will be disrupted by
hyposensitivity to increases in PTH (29) or a negative-feedback mechanism for
lowering serum Mg (30,31), also implicated in calcium regulation.
Therefore, patients with higher levels of PTH or PTH-rP may have
more difficulties with hypomagnesemia and suppressing
cisplatin-induced nephrotoxicity than patients with lower PTH or
PTH-rP levels.
Approximately 8 mEq of Mg is excreted into the urine
each day and reabsorbed during Mg deprivation (13). On this basis, 8 mEq of magnesium
sulfate was administered prior to the administration of cisplatin,
which was demonstrated to be similar to the volume excreted each
day in a previous study (11).
However, it currently remains unclear whether the volume and route
of supplemented Mg was adequate.
Kidera et al (10) suggested that Mg supplementation was
effective for protecting against renal toxicity induced by
cisplatin. They also demonstrated that nephrotoxicity was more
likely for patients with esophageal cancer. Although detailed
tissue types were not considered in the present study, the results
regarding increases in PTH or PTH-rP levels in patients with ESCC
may be associated with this specificity. However, previous studies
reported that Mg supplementation did not affect the tumor response
to cisplatin-based chemotherapy (9,11,25). It was also concluded in the present
study that Mg supplementation did not affect the efficacy of
cisplatin-containing chemotherapy, as the overall survival time was
similar between the patients with or without Mg supplementation
(Fig. 4).
However, certain points in the present study remain
undetermined. The reason why the level of PTH was increased in
patients with ESCC was not identified. In addition to high
sensitive PTH, the level of intact PTH was also measured, and the
frequency of its increase being detected was low (data not shown).
The frequency of intact PTH or PTH-rP increase was markedly lower
than high sensitive PTH, although the results of high sensitive PTH
may be influenced by other PTH subtypes, including PTH-rP, due to
their similar structures. Although the levels of PTH and PTH-rP
were analyzed separately in the present study, their effects on
creatinine increases may be similar.
Alternatively, PTH levels may be influenced by
chronic renal dysfunction. In the present study, pre-therapeutic
creatinine concentrations were significantly higher in the patients
with high levels of PTH, and not for patients with high levels of
PTH-rP (Fig. 3A). The effects of Mg
supplementation on creatinine alterations were also slightly
different between patients with high levels of PTH and PTH-rP.
These results require further investigation in order to confirm the
influence of PTH or PTH-rP on cisplatin-induced nephrotoxicity.
In conclusion, although performed on a small scale,
the present prospective study revealed that intravenous magnesium
supplementation conferred protective effects against
cisplatin-induced nephrotoxicity in patients with ESCC.
Furthermore, increases in PTH or PTH-rP levels may influence
nephrotoxicity.
Glossary
Abbreviations
Abbreviations:
|
CRT
|
chemoradiotherapy
|
|
ESCC
|
esophageal squamous cell carcinoma
|
|
Mg
|
magnesium
|
|
PTH
|
parathyroid hormone
|
|
PTH-rP
|
parathyroid hormone-related
protein
|
|
ULN
|
upper limit of normal
|
References
|
1
|
Hofstetter W, Swisher SG, Correa AM, Hess
K, Putnam JB Jr, Ajani JA, Dolormente M, Francisco R, Komaki RR,
Lara A, et al: Treatment outcomes of resected esophageal cancer.
Ann Surg. 236:376–384. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sjoquist KM, Burmeister BH, Smithers BM,
Zalcberg JR, Simes RJ, Barbour A and Gebski V; Australasian
Gastro-Intestinal Trials Group, : Survival after neoadjuvant
chemotherapy or chemoradiotherapy for resectable oesophageal
carcinoma: An updated meta-analysis. Lancet Oncol. 12:681–692.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang D and Lippard SJ: Cellular processing
of platinum anticancer drugs. Nat Rev Drug Discov. 4:307–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Japanese Society for Esophageal Diseases,
. Japanese Classification of Esophageal Cancer. 10th. Kanehara
& Co., Ltd.; Tokyo, Japan: 2007
|
|
5
|
Ando N, Kato H, Igaki H, Shinoda M, Ozawa
S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lajer H and Daugaard G: Cisplatin and
hypomagnesemia. Cancer Treat Rev. 25:47–58. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lam M and Adelstein DJ: Hypomagnesemia and
renal magnesium wasting in patients treated with cisplatin. Am J
Kidney Dis. 8:164–169. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Evans TR, Harper CL, Beveridge IG,
Wastnage R and Mansi JL: A randomised study to determine whether
routine intravenous magnesium supplements are necessary in patients
receiving cisplatin chemotherapy with continuous infusion
5-fluorouracil. Eur J Cancer. 31A:1–178. 1995.
|
|
9
|
Bodnar L, Wcislo G, Gasowska-Bodnar A,
Synowiec A, Szarlej-Wcisło K and Szczylik C: Renal protection with
magnesium subcarbonate and magnesium sulphate in patients with
epithelial ovarian cancer after cisplatin and paclitaxel
chemotherapy: A randomised phase II study. Eur J Cancer.
44:2608–2614. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kidera Y, Kawakami H, Sakiyama T, Okamoto
K, Tanaka K, Takeda M, Kaneda H, Nishina S, Tsurutani J, Fujiwara
K, et al: Risk factors for cisplatin-induced nephrotoxicity and
potential of magnesium supplementation for renal protection. PLoS
One. 9:e1019022014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muraki K, Koyama R, Honma Y, Yagishita S,
Shukuya T, Ohashi R, Takahashi F, Kido K, Iwakami S, Sasaki S, et
al: Hydration with magnesium and mannitol without furosemide
prevents the nephrotoxicity induced by cisplatin and pemetrexed in
patients with advanced non-small cell lung cancer. J Thorac Dis.
4:562–568. 2012.PubMed/NCBI
|
|
12
|
Yoshida T, Niho S, Toda M, Goto K, Yoh K,
Umemura S, Matsumoto S, Ohmatsu H and Ohe Y: Protective effect of
magnesium preloading on cisplatin-induced nephrotoxicity: A
retrospective study. Jpn J Clin Oncol. 44:346–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rude RK: Magnesium metabolism and
deficiency. Endocrinol Metab Clin North Am. 22:377–395.
1993.PubMed/NCBI
|
|
14
|
Rabbani SA: Molecular mechanism of action
of parathyroid hormone related peptide in hypercalcemia of
malignancy: Therapeutic strategies (Review). Int J Oncol.
16:197–206. 2000.PubMed/NCBI
|
|
15
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union Against Cancer: TNM Classification of
Malignant Tumours. 7th. Wiley-Blackwell; Hoboken, NJ: pp. 66–72.
2010
|
|
16
|
Hara H, Tahara M, Daiko H, Kato K, Igaki
H, Kadowaki S, Tanaka Y, Hamamoto Y, Matsushita H, Nagase M and
Hosoya Y: Phase II feasibility study of preoperative chemotherapy
with docetaxel, cisplatin, and fluorouracil for esophageal squamous
cell carcinoma. Cancer Sci. 104:1455–1460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishida K, Ando N, Yamamoto S, Ide H and
Shinoda M: Phase II study of cisplatin and 5-fluorouracil with
concurrent radiotherapy in advanced squamous cell carcinoma of the
esophagus: A Japan Esophageal Oncology Group (JEOG)/Japan Clinical
Oncology Group trial (JCOG9516). Jpn J Clin Oncol. 34:615–619.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stewart DJ, Dulberg CS, Mikhael NZ,
Redmond MD, Montpetit VA and Goel R: Association of cisplatin
nephrotoxicity with patient characteristics and cisplatin
administration methods. Cancer Chemother Pharmacol. 40:293–308.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Cancer Institute, . Common
Terminology Criteria for Adverse Events v.3.0 and v.4.0 (CTCAE).
2011, http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
|
|
20
|
Lajer H, Kristensen M, Hansen HH, Nielsen
S, Frøkiaer J, Ostergaard LF, Christensen S, Daugaard G and
Jonassen TE: Magnesium depletion enhances cisplatin-induced
nephrotoxicity. Cancer Chemother Pharmacol. 56:535–542. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Angelen AA, Glaudemans B, van der Kemp
AW, Hoenderop JG and Bindels RJ: Cisplatin-induced injury of the
renal distal convoluted tubule is associated with hypomagnesaemia
in mice. Nephrol Dial Transplant. 28:879–889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Solanki MH, Chatterjee PK, Gupta M, Xue X,
Plagov A, Metz MH, Mintz R, Singhal PC and Metz CN: Magnesium
protects against cisplatin-induced acute kidney injury by
regulating platinum accumulation. Am J Physiol Renal Physiol.
307:F369–F384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vokes EE, Mick R, Vogelzang NJ, Geiser R
and Douglas F: A randomised study comparing intermittent to
continuous administration of magnesium aspartate hydrochloride in
cisplatin-induced hypomagnesaemia. Br J Cancer. 62:1015–1017. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin M, Diaz-Rubio E, Casado A, López
Vega JM, Sastre J and Almenarez J: Intravenous and oral magnesium
supplementations in the prophylaxis of cisplatin-induced
hypomagnesemia. Results of a controlled trial. Am J Clin Oncol.
15:348–351. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Willox JC, McAllister EJ, Sangster G and
Kaye SB: Effects of magnesium supplementation in testicular cancer
patients receiving cis-platin: A randomised trial. Br J Cancer.
54:19–23. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carney SL, Ray C, Ebeling PR, Martin TJ
and Gillies AH: Synthetic human parathyroid hormone-related protein
and rat renal electrolyte transport. Miner Electrolyte Metab.
17:41–45. 1991.PubMed/NCBI
|
|
27
|
Jaïs P, Bouizar Z, Binn M, Vissuzaine C,
Hayem G, Mignon M and Lewin MJ: Parathyroid hormone-related protein
in an esophageal squamous cell carcinoma with tumor-induced
hypercalcemia. Am J Gastroenterol. 92:343–346. 1997.PubMed/NCBI
|
|
28
|
Arany I and Safirstein RL: Cisplatin
nephrotoxicity. Semin Nephrol. 23:460–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Naafs MA, Hackeng WH, Koorevaar G and
Silberbusch J: Abnormal responsiveness of nephrogenous cyclic AMP
excretion following intravenously administered calcium in
normocalcaemic squamous cell cancer patients. Bone Miner.
4:289–298. 1988.PubMed/NCBI
|
|
30
|
Suh SM, Tashjian AH Jr, Matsuo N,
Parkinson DK and Fraser D: Pathogenesis of hypocalcemia in primary
hypomagnesemia: Normal end-organ responsiveness to parathyroid
hormone, impaired parathyroid gland function. J Clin Invest.
52:153–160. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yap AS, Mortimer RH, Jacobi JM, Galligan
JP, Perry-Keene DA and Khafagi FA: Blunted parathyroid response to
correction of hypercalcemia in subjects with squamous cell
carcinoma. Horm Res. 40:222–226. 1993. View Article : Google Scholar : PubMed/NCBI
|